Abstract
Dendritic cell (DC) apoptosis has been shown to play a role in maintaining a balance between tolerance and immunity. However, the mechanisms of how DC apoptosis affects the immune response are unclear. We have shown that in vitro culture of apoptotic DCs with immature DCs, results in their uptake by immature DCs, which subsequently turn into tolerogenic DCs, which then secrete TGF-β1 and induce Foxp3+ regulatory T cells (Tregs). In this study we looked at the effects of apoptotic DCs in vivo. Here we show that apoptotic DCs are taken up by viable DCs in vivo, which suppresses the ability of viable DCs to undergo maturation and subsequent migration to the lymph nodes in response to LPS. Additionally, delivery of apoptotic DCs to LPS inflamed lungs results in resolution of inflammation, which is mediated by the ability of apoptotic DCs to suppress response of viable DCs to LPS. Additionally, apoptotic DCs also induce TGF-β1 secretion in the mediastinal lymph nodes, which results in expansion of Foxp3+ Tregs. Most importantly, we show that delivery of apoptotic DCs followed by OVA in CFA to mice suppresses T cell response to OVA and instead induces de novo generation of OVA-specific Tregs. Furthermore, delivery of apoptotic DCs followed by OVA in CFA results in expansion of Tregs in TCR transgenic (OT-II) mice. These findings demonstrate that apoptotic DCs are taken up by viable DCs in vivo, which promotes tolerance through suppression of DC maturation and induction of Tregs.
Dendritic cells (DCs)3 are potent APCs with the ability to initiate T cell responses. DCs are present in the peripheral tissues where they are constantly engulfing Ags along with dying cells. However, the ability to initiate a T cell response depends on the ability of DC to undergo maturation and subsequently migrate to draining lymph nodes, where in the presence of appropriate cytokines, T cells can be differentiated into a particular lineage in an Ag-specific manner. In addition to priming effector T cell responses, DCs also play a role in induction of tolerance. Due to the ability of DCs to engulf dead cells, they are affected by the death of other cells in close proximity (1–3). According to the danger hypothesis, it has been suggested that injured cells, analogous to necrotic cells provide danger signals which induce activation of DCs and subsequent induction of T cells (4). In contrast to necrotic cells, apoptotic cells are thought to be cleared rapidly without any immunological response (3, 5). Studies have identified necrotic cells acting as adjuvants whereas apoptotic cells have been reported as immunogenic (6–8) or immunosuppressive (9, 10).
Though studies have been conducted to look at the effects of apoptotic or necrotic cells on DCs, there is lack of information on the effects of apoptotic or necrotic DCs on viable DCs. DC apoptosis in itself is thought to be an important event for maintenance of tolerance. Transgenic mice with DC-specific apoptosis defects go on to develop a systemic autoimmune response, which is not observed in transgenic mice harboring apoptosis defects in T and B cells (11–14). Additionally, selective apoptosis of DCs is observed in sepsis patients along with breast cancer patients, with its significance being relatively unclear (15–18).
Immature DCs are highly phagocytic and have the ability to ingest entire cells. Previous studies have reported that ingestion of necrotic cells is recognized as immunostimulatory, whereas ingestion of apoptotic cells appears to be an immunologically null event (3, 19). Despite the importance of DC apoptosis in the immune response, studies have not investigated the effects of ingestion of apoptotic or necrotic DCs by viable DCs. To the best of our knowledge, in addition to our current study, the only other study that has investigated this phenomena is our other work, where we demonstrated that immature DCs efficiently uptake both apoptotic as well as necrotic DCs in vitro (manuscript submitted). The uptake of apoptotic DCs suppresses subsequent LPS-induced maturation and induces secretion of TGF-β1 and up-regulation of TGF- β2 which induces differentiation of naive T cells into Foxp3+ Tregs. Furthermore, we also showed that it is the uptake of only apoptotic DCs that induces TGF-β1 secretion and induction of Foxp3+ Tregs in vitro, for uptake of apoptotic splenocytes did not produce the same effects, indicating that the observed suppression is likely not phosphatidylserine dependent, since even apoptotic splenocytes have phosphatidylserine exposed on the surface which can then interact with its receptor on viable DCs. Moreover, previous studies have also indicated that exposure of murine DCs to apoptotic cells does not induce TGF-β1 secretion (20–22). The aim of our current study was to study the effects of apoptotic DCs on viable DCs in vivo and to test whether they can be used for suppression of existing inflammation and induction of Ag-specific tolerance in mice.
In this study, we show that delivery of apoptotic DCs in mice results in their rapid uptake by viable DCs both in the spleen as well as the draining lymph nodes. Though, the uptake of either necrotic or apoptotic DCs by viable DCs in vivo does not effect their phenotype, albeit, it has an effect on subsequent response to LPS. Apoptotic DCs are able to suppress subsequent LPS-induced DC maturation and DC migration from the periphery to the lymph nodes. Furthermore, apoptotic DCs are able to suppress LPS-induced airway inflammation when they are given together with LPS or after LPS delivery. This has to do with the ability of apoptotic DCs to suppress IL-12 production by viable DCs in the lymph nodes and their ability to instead promote secretion of TGF-β1 from the draining lymph nodes, which contributes to immunosuppression and induces expansion of Foxp3+ Tregs. In addition, we also show that delivery of apoptotic DCs followed by OVA-CFA in mice results in induction of tolerance, via generation of OVA-specific Tregs. Taken together, our results demonstrate that apoptotic DCs can be potentially used for induction of tolerance via generation of Ag-specific Tregs and for suppression of pre-existing inflammation.
Materials and Methods
Mice, Abs and other reagents
C57BL/6 mice were purchased from Charles River Laboratories, and B6.SJL mice were purchased from Taconic and maintained per guidelines of SickKids animal facilities. All the animal studies were reviewed and approved by the SickKids Institutional Committee for humane use of laboratory animals. OT-II mice were purchased from The Jackson Laboratory. OT-II transgenic mice express the mouse α-chain and β-chain TCR that pairs with the CD4 coreceptor and is specific for chicken OVA 323–339 in the context of I-Ab. All the Abs used were directed against mouse Ags. The following Abs were purchased from eBioscience: CD11c PE-Cy7, CD11c PE, CD86 PE, CD80 PE, MHC class II (MHC II) PE, IL-10 Alexa647, IL-12 allophycocyanin, CD25 PE-Cy7, BrdU FITC, Foxp3 PE and the following from BD Biosciences: CD11c FITC, CD25 PE, CD4 FITC, CD3 PE. Isotype control IgGs were obtained from eBioscience and/or Serotec. CFSE was obtained from Molecular Probes; BrdU, OVA, CFA, and propidium iodide (PI) was obtained from Sigma-Aldrich. GM-CSF was obtained from R&D Systems. Cell proliferation ELISA based on BrdU incorporation and chemiluminescent detection was obtained from Roche. Aerrane was obtained from Baxter. Triton X-100 buffer was used for permeabilization for intracellular Foxp3 staining.
Generation of bone marrow-derived DCc
Bone marrow cells were isolated from tibias and femurs of adult mice and cultured in presence of GM-CSF as described previously (23). On day 3, the non-adherent granulocytes, and T and B cells were gently removed and the respective fresh media were added, and two days later the loosely adherent proliferating DC aggregates were dislodged and replated. On day 7, the released, weakly adherent cells were harvested. Of the cells, 85–90% were confirmed to be CD11c+ DCs via FACS analysis.
Isolation of naive CD4+ CD25− T cells
Naive CD4+ CD25− CD62L+ T cells were isolated from spleens of wild-type mice or OT-II mice using CD4+ CD62L+ naive T cell isolation kit in conjunction with MACS columns from Miltenyi Biotec, according to the manufacturer’s instructions.
Induction of DC apoptosis and necrosis
Dendritic cells were cultured on a 6-well dish and irradiated for 2 min with a UV transilluminator, with a peak intensity of 9000 mW/cm2 at the filter surface and a peak emission of 313 nm. Induction of apoptosis was confirmed using apoptosis, necrosis and healthy cell quantification kit (Biotium), following the manufacturer’s instructions. Additionally, staining with annexin V-FITC and PI was also used to confirm apoptosis. Necrosis was induced by first pelleting cells, followed by freeze and thaw.
CFSE labeling of DCs
DCs were harvested and suspended in balanced salt solution (BSS) containing 1 μM CFSE at a concentration of 106 cells/ml. Subsequently, the cells were incubated at 37°C for 10 min. FCS was added to a concentration of 5% and the cells were washed twice.
In vivo assays to assess suppression by apoptotic DCs
Wild-type C57BL/6 mice were injected with 100 μl of saline alone or 4 × 106 apoptotic/necrotic DCs in 100 μl volume saline in footpads, and 6 h later, 1 μg of LPS was injected in a volume of 50 μl. FITC-dextran was injected before LPS delivery, to label DCs in the periphery. Twenty four hours later, popliteal lymph nodes (PLN) were isolated and assessed for migration of FITC-dextran+ CD11c+ DCs along with CD86 expression on CD11c+ DCs via FACS analysis. Furthermore, experiments were also repeated by delivering CFSE-labeled apoptotic or necrotic DCs, followed by assessment of CD86, CD80, and MHC II expression on CFSE+ CD11c+ DCs (indicative of DCs that had taken up apoptotic/necrotic DCs). Additionally, experiments were also performed to look at IL-12+ DCs in the PLN. Single cell suspension from PLN was prepared and cells were cultured in medium containing brefeldin A (1 μg/ml) for 3 h. Subsequently, cells were stained for CD11c, fixed and permeabilized using buffer containing saponin and stained with IL-12 Ab or isotype control, followed by subsequent analysis on a FACSCalibur.
