Abstract
In the enteric nervous system, there exist a lot of local intrinsic neurons which control the gastrointestinal functions. Culture of enteric neurons provides a good model system for physiological, electrophysiological, and pharmacological studies. Here, we describe two methods to obtain sufficient enteric neurons from mouse myenteric plexuses by directly culturing primary neurons or inducing neuronal differentiation of enteric neural stem/progenitor cells.
Keywords: Enteric neurons, Neural stem/progenitor cells, Neuronal differentiation, Cell culture, Mouse
1 Introduction
The enteric nervous system, also called “the second brain,” is embedded within the wall of gut, which control the complex functions of gastrointestinal tract such as peristalsis, blood flow, and secretion [1–3]. The enteric nervous system consists of at least two major ganglionated plexuses, the myenteric plexus between the circular and longitudinal muscle layers and the submucosal plexus between the circular muscle and mucosal layers [2, 3]. The cell bodies of enteric neurons are surrounded and protected by enteric glial cells [4]. Enteric neurons from different plexuses have different functions and many subtypes of enteric neurons exist in the plexuses [5–7].
There are some difficulties in isolating and culturing enteric neurons in contrast to brain neurons: food debris, bacteria, and fungi in the lumen of the gut; mixed cell types in the tissue or plexus preparation; long network between various plexuses; and anatomically diffuse distribution of plexuses embedded into smooth muscle layers and mucosa layers. Most early studies used guinea pig for enteric neuronal culture due to relatively easy isolation of the loose and large plexuses [8–10]. Also, culture of enteric neurons in rat and human has been established [9, 11–13]. Recently, successful culture of mouse enteric neurons has been reported [14, 15]. The mouse model attracts wide attention because of the versatility in inbred and genetically engineered strains and the economic cost. In this chapter, we describe a unique isolation of mouse myenteric plexuses, the primary culture of mouse enteric neurons as well as the culture and neuronal differentiation of enteric neural stem/progenitor cells.
2 Materials
2.1 Isolation of Smooth Muscle/Myenteric Plexus (SM/MP) Strips
Fine scissors and fine forceps.
Stereomicroscope.
Agar plate (2 % agar PBS in 10 cm sterile petri dish, see Note 1).
Ice box.
Sterile petri dish.
2.2 Reagents
Ice cold HBSS with 10 mM HEPES (calcium and magnesium free).
- Enteric neuronal culture medium (equal to enteric neural stem/progenitor cell differentiation medium):
- To make 50 ml of complete medium:
DMEM/F12 medium 39.5 ml
Chick Embryo Extract (CEE) 7.5 ml
Penn/Strep (100×) 0.5 ml
Gentamicin (500×) 100 μl
Amphotericin (100×) 0.5 ml
N2 (100×) 0.5 ml
B27 (50×) 1