In vivo lung assays
Mice that were 8–11 wks of age, were lightly anesthetized by Aerrane inhalation, and 100 μl of saline alone or 20 μg of LPS in a volume of 100 μl saline with or without apoptotic DCs was delivered intranasally; 10 × 106 apoptotic DCs were delivered together with LPS or 1 day after LPS delivery; 6 h or 24 h later, mice were sacrificed and bronchoalveolar lavage fluid (BALF) along with the mediastinal lymph nodes (MLN) were isolated. To isolate BALF, mouse tracheas were briefly exposed and cannulated with a catheter and a syringe; using ice-cold PBS with 5 mM EDTA, the lower respiratory tract was rinsed 6 times using 1 ml volume to collect inflammatory cells from the airspaces. Total cell counts in BALF were determined by light microscopy, and supernatants were used for determination of TGF- β1 levels by ELISA. Single cell suspension was prepared from MLN and cells were stained with CD4 and Foxp3 to assess percent-age of Tregs via FACS analysis as described previously (23). Additionally, in another set of mice, 48 h after delivery, mice were sacrificed and lungs were isolated for histological analysis. Formalin-fixed, paraffin-embedded mouse lung tissue samples were sectioned at 4 μm and stained with H&E for histological examination of LPS-induced inflammation under a light microscope as described previously (24).
Cytokine assays
We quantified levels of TGF- β1 in BALF and culture supernatants by ELISA using a commercial kit following the manufacturer’s instructions (TGF-β1 kit; R&D Systems). A total of 5 × 105 cells/well were cultured in X-VIVO 20 serum-free medium (Cambrex), and 48 h later, culture supernatants were used for ELISA.
Immunization with OVA and assessment of T cell proliferation in vivo
Mice were immunized with 100 μg of OVA in a 100-μl volume of saline, emulsified in an equal volume of CFA. BrdU-labeling solution was prepared in PBS with a final concentration of 5 mg/ml. After treatment with OVA-CFA, mice were given daily intraperitoneal injections of BrdU (1 mg/mouse) for 7 days. On day 7, mice were sacrificed and draining lymph nodes were isolated. Single cell suspension was prepared, and cells were stained for CD3 and BrdU, as described previously (23).
Suppression assay
OVA-pulsed (0.5 mg/ml) DCs were used as stimulators and naive OT-II CD4+ T cells were used as responders. The stimulators (2.5 × 105 cells/ well) and responder cells (2.5 × 104 cells/well) were cultured in 96-well round-bottom plates at a ratio of 10:1 and suppressors (CD25+), isolated from draining lymph nodes of mice, were added at a ratio of 1:2, 1:10, or 1:30. Proliferation was assessed at day 4 of coculture using BrdU cell proliferation assay. To isolate CD4+ CD25+ T cells, lymph nodes from immunized mice were isolated and a single cell preparation was prepared. Cell suspension was first enriched for CD4+ T cells by depletion of non-CD4+ T cells using CD4+ T cell isolation kit (Miltenyi Biotec) in conjunction with LS columns. The flow-through consisted of enriched CD4+ T cells. Cells were stained for CD25 and cell-sorting was conducted to isolate CD4+ CD25high T cells. FACS analysis confirmed that greater than 94% of the T cells were CD4+ CD25+. Proliferation was assessed at day 4 of coculture using BrdU cell proliferation assay following manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using Student’s t test to compare two groups and ANOVA to compare multiple groups (SPSS 16.0). Significance was set at p < 0.05.
Results
Uptake of apoptotic DCs by viable DCs in vivo
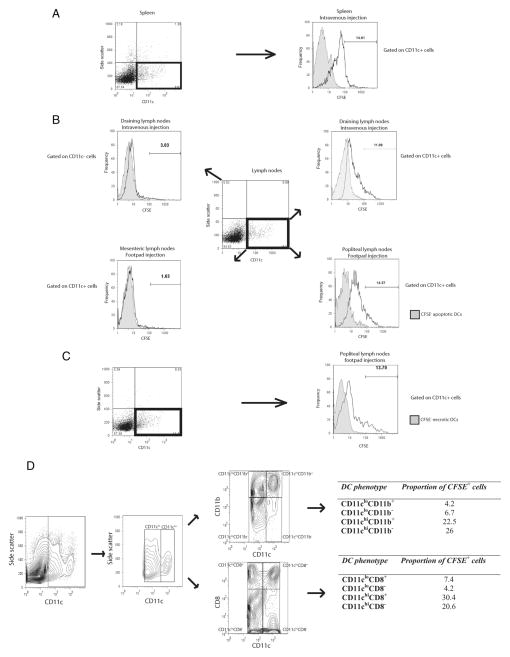
UV-radiation was used to induce DC apoptosis, and apoptotic DCs were characterized as annexin V+ PI−. Our data indicate that upon induction of apoptosis in DCs via UV exposure, most of the DCs become annexin V+ PI+ by 6–10 h after UV exposure (data not shown). To assess whether viable DCs can take up apoptotic DCs in vivo, CFSE-labeled apoptotic DCs were injected i.v. in mice through the tail vein, and 24 h later, spleen and draining lymph nodes were isolated to assess for uptake of CFSE+ apoptotic DCs. We gated on PI− CD11c+ DCs for viable DCs and among them we assessed the proportion of CFSE+ DCs, which were indicative of viable CD11c+ DCs that had taken up apoptotic DCs. Our data show that approximately 14 –15% of CD11c+ DCs in spleen were able to take up apoptotic DCs, whereas 11% of CD11c+ DCs in draining lymph nodes had taken up apoptotic DCs (Fig. 1, A and B). In contrast, only 3% of CD11c− cells (i.e., non-DCs) had taken up CFSE-labeled apoptotic DCs, indicating that the uptake of apoptotic DCs was largely restricted to CD11c+ DCs (Fig. 1B).
FIGURE 1.
Apoptotic or necrotic DCs are taken up by viable DCs in vivo. CFSE-labeled apoptotic DCs were injected i.v. or into footpads of mice, and 24 h later, spleen and draining lymph nodes or PLN were isolated. FACS analysis was performed to look at uptake of apoptotic CFSE+ DCs by CD11c+ viable DCs from spleen and draining lymph nodes (mediastinal, mesenteric, inguinal, brachial, and superficial cervical nodes) (A and B). Similarly FACS analysis was also conducted to look at uptake of necrotic CFSE+ DCs by CD11c+ viable DCs in PLN (C). PI exclusion was performed to gate on viable CD11c+ DCs. Among the PI− viable cells, gating was performed on CD11c+ cells to assess for presence of CFSE+ CD11c+ DCs. Controls included injection of unlabeled apoptotic or unlabeled necrotic DCs along with assessment of CFSE+ cells among CD11c+ viable DCs in the MLN upon footpad injections (B). D, FACS analysis was performed to assess uptake of CFSE+ DCs by different subsets of DCs based on different levels of CD11c, CD11b, and CD8 expression. Data shown is representative of three to four independent experiments.
To further, eliminate the possibility of CFSE+ apoptotic DCs being PI− upon in vivo delivery, we performed delivery of CFSE+ CD45.1 apoptotic DCs to CD45.2 mice (supplemental Fig. 1A)4 and delivery of CFSE+ CD45.2 apoptotic DCs to CD45.1 mice (supplemental Fig. 1B). Our results show that upon delivery of CD45.1+ DCs to CD45.2 mice, 99% of PI− CD11c+ DCs were CD45.2+, among which 11–12% were CFSE+, indicating that it was in fact the host CD45.2+ DCs which took up donor CFSE+ apoptotic DCs (supplemental Fig. 1A). Similarly, upon delivery of CFSE+ CD45.2 apoptotic DCs to CD45.1 mice, 98–99% of PI− CD11c+ DCs were CD45.1+, among which 13% were CFSE+, indicating that it was the host CD45.1+ DCs which took up donor CFSE+ apoptotic DCs (supplemental Fig. 1B).
CFSE-labeled apoptotic DCs were also injected into footpads of wild-type mice. Twenty four hours later PLN were isolated, and the uptake of CFSE+ apoptotic DCs by CD11c+ viable DCs was assessed via FACS analysis. We gated on PI− CD11c+ DCs for viable DCs, among which approximately 15% DCs were CFSE+ (Fig. 1B). In contrast, when we gated on PI− CD11c+ DCs from MLN, only 1.6% of the cells were CFSE+, which was expected, since most of the lymphatic drainage from the footpads is into the PLN (Fig. 1B). Taken together, our findings demonstrate that delivery of apoptotic DCs in mice results in their uptake by viable CD11c+ DCs in both the spleen as well as in the draining lymph nodes. Similarly, CFSE-labeled necrotic DCs were delivered via footpad injections to mice and 24 h later, approximately 11–12% of PI− CD11c+ DCs were CFSE+, indicating uptake of necrotic DCs by viable DCs in vivo (Fig. 1C).
Next, we went on to assess the phenotype of DCs that take up apoptotic DCs (Fig. 1D). CFSE-labeled apoptotic DCs were injected i.v. in mice and 24 h later FACS analysis was performed to assess the uptake of CFSE-labeled apoptotic DCs by different DC subsets. Gating was performed based on CD11c expression to classify CD11c+ DCs as CD11clow or CD11chigh. Among, these two subsets, further gating was performed based on CD11b and CD8 expression. Our findings identify, that it is primarily the CD11chigh DCs that take up apoptotic DCs (48–50%), with much lower proportions of CD11clow DC taking up apoptotic DCs (10–11%). When we further classified DCs based on CD11b expression, no differences were observed, with similar proportions of both CD11chighCD11b− and CD11chighCD11b+ DCs taking up apoptotic DCs. Further gating based on CD8 expression revealed that higher proportions of CD11chighCD8+ (30.4%) and CD11clowCD8+ (7.4%) took up apoptotic DCs compared with CD11chighCD8− (20.6%) and CD11clowCD8− (4.2%), respectively. Overall, these findings indicate that CD11chigh DCs are primarily responsible for taking up apoptotic DCs, with preferential uptake by CD11chighCD8+ DCs.
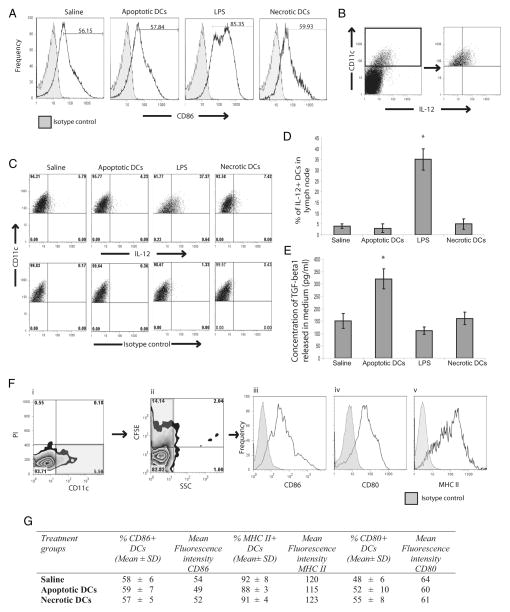
Delivery of apoptotic or necrotic DCs does not result in maturation of viable DCs
To test whether apoptotic or necrotic DCs have any inflammatory effect on viable DCs, footpad injections of either saline, apoptotic DCs, LPS or necrotic DCs were performed in mice and 24 h later, PLN were isolated to assess for maturation of viable DCs (Fig. 2). Gating was performed on PI− CD11c+ DCs, indicative of viable DCs. In response to saline, approximately 56% of DCs in the PLN were CD86+, which indicates the basal levels of CD86 expression on DCs in the lymph nodes. Similarly, delivery of apoptotic DCs did not alter the proportion of CD86+ DCs. In contrast, delivery of LPS resulted in an increase in the levels of CD86+ DCs, which increased from 56% at basal levels to 85% in response to LPS. However, similar to delivery of apoptotic DCs, injection of necrotic DCs did not result in up-regulation of CD86 expression on DCs in the PLN (Fig. 2A). Furthermore, we also looked at the proportion of IL-12+ DCs in the PLN (Fig. 2, B–D). At basal levels and upon injection of apoptotic or necrotic DCs, only 5% of the DCs were IL-12+. In contrast, delivery of LPS resulted in IL-12 production by approximately 30–35% of DCs in the PLN. Overall these results indicated that delivery of apoptotic or necrotic DCs does not result in maturation of viable DCs in vivo. We also isolated the PLN and looked at TGF-β1 secretion from PLN-derived cells in vitro (Fig. 2E). Our findings indicate that PLN cells from mice that received apoptotic DCs secreted significantly elevated levels of TGF-β1.
FIGURE 2.
Delivery of apoptotic DCs to mice results in their uptake by viable DCs, which do not undergo maturation but produce TGF-β1. Mice were injected in footpads with saline, apoptotic DCs, LPS, or necrotic DCs, and 24 h later, PLN were isolated. A, Representative FACS histograms depicting CD86 expression on viable PI− CD11c+ DCs in PLN upon delivery as described above. B, Representative FACS plots depicting gating strategy, used for gating on CD11c+ DCs from the PLN. C, Representative FACS plots depicting IL-12+ cells among gated CD11c+ cells from the PLN. D, Comparison of proportions of IL-12+ CD11c+ DCs in PLN upon delivery as described above. E, Release of TGF-β1 from single cell suspension of PLN cells isolated from mice treated as above upon culture in vitro. F and G, Mice were injected as above, but with CFSE-labeled apoptotic or necrotic cells and 24 h later assessed for CD86 levels on viable DCs in PLN that had taken up apoptotic DCs. F, Gating strategy to gate on viable CD11c+ DCs that had taken up CFSE-labeled apoptotic or necrotic DCs. Gating was first performed on PI− CD11c+ DCs to gate on viable DCs (i), followed by gating on CFSE+ population among PI− CD11c+ DC population (ii), then expression of CD86 (iii), CD80 (iv), and MHC II (v) was assessed on the gated PI− CD11c+ CFSE+ population (iii). G, Proportions of CD86+, CD80+, and MHC II+ DCs among PI− CD11c+ CFSE+ population along with respective MFI. All data are mean ± SD, and representative of n = 4–5 and representative of four independent experiments. *, p < 0.05, statistically significant compared with all the other groups.
We also looked at the phenotype of viable CD11c+ DCs in lymph nodes that had taken up apoptotic or necrotic DCs. To test this, CFSE+ apoptotic or necrotic DCs were delivered by footpad injections and gating was performed first on PI− CD11c+ DCs from the lymph nodes, indicative of viable DCs and among them further gating was performed on CFSE+ DCs to look at CD86, CD80, and MHC II expression levels on viable DCs that had taken up apoptotic or necrotic DCs (Fig. 2F). Viable DCs (59%) that had taken up apoptotic DCs were CD86+, which was similar to the proportion of CD86+ DCs normally observed in lymph nodes upon saline injections (Fig. 2G). Similar proportions of CD86+ DCs were observed among viable DCs that had taken up necrotic DCs (57%). Similarly the proportions of CD80+ and MHC II+ viable CD11c+ DCs that had taken up apoptotic or necrotic DCs was similar to the proportions of lymph node DCs observed upon saline injections (Fig. 2G) Additionally, we also assessed for mean fluorescent intensity (MFI) of CD86, CD80 and MHC II on viable DCs that had taken up apoptotic or necrotic DCs and our results show that uptake of apoptotic or necrotic DCs did not change the MFI of CD86, CD80, or MHC II compared with MFI of these markers on lymph node DCs of mice that received saline only (Fig. 2G). Taken together, these findings indicate that uptake of apoptotic or necrotic DCs by viable DCs in vivo is not recognized as inflammatory.
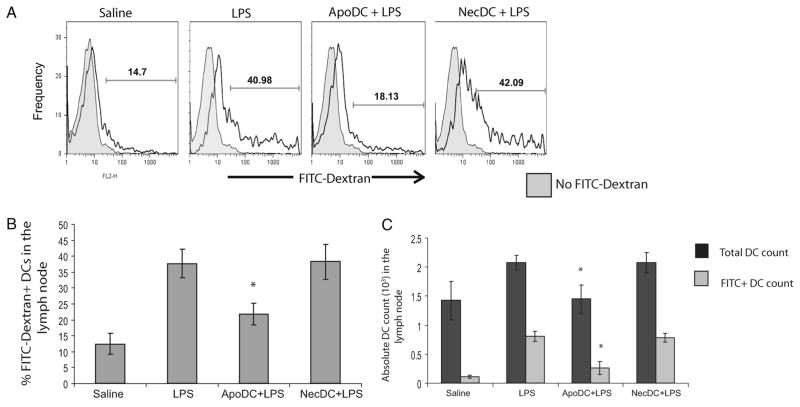
Apoptotic DCs can suppress LPS-induced viable DC migration in vivo
To confirm the immunosuppressive properties of apoptotic DCs in vivo, we looked at the effects of apoptotic or necrotic DCs on LPS-induced DC migration from the periphery to PLN upon footpad injections by gating on CD11c+ PI− cells (viable cells) (Fig. 3). FITC-dextran injection was used to label DCs in the periphery to monitor their migration to the lymph nodes as described previously (23). Upon delivery of saline, approximately 14–15% of CD11c+ DCs in the lymph nodes were FITC-dextran+, which indicates the basal levels of DC migration from the periphery to the lymph nodes (Fig. 3, A and B). In contrast, in response to LPS delivery, approximately 40% of CD11c+ DCs in PLN were FITC-dextran+, indicating increased migration of DCs from the periphery to PLN. This was expected, as LPS in a known inducer of DC maturation and migration. Similarly, delivery of necrotic DCs before LPS delivery produced similar results, indicating that necrotic DC delivery was not able to dampen LPS-induced DC migration from the periphery to the PLN. However, upon injection of apoptotic DCs before LPS administration, only 18% of the DCs in PLN were FITC-dextran+, i.e., were from the periphery, indicating that apoptotic DCs suppressed LPS-induced DC migration (Fig. 3, A and B). This was further confirmed by measuring absolute count of total DCs in the PLN along with the absolute count of FITC-dextran+ DCs (indicative of DCs that had migrated from the periphery to PLN); both were significantly reduced upon injection of apoptotic DCs before LPS delivery compared with mice that had received LPS alone (Fig. 3C). In fact, the levels of DC migration observed upon delivery of apoptotic DCs before LPS delivery was similar to the basal levels, seen upon injection of saline only. Overall, the findings indicate that injection of apoptotic DCs before LPS delivery was able to suppress LPS-induced DC migration from the periphery to the lymph nodes. In contrast, delivery of necrotic DCs before LPS delivery had no effect on LPS-induced DC migration from the periphery to PLN.
FIGURE 3.
Apoptotic DCs suppress LPS-induced DC migration from periphery to draining lymph nodes in vivo. Mice were injected in footpads with FITC-dextran along with saline, FITC-dextran before LPS delivery (LPS), FITC-dextran along with apoptotic DCs before LPS delivery (ApoDC + LPS), or FITC-dextran along with necrotic DCs (NecDC + LPS) before LPS delivery. Twenty four hours later, percentage of FITC-dextran+ cells among viable CD11c+ DCs were analyzed in the PLN using FACS analysis. PI exclusion was used to exclude injected apoptotic/necrotic DCs from viable DCs. A, Representative FACS histograms looking at FITC-dextran+ DCs among CD11c+ DCs from the PLN of mice treated, as described above. B, Comparison of proportion of FITC-dextran+ DCs in the PLN of mice treated, as described above. C, Absolute DC count in PLN was measured using FACS analysis with total DC count corresponding to total CD11c+ count and FITC-dextran+ CD11c+ DC count corresponding to DCs that had migrated from the periphery to PLN. All data are means ± SD obtained from n = 4–5 for each group. *, p < 0.05, statistically significant compared with NecDC + LPS and LPS.
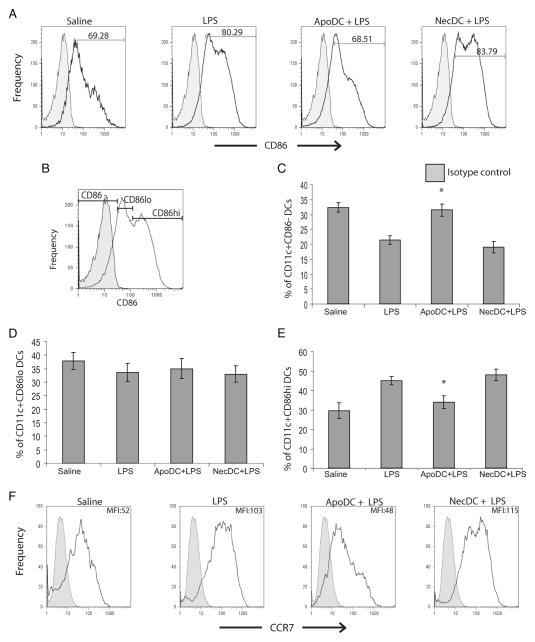
Apoptotic DCs suppress LPS-induced DC maturation in vivo
To assess the effects of apoptotic DCs on DC maturation in vivo, we looked at the expression of CD86 and CCR7 on DCs in PLN after delivery of apoptotic or necrotic DCs before LPS delivery (Fig. 4). DCs normally express very low levels of CD86, which is up-regulated in response to inflammatory stimuli, such as LPS. Upon saline delivery, approximately 69% of CD11c+ DCs in PLN were CD86+, which increased to 80–83% upon injection of LPS or necrotic DCs before LPS delivery (Fig. 4A). In contrast, injection of apoptotic DCs before LPS delivery reduced this to 68%, which was similar to the proportion of CD86+ DCs observed in PLN of mice which received saline only. In addition, a higher proportion of DCs were CD86− upon injection of apoptotic DCs before LPS delivery (30%) compared with LPS alone (21%) or LPS after necrotic DC injection (20%) (Fig. 4C). Moreover, though the proportion of CD86low DCs was similar among all the groups, the proportion of mature DCs (i.e., CD86high DCs) was significantly reduced upon injection of apoptotic DCs before LPS delivery compared with the rest of the groups, and was similar to the levels seen with saline injections (Fig. 4, D and E). We also looked at the expression levels of CCR7, which is a chemokine receptor, up-regulated on DCs as they undergo maturation (Fig. 4F). Our results indicate that though there was not a significant difference in the proportion of DCs that were CCR7+, there was indeed a difference in the levels of CCR7 expression. Upon saline delivery, the MFI of CCR7 on CD11c+ DCs in PLN was 52, which increased to 103 upon LPS delivery and increased to 115 upon delivery of necrotic DCs before LPS delivery. In contrast, injection of apoptotic DCs before LPS delivery reduced the MFI to 48, which was indeed similar to the MFI of CCR7 expression seen only with saline delivery. These results clearly show that apoptotic DCs but not necrotic DCs can suppress maturation of viable DCs in response to an inflammatory stimulus in vivo.
FIGURE 4.
Apoptotic DCs suppress LPS-induced DC maturation in vivo. Mice were injected in footpads with saline, LPS, apoptotic DCs before LPS delivery (ApoDC + LPS), or necrotic DCs (NecDC + LPS) before LPS delivery. Twenty four hours later, FACS staining was performed to assess expression of CD86 by CD11c+ viable DCs in the PLN. PI exclusion was used to gate out dead DCs. A, Representative FACS expression profile of CD86 on PI− CD11c+ DCs from treated animals as described above. B, Shown here is the gating strategy used to classify DCs as CD86−, CD86low, and CD86high. Proportions of CD86− (C), CD86low (D), and CD86high (E) CD11c+ DCs in PLN of groups of mice injected as described. F, Representative FACS expression profile of CCR7 on PI− CD11c+ DCs along with corresponding MFI from treated animals as described above. All data are means ± SD obtained from n = 4–5 for each group. *, p < 0.05 for ApoDC + LPS vs LPS and NecDC + LPS for C and E.
Intranasal delivery of apoptotic DCs can suppress LPS-induced inflammation and induce expansion of Tregs
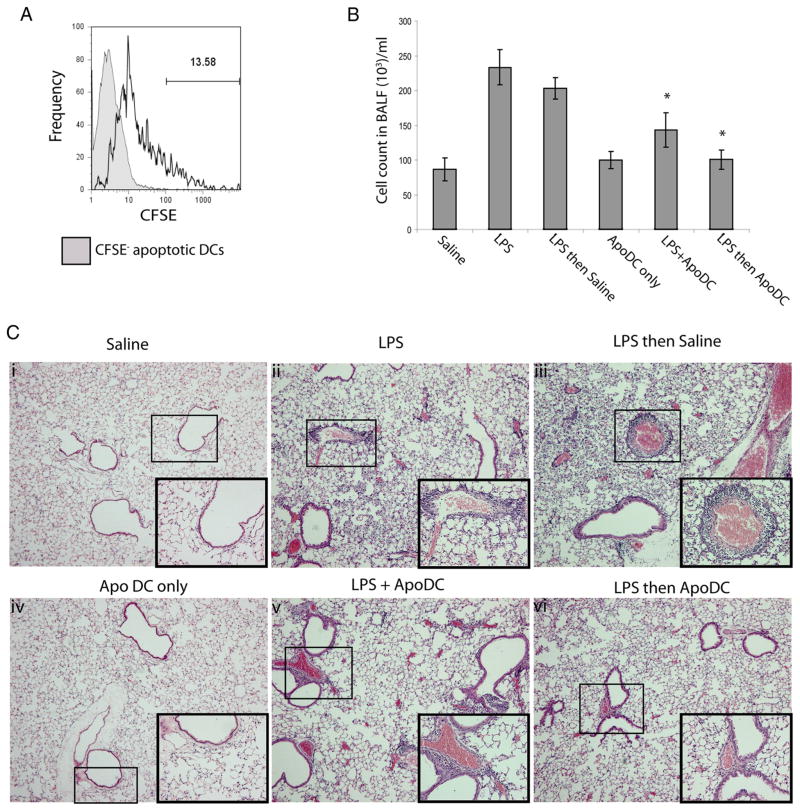
To further confirm the immunosuppressive and tolerogenic properties of apoptotic DCs, we assessed the effects of apoptotic DCs in suppression of airway inflammation (Fig. 5). Delivery of CFSE+ apoptotic DCs resulted in uptake by viable CD11c+ DCs that could be detected in the MLN (Fig. 5A). Using wild-type mice, we performed intranasal delivery of apoptotic DCs both with LPS or 1 day after LPS delivery. In response to delivery of LPS, there was approximately a 3-fold increase in absolute cell count in BALF compared with mice that received saline only (Fig. 5B). In contrast, delivery of LPS with apoptotic DCs or delivery of apoptotic DCs 1 day after LPS delivery resulted in significant reductions in absolute cell count in BALF compared with LPS delivery alone. However, there was a slightly greater reduction in absolute cell count in BALF upon delivery of apoptotic DCs at 1 day after LPS delivery compared with delivery of LPS together with apoptotic DCs. In addition, we also performed histopathological assessment of inflammation in the airways by staining lung sections with H&E, followed by observation under a light microscope (Fig. 5C). Intranasal delivery of saline or apoptotic DCs alone did not result in any infiltration within the airways. However, upon delivery of LPS, perivascular inflammation as well as both parenchymal and intraalveolar infiltrates of neutrophils, macrophages and lymphocytes could be observed throughout the lungs, which was also the case upon delivery of LPS followed by saline. In contrast, delivery of LPS along with apoptotic DCs resulted in reduced levels of cellular infiltrates compared with LPS delivery alone. This reduction in the extent of cellular infiltrate was even more profound upon delivery of apoptotic DCs 1 day after LPS delivery.
FIGURE 5.
Apoptotic DCs can suppress LPS-induced airway inflammation. The following treatments were delivered to mice by intranasal administration: (i) saline alone, (ii) LPS alone, (iii) LPS then saline, (iv) apoptotic DC alone (ApoDC only), (v) LPS with apoptotic DCs (LPS + ApoDC), or (vi) LPS followed by delivery of apoptotic DCs after 1 day (LPS then ApoDC). A, Representative FACS histogram looking at uptake of CFSE-labeled apoptotic DCs by viable PI+ CD11c+ DCs in the mediastinal lymph node upon intranasal delivery. B, Total cell count in BALF of mice, 24 h post delivery. C, Histopathological analysis of H&E stained lung sections to assess inflammation in the airways 48 h after delivery conditions as described above (i–vi). Upon intranasal delivery of saline or apoptotic DCs alone, no inflammation was present within the airways. Perivascular inflammation as well as both parenchymal and intraalveolar infiltrates of neutrophils, macrophages, and lymphocytes were detected throughout the lungs upon delivery of LPS, which was also the case upon delivery of LPS followed by saline. In contrast, delivery of LPS along with apoptotic DCs resulted in a reduction of inflammatory cell infiltrate compared with LPS delivery alone. This reduction was even more obvious for delivery of LPS followed by apoptotic DCs. Images were taken at 50× magnification and insets refer to a 200×-magnified region of the perivascular inflammation indicated by a box within the same image. Images are representative of n = 3–4 mice for each group. Data is presented as mean ± SD, obtained from n = 4 mice per group, representative of three independent experiments. *, p < 0.05, LPS + ApoDC or LPS then Apo DC vs LPS and LPS then saline.
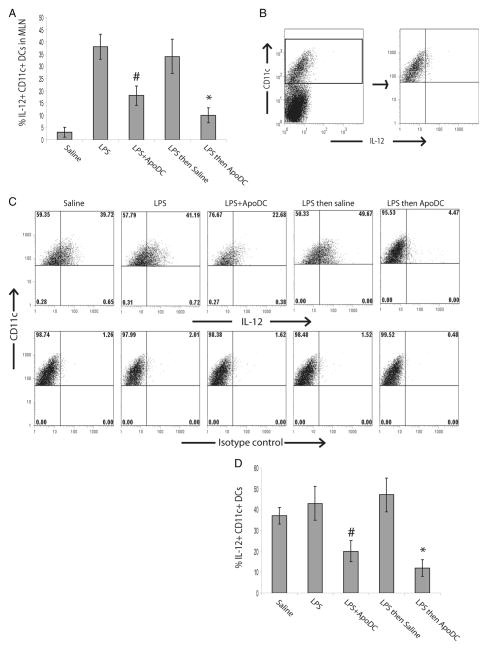
To further study the extent of immunosuppression induced by apoptotic DCs, we also looked at the proportion of IL-12+ DCs in MLN of treated mice (Fig. 6A). Approximately 5% of CD11c+ DCs in the MLN were detected as IL-12+ DCs upon saline delivery, which increased to 35–40% upon intranasal delivery of LPS. Similarly, 35–40% of DCs were IL-12+ in MLN of mice that received LPS followed by saline delivery. In contrast, delivery of apoptotic DCs with LPS or LPS followed by apoptotic DCs resulted in suppression of IL-12 production by DCs in MLN (Fig. 6A). Approximately, 15–17% of the DCs were IL-12+ in MLN of mice which received LPS with apoptotic DCs and 8–10% were IL-12+ in MLN of mice which received LPS followed by apoptotic DCs. This was overall a significant reduction compared with the 35–40% IL-12+ DCs observed in MLN of mice which received LPS only. Next, we assessed the capacity of CD11c+ DCs from MLN of treated mice to respond to LPS restimulation in vitro. MLN from treated mice were isolated and single cell suspension was prepared, which was cultured in presence of LPS and 24 h later, proportion of IL-12+ CD11c+ DCs was assessed by FACS analysis (Fig. 6, B–D). Exposure of cells from MLN of mice which received saline alone had a robust response to LPS, which approximately 35–40% of DCs as IL-12+. Similarly, LPS exposure of cells from MLN of mice which received LPS only or LPS followed by saline, resulted in approximately 40–50% of DCs producing IL-12. In contrast, cells from mice treated with LPS in combination with apoptotic DCs or LPS followed by apoptotic DCs had a significantly diminished ability to respond to LPS (Fig. 6 B–D). Only 20% of DCs from mice that received LPS with apoptotic DCs were IL-12+ upon restimulation with LPS. Similarly, only 10–12% of DCs from mice which received LPS followed by apoptotic DCs were IL-12+ upon LPS restimulation. Taken together, these findings indicate that delivery of apoptotic DCs with LPS or after LPS exposure, results in suppression of inflammation. This may be due in part to the suppressive effects of apoptotic DCs on IL-12 production by CD11c+ DCs in the lymph nodes in response to LPS.
FIGURE 6.
Intranasal delivery of apoptotic DCs suppresses LPS-induced IL-12 production in DCs in MLN. Mice were administered the following by intranasal delivery: saline, LPS, LPS with apoptotic DCs (LPS + ApoDC), LPS followed by saline after 1 day (LPS then saline), or LPS followed by delivery of apoptotic DCs after 1 day (LPS then ApoDC). A, Proportion of IL-12+ CD11c+ DCs in MLN, 24 h after delivery. B—D, After 24 h, MLN were isolated and a single cell suspension was prepared that was cultured in the presence of LPS for 24 h. B, Representative FACS plots depicting gating strategy, used for gating on CD11c+ DCs from the MLN single cell suspension. C, Representative FACS plots looking at IL-12+ CD11c+ DCs in culture after LPS exposure. D, The graph compares the proportion of IL-12+ CD11c+ DCs in culture after LPS exposure. Data are presented as mean ± SD, obtained from n = 4 mice for each group, representative of four independent experiments. *, p < 0.05, LPS then ApoDC vs all the other groups except LPS + ApoDC, #, p < 0.05, LPS + ApoDC vs all other groups except LPS then ApoDC.
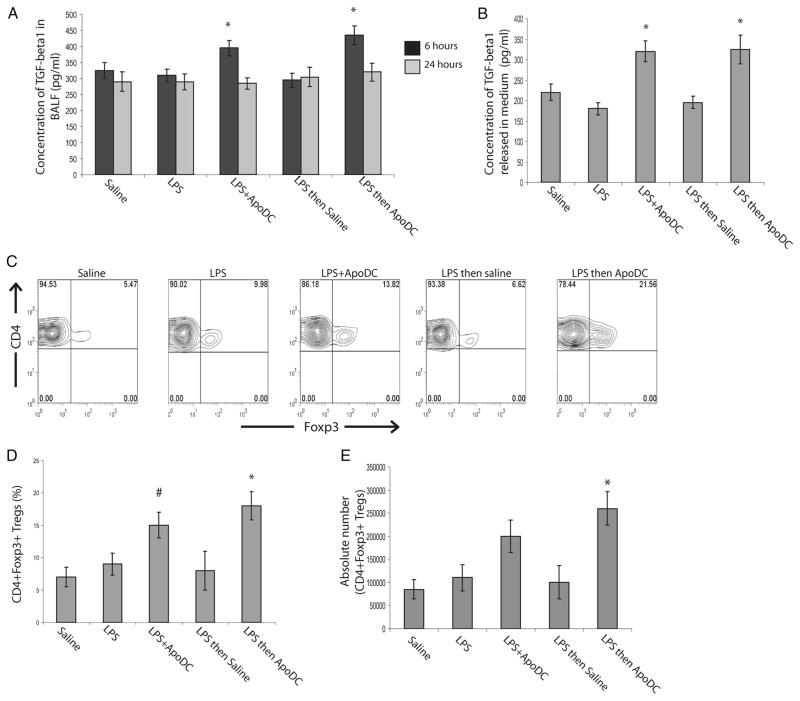
Since, our experiments showed that delivery of apoptotic DCs resulted in TGF-β1 secretion from the lymph nodes, we looked at the secretion of TGF-β1 in BALF fluid after intranasal delivery of apoptotic DCs (Fig. 7A). Delivery of LPS or LPS followed by saline did not result in any significant change in total TGF-β1 levels in BALF compared with the levels in BALF of mice that received saline alone. In contrast, delivery of apoptotic DCs (both in combination with LPS or 24 h after LPS delivery) resulted in significant increase in TGF-β1 in BALF (Fig. 7A). However, the increase was transient, since it could only be observed at 6 h after delivery and returned to normal levels after 24 h. We also looked at TGF-β1 secretion from the MLN of mice that received apoptotic DCs (Fig. 7B). MLN were isolated 24 h after delivery of apoptotic DCs in combination with LPS or apoptotic DCs 1 day after LPS delivery; a single cell suspension was made, which was cultured in serum free media for 48 h, after which TGF-β1 levels in the medium were assessed by ELISA. Our results indicate that there was a significant increase in TGF-β1 secretion from MLN cells isolated from mice that received LPS in combination with apoptotic DCs or apoptotic DCs alone 24 h after LPS delivery as compared with mice that received saline alone, LPS alone or LPS followed by saline delivery (Fig. 7b). Since there was enhanced TGF-β1 secretion from lymph nodes, we assessed whether there was Treg expansion occurring in MLN upon apoptotic DC delivery (Fig. 7, C and D). Approximately 5–8% of CD4+ T cells in MLN of mice that received saline alone, LPS alone or LPS followed by saline delivery were CD4+ Foxp3+ Tregs. In contrast, in mice that received LPS in combination with apoptotic DCs, the proportion of CD4+ Foxp3+ Tregs increased to 15%. However, in mice that had inflammation before apoptotic DC delivery (i.e., received LPS followed by apoptotic DCs), there was a significant increase in the proportion of CD4+ Foxp3+ Tregs, which increased to approximately 18–22% of CD4+ T cells in the MLN (Fig. 7, C and D). Similarly, increase in the absolute count of CD4+ Foxp3+ Tregs was also observed in the MLN (Fig. 7E). This increase in the proportion of CD4+ Foxp3+ Tregs was specific to MLN, and was not observed in the spleen or the distal lymph nodes (data not shown). Therefore, our findings indicate that intranasal delivery of apoptotic DCs can mediate resolution of inflammation firstly through induction of TGF-β1 secretion, which itself is known to be immunosuppressive and secondly through their ability to suppress IL-12 production by DCs in lymph nodes. Additionally, our data also indicates that upon intranasal delivery of apoptotic DCs, there is secretion of TGF-β1 in MLN, which promotes expansion of Tregs.
FIGURE 7.
Intranasal delivery of apoptotic DCs results in the secretion of TGF-β1 and Treg expansion. Mice were administered the following by intranasal delivery: saline, LPS, LPS with apoptotic DCs (LPS + ApoDC), LPS followed by saline after 1 day (LPS then saline), or LPS followed by delivery of apoptotic DCs after 1 day (LPS then ApoDC). A, Concentration of total TGF-β1 levels in BALF, 6 h and 24 h after delivery. B, Concentration of total TGF-β1 that is released into medium 48 h after culture of MLN cells isolated from the mice treated as above. C, Representative FACS plots looking at Foxp3+ cells among CD4+ cells from the MLN of mice, 1 day after above-mentioned treatments. D, Histogram compares the percentage of CD4+ Foxp3+ Tregs normalized to total CD4+ T cells in the MLN of mice, 1 day after above-mentioned treatments, assessed by FACS analyses. E, Comparison of absolute count of CD4+ Foxp3+ Tregs from MLN of mice, 1 day after above-mentioned treatments. All data are presented as mean ± SD, obtained from n = 4–5 mice per group, representative of four independent experiments. *, p < 0.05, LPS + ApoDC or LPS then ApoDC vs all other groups for A and B; LPS then ApoDC vs all other groups except LPS + ApoDC for D. #, p < 0.08, LPS + ApoDC vs LPS then saline.
Apoptotic DCs can induce immune tolerance in vivo
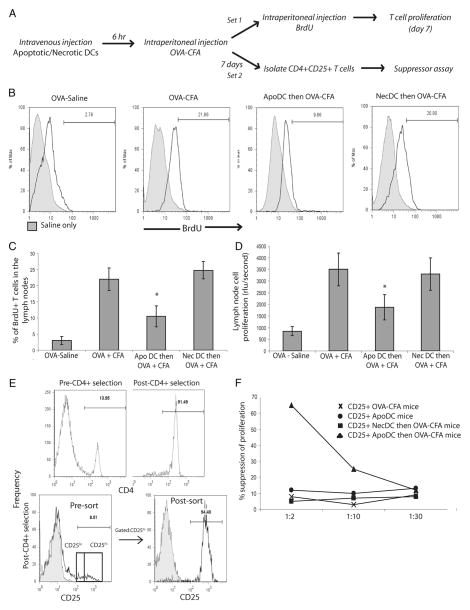
Since our in vitro findings suggested that viable DCs prime differentiation of naive T cells into Tregs and do not induce T cell proliferation upon apoptotic DC uptake, we assessed whether apoptotic DCs can suppress T cell proliferation in vivo (Fig. 8A). C57BL/6 mice were injected with OVA in saline or OVA in CFA, which would prime a robust T cell response directed against OVA. To test the efficacy of apoptotic DCs in inducing tolerance, 6 h before injection of OVA in CFA, mice were injected with apoptotic DCs or necrotic DCs i.v.. Mice were given intraperitoneal injections of BrdU to label proliferating cells and 7 days after delivery of OVA in CFA, mice were sacrificed and T cell proliferation was assessed in draining lymph nodes by looking at the proportion of BrdU+ CD3+ T cells. Results indicate that upon delivery of OVA in saline, approximately 3– 4% of the total CD3+ T cells in draining lymph nodes were proliferating (i.e., BrdU+) (Fig. 8, B and C). However, upon injection of OVA in CFA, the number increased to approximately 21–22%, indicating that CFA primed a robust immune response against OVA, as expected. Similarly, 22–23% of the T cells in draining lymph nodes of mice that received necrotic DCs before delivery of OVA in CFA were BrdU+, indicating that necrotic DCs had no effect on the ability of viable DCs to prime a T cell response in vivo. In contrast, upon delivery of apoptotic DCs before delivery of OVA in CFA, approximately 10% of the T cells were BrdU+ (i.e., proliferating), which was a marked reduction compared with 23% BrdU+ T cells seen with delivery of OVA in CFA (Fig. 8, B and C). Therefore, our results demonstrate that delivery of apoptotic DCs before delivery of OVA in CFA was able to suppress the priming of immune response directed toward OVA. Furthermore, 7 days after delivery, draining lymph nodes were isolated and total cells were cultured in the presence of OVA for 4 days to assess for OVA-specific T cell proliferation (Fig. 8D). Cells isolated from lymph nodes of mice that received apoptotic DCs before delivery of OVA in CFA had significantly lower levels of proliferation compared with the cells isolated from mice which received OVA in CFA alone, or necrotic DCs before delivery of OVA in CFA (Fig. 8D).
FIGURE 8.
Apoptotic DCs induce Ag-specific Tregs in vivo. A, Protocol for induction of OVA-specific tolerance in mice. C57BL/6 mice were injected i.v. with OVA in saline (OVA-saline), OVA in CFA (OVA-CFA), apoptotic DCs followed by OVA-CFA after 6 h (ApoDC then OVA-CFA), or necrotic DCs followed by OVA-CFA after 6 h (NecDC then OVA-CFA). For the next 7 days, one set of mice were given intraperitoneal injections of BrdU as described in Materials and Methods and the other set of mice was used after 7 days for isolation of CD4+ CD25+ cells. One week later, T cell proliferation in draining lymph nodes (inguinal and mesenteric) was assessed via BrdU incorporation. B, Representative histograms depicting BrdU incorporation by CD3+ T cells in the draining lymph nodes of immunized mice. C, Comparison of percentage of BrdU+ T cells, expressed as percentage of total CD3+ T cells, from draining lymph nodes of immunized mice. D, After 7 days, draining lymph nodes were isolated and total cells were cultured with OVA protein for 4 days and Ag-specific T cell proliferation was determined by BrdU incorporation assay. E, After 7 days, CD4+ CD25+ T cells were isolated from draining lymph nodes of mice injected with OVA-CFA (CD25+ OVA-CFA mice), apoptotic DCs (CD25+ ApoDC mice), necrotic DC followed by OVA-CFA (CD25+ NecDC then OVA-CFA mice), or apoptotic DCs followed by OVA-CFA (CD25+ ApoDC then OVA-CFA). Magnetic selection was used to enrich for CD4+ cells (91%) purity, which was subjected to cell sorting for isolation of CD25high population (94% purity). F, The isolated CD4+ CD25+ T cells were then added to a coculture of naive OT-II CD4+ T cells and OVA-pulsed BMDCs at three different ratios of 1:2, 1:10, and 1:30. Four days later, cell proliferation was assessed by BrdU incorporation assay and data is presented as percentage of suppression of T cell proliferation compared with that of OT-II CD4+ T cells cultured in presence of OVA-pulsed BMDCs without addition of any CD4+ CD25+ T cells. All data presented as mean ± SD, representative of n = 4 mice per group and representative of three independent experiments. *, p < 0.05, ApoDC then OVA-CFA vs all other groups except OVA-saline.
We also wanted to confirm the ability of apoptotic DCs to induce Ag-specific Tregs in vivo (Fig. 8, E and F). Mice were injected with OVA in saline, OVA in CFA, apoptotic DCs before delivery of OVA in CFA or necrotic DCs before delivery of OVA in CFA. After 7 days, draining lymph nodes were isolated, and magnetic separation was performed to enrich for CD4+ T cells, followed by cell sorting for CD4+ CD25high cells, which would include CD4+ Foxp3+ Tregs (Fig. 8E). Since wild-type mice are not expected to have tolerance toward OVA, they should not have any Tregs specific to OVA. In contrast, if apoptotic DCs are able to induce OVA-specific Tregs, then Tregs from mice treated with apoptotic DCs before OVA-CFA delivery should be able to suppress OVA-specific T cell proliferation in vitro. To test this, CD4+ CD25+ T cells isolated from different groups of mice were added as “suppressors” to a coculture of naive CD4+ CD25− T cells isolated from OT-II mice which would have specificity for OVA, acting as “responders” along with dendritic cells pulsed with OVA to stimulate OT-II cells, acting as “stimulators”. CD4+ CD25+ were added at ratios of 1:2, 1:10 and 1:30 to naive OT-II CD4+ CD25− T cells in the coculture. CD4+ CD25+ T cells isolated from mice immunized with OVA-CFA failed to suppress proliferation, indicating that there were no Tregs specific for OVA in the immunized mice (Fig. 8F). This was expected because immunization with OVA in CFA led to a robust immune response. Similarly, CD4+ CD25+ T cells isolated from mice, which only received apoptotic DCs, failed to suppress T cell proliferation. This was again expected, since the animals, which received apoptotic DCs, were not exposed to OVA. Therefore, there was no differentiation of naive T cells into Tregs with specificity for OVA. CD4+ CD25+ T cells isolated from mice, which received necrotic DCs before OVA-CFA delivery, were again not able to suppress T cell proliferation, indicating that there were no OVA-specific Tregs generated in mice upon delivery of necrotic DCs before OVA-CFA. In contrast, CD4+ CD25+ T cells isolated from animals, which received apoptotic DCs before OVA-CFA delivery, were able to suppress proliferation of OT-II CD4+ CD25− T cells in a dose-dependent manner with approximately 70% suppression at 1:2 and 30% suppression at 1:10 ratio of suppressors to responders (Fig. 8F). This indicates that delivery of apoptotic DCs, before delivery of OVA-CFA, was able to induce de novo generation of OVA- specific Tregs in mice.
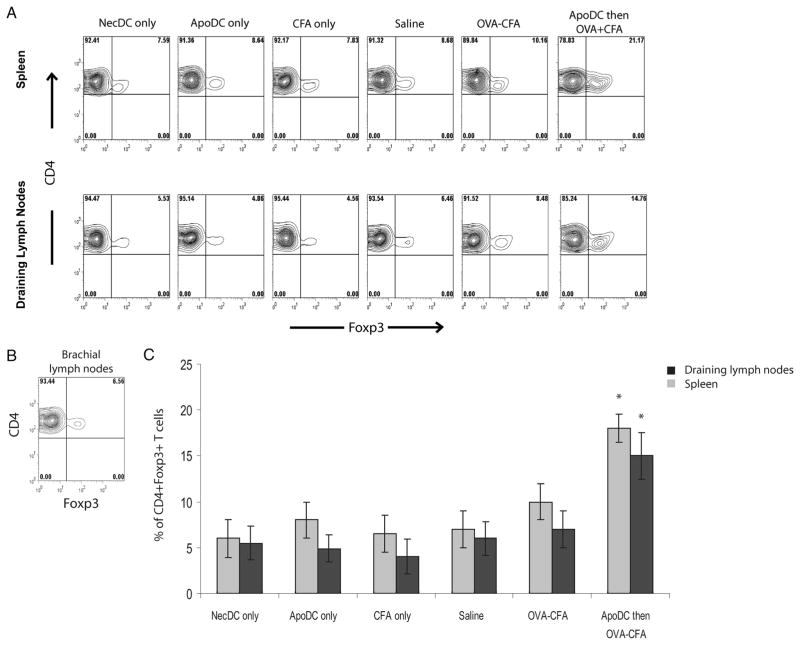
In addition to the ability of apoptotic DCs to induce de novogeneration of Tregs, we also wanted to assess their ability to induce expansion of Tregs. OT-II mice were given i.v. injections of saline, OVA-CFA or apoptotic DCs followed by OVA-CFA. 24 h later, the proportions of Tregs in spleen as well as the draining lymph nodes were assessed via FACS analysis. Upon injection of saline, the proportions of Tregs relative to total CD4+ T cells were approximately 8% and 5% in spleen and draining lymph nodes, respectively (Fig. 9). After injection of OVA-CFA, there was a slight increase in the proportion of Tregs, which increased to approximately 10% in the spleen and 7% in the draining lymph nodes, albeit the difference compared with saline delivery was not statistically significant. In contrast, 24 h after delivery of apoptotic DCs before OVA-CFA delivery, the proportions of Tregs increased to approximately 20% in the spleen and 15% in the draining lymph nodes, which was around 2–3 fold expansion compared with the proportions observed in mice that received saline only. The expansion of Tregs upon delivery of apoptotic DCs before OVA-CFA was not observed in brachial lymph nodes, which was expected since OVA-CFA was delivered via the intraperitoneal route and it is primarily the lymphatic drainage from the thoracic region that reaches the brachial lymph nodes (Fig. 9B). Therefore, these results clearly indicate that delivery of apoptotic DCs before Ag stimulation can induce expansion of Ag-specific Tregs. Collectively, our results indicate that delivery of apoptotic DCs before Ag delivery in vivo can induce de novo generation of Ag-specific Tregs along with expansion of existing Tregs, thereby preventing induction of a T cell response against the delivered Ag.
FIGURE 9.
Apoptotic DCs induce expansion of Tregs in vivo. A, Representative FACS profile of Foxp3 expression on CD4+ cells from spleens and draining lymph nodes of OT-II mice immunized with necrotic DCs (NecDC only), apoptotic DCs (ApoDC only), CFA (CFA only), saline (saline only), OVA-CFA or with apoptotic DCs followed by OVA-CFA (ApoDC then OVA-CFA). Immunization was performed as shown in Fig. 8A. One day after immunization, proportions of Tregs were assessed in spleen along with draining lymph nodes (inguinal and mesenteric) via FACS analysis. B, Representative FACS profile of Foxp3 expression on CD4+ cells from brachial lymph nodes of mice immunized i.v. with apoptotic DCs followed by OVA-CFA. C, Comparison of percentage of CD4+ Foxp3+ Tregs in spleen and draining lymph nodes (inguinal and mesenteric) from OT-II mice immunized as described above. Data presented as mean ± SD, representative of n = 3 mice per group. *, p < 0.05, ApoDC then OVA-CFA vs all the groups.
Discussion
Cumulatively, our results clearly indicate that apoptotic DCs have a tolerogenic effect on the immune response in vivo. To our knowledge, this is the first study, which assessed the effects of apoptotic DCs on immune response in vivo. Our findings indicate that delivery of apoptotic DCs in mice results in their rapid engulfment by viable DCs in both the spleen as well as draining lymph nodes. However, the delivery of apoptotic or necrotic DCs has no effect on the surface phenotype of viable DCs which take them up and nor is their uptake recognized as inflammatory by viable DCs. In contrast to the delivery of necrotic DCs, delivery of apoptotic DCs results in TGF-β1 production in the draining lymph nodes. If an inflammatory stimulus such as LPS is delivered after delivery of apoptotic DCs, then apoptotic DCs suppress migration of DCs from the periphery to the draining lymph nodes. In addition, the maturation of DCs in response to the inflammatory stimuli is also inhibited. Furthermore, this results in suppression of T cell response to the inflammatory agent and instead results in de novo generation of Ag-specific Tregs along with expansion of existing Tregs. Overall, our findings identify that priming of immune system in vivo with apoptotic DCs results in tolerance induction to a subsequent immunogen.
In addition to induction of tolerance, our findings also identify that apoptotic DCs can be used for suppression of existing inflammation. Our findings show that apoptotic DCs are quite potent at suppression of LPS-induced inflammation in the airways. The suppression is actually mediated in three different steps. Firstly, apoptotic DCs suppress the induction of IL-12 by viable DCs in the draining MLN and make them non-responsive to a further inflammatory insult. Secondly, apoptotic DCs also induce transient TGF- β1 secretion in BALF, which is known to be immunosuppressive. Thirdly, apoptotic DCs induce TGF-β1 secretion in the MLN, which results in Treg expansion. Taken together, these findings indicate that apoptotic DCs are very potent in suppression of existing inflammation. The tolerogenic effects of DCs reported in this study could be relevant for generation of immunosuppression and Ag-specific tolerance for many applications in transplantation, allergy and autoimmunity. These findings could have implications in suppression of airway immune response as is the case in asthma, lung transplantation and also upon delivery of gene therapy vectors. In human lung transplantation, it has been shown that grafts with high levels of inflammatory cytokines develop severe graft dysfunction following reperfusion (25–27). Perhaps, delivery of apoptotic DCs before harvesting the organ may prevent induction of reperfusion injury by suppressing inflammatory response, which could potentially enhance graft survival and limit lung inflammation. In addition, airway immune responses are a barrier to the success of airway gene therapy (28). Our previous work has shown that even, so called non-immunogenic vectors such as helper-dependent adenoviral vectors are able to potentiate cytotoxic T cell response upon airway delivery at low doses (23). This poses a serious barrier toward readministration of gene therapy vectors. Therefore, it is feasible that using apoptotic DCs perhaps transient immunosuppression and tolerance can be induced, which would prevent subsequent immune response against gene therapy vectors.
Though, some earlier studies were conducted to look at the effects of apoptotic cells in vivo, most relied on the use of apoptotic splenocytes as a source of apoptotic cells. Moreover, some studies have even concluded that apoptotic cells are better than necrotic cells to induce immune responses. Recently, a study was conducted where DCs were treated with apoptotic splenocytes and subsequently delivered to mice. It was identified that apoptotic cells impact the immune response by triggering DCs to produce high levels of NO which attracts T cells and renders them non-responsive (29). Earlier studies also looked at the effects of delivering apoptotic splenocytes loaded with OVA to mice (30). Upon delivery, these splenocytes were rapidly taken up by DC and contributed to deletion of T cells. In addition to the ability of apoptotic splenocytes to induce deletional tolerance, some studies have also reported that apoptotic splenocytes can induce immunological tolerance through induction of Tregs (31–33). Along with apoptotic splenocytes, apoptotic pancreatic cells have also been shown to promote tolerance to islet Ags and prevent diabetes development (34). It is important to note that none of these studies looked at the effects of apoptotic DCs in vivo. Therefore, our studies are the first ones, which specifically looked at the effects of apoptotic DCs and clearly showed their potential in inducing Ag-specific tolerance in vivo through induction of Tregs. Furthermore, studies have also shown that lack of DC apoptosis can promote autoimmunity, with the mechanism being relatively unclear. Our study shows that in addition to the dogma of DC apoptosis as a mechanism to eliminate activated DCs to prevent hyperactivation of the immune response, DC apoptosis also plays an active role in induction and maintenance of tolerance through induction of Tregs. Studies have also shown that exposure to necrotic cells results in maturation of DCs, which then acquire a potent T cell stimulatory capacity (35–38). Surprisingly, our findings show that necrotic DCs are not immunostimulatory, which may be due to the paucity of presence of certain immunosuppressive factors in primary DCs, which renders them non-immunogenic even after the cellular contents are released into the extracellular milieu. However, such factors still need to be identified.
Studies have documented a substantial depletion of DCs, both in sepsis patients and septic mice linking this event to immunosuppression, which is one of the main reasons for the fatality observed in sepsis patients (39, 40). In addition, increased levels of circulating Tregs have also been observed in sepsis patients (41). Mice over expressing anti-apoptotic proteins (selectively in DCs) are resistant to sepsis induced immunosuppression (42). However, the mechanism of how DC apoptosis can contribute to immunosuppression is unclear. Our study sheds light on immunosuppression induced by apoptotic DCs and demonstrates that DC apoptosis in sepsis may impact the ability of viable DCs to undergo maturation and instead promote induction and expansion of Tregs, thereby offering a potential explanation for the observation of enhanced circulating levels of Tregs in sepsis patients. In addition to sepsis, high levels of spontaneous DC apoptosis has also been observed in breast cancer patients (16, 17). Our study indicates that DC apoptosis in cancer patients may play a role in suppressing immune responses against the tumor by inducing immunosuppression and tolerance. Therefore, prevention of DC apoptosis may enhance the therapeutic effects of chemotherapy in tumor eradication (16, 17).
Taken together, our findings clearly show that apoptotic DCs have a potent ability to induce immunological tolerance in vivo. Furthermore, even in cases of pre-existing inflammation, apoptotic DCs are quite potent in suppressing inflammation and inducing expansion of Tregs. Our findings may represent a therapeutic strategy in prevention of unwanted immune responses in autoimmune diseases and transplantation along with inhibition of DC apoptosis to assist in tumor eradication.
Supplementary Material
Acknowledgments
We thank Dr. Michel C. Nussenzweig (Rockefeller University) for reading the manuscript.
Footnotes
This work was supported in part by Operating Grants from the Canadian Institutes of Health Research, the Canadian Cystic Fibrosis Foundation, and the Foundation Fighting Blindness-Canada (to J.H.). J.H. was a Canadian Cystic Fibrosis Foundation Scholar and a recipient of the Canadian Cystic Fibrosis Foundation Zellers Senior Scientist Award, and holds a Premier’s Research Excellence Award of Ontario, Canada. R.K. is a recipient of Canadian Cystic Fibrosis Foundation Doctoral Award.
Abbreviations used in this paper: DC, dendritic cell; Treg, regulatory T cell; BALF, bronchoalveolar lavage fluid; MLN, mediastinal lymph node; PLN, popliteal lymph node; PI, propidium iodide; MFI, mean fluorescent intensity.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007;29:303–309. doi: 10.1016/j.jaut.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 8.Johansson U, Walther-Jallow L, Smed-Sorensen A, Spetz AL. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 9.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Frank ME, Jin W, Wahl SM. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 12.Doerfler P, Forbush KA, Perlmutter RM. Caspase enzyme activity is not essential for apoptosis during thymocyte development. J Immunol. 2000;164:4071–4079. doi: 10.4049/jimmunol.164.8.4071. [DOI] [PubMed] [Google Scholar]
- 13.Walsh CM, Wen BG, Chinnaiyan AM, O’Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 14.Newton K, Harris AW, Bath ML, Smith KG, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, Imai K, Hirokawa M, Ogawa J. Tumor-derived TGFβ-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol. 2006;176:5637–5643. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 16.Pinzon-Charry A, Maxwell T, McGuckin MA, Schmidt C, Furnival C, Lopez JA. Spontaneous apoptosis of blood dendritic cells in patients with breast cancer. Breast Cancer Res. 2006;8:R5. doi: 10.1186/bcr1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinzon-Charry A, Ho CS, Maxwell T, McGuckin MA, Schmidt C, Furnival C, Pyke CM, Lopez JA. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer. 2007;97:1251–1259. doi: 10.1038/sj.bjc.6604018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–2994. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Kobayashi Y. Cytokine production in association with phagocytosis of apoptotic cells by immature dendritic cells. Cell Immunol. 2003;226:105–115. doi: 10.1016/j.cellimm.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:11–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 23.Kushwah R, Cao H, Hu J. Characterization of pulmonary T cell response to helper-dependent adenoviral vectors following intranasal delivery. J Immunol. 2008;180:098–4108. doi: 10.4049/jimmunol.180.6.4098. [DOI] [PubMed] [Google Scholar]
- 24.Kushwah R, Oliver JR, Cao H, Hu J. Nacystelyn enhances adenoviral vector-mediated gene delivery to mouse airways. Gene Ther. 2007;14:243–1248. doi: 10.1038/sj.gt.3302968. [DOI] [PubMed] [Google Scholar]
- 25.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, Wigle DA, Keshavjee S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 26.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, Keshavjee S. Interleukin-8 release during ischemia-reperfusion correlates with early graft function in human lung transplantation. J Heart Lung Transplant. 2001;20:175–176. doi: 10.1016/s1053-2498(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 27.Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, Dark JH, Corris PA. Elevated levels of interleukin-8 in donor lungs is associated with early graft failure after lung transplantation. Am J Respir Crit Care Med. 2001;163:259–265. doi: 10.1164/ajrccm.163.1.2005093. [DOI] [PubMed] [Google Scholar]
- 28.Kushwah R, Cao H, Hu J. Potential of helper-dependent adenoviral vectors in modulating airway innate immunity. Cell Mol Immunol. 2007;4:81–89. [PubMed] [Google Scholar]
- 29.Ren G, Su J, Zhao X, Zhang L, Zhang J, Roberts AI, Zhang H, Das G, Shi Y. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-γ and nitric oxide. J Immunol. 2008;181:3277–3284. doi: 10.4049/jimmunol.181.5.3277. [DOI] [PubMed] [Google Scholar]
- 30.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Larregina AT, Shufesky WJ, Perone MJ, Montecalvo A, Zahorchak AF, Thomson AW, Morelli AE. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant. 2006;6:1297–1311. doi: 10.1111/j.1600-6143.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Shufesky WJ, Montecalvo A, Divito SJ, Larregina AT, Morelli AE. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS ONE. 2009;4:e4940. doi: 10.1371/journal.pone.0004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia CQ, Qiu Y, Peng R, Lo-Dauer J, Clare-Salzler MJ. Apoptotic non-β cells suppress β cell antigen-reactive T cells and induce β cell antigen-specific regulatory T cells. Ann NY Acad Sci. 2008;1150:167–170. doi: 10.1196/annals.1447.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hugues S, Mougneau E, Ferlin W, Jeske D, Hofman P, Homann D, Beaudoin L, Schrike C, Von Herrath M, Lehuen A, Glaichenhaus N. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity. 2002;16:169–181. doi: 10.1016/s1074-7613(02)00273-x. [DOI] [PubMed] [Google Scholar]
- 35.Paczesny S, Beranger S, Salzmann JL, Klatzmann D, Colombo BM. Protection of mice against leukemia after vaccination with bone marrow-derived dendritic cells loaded with apoptotic leukemia cells. Cancer Res. 2001;61:2386–2389. [PubMed] [Google Scholar]
- 36.Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol. 2003;171:5940–5947. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- 37.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 38.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998;4:581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 39.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 40.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 41.Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, Lepape A. Marked elevation of human circulating CD4+ CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F. [DOI] [PubMed] [Google Scholar]
- 42.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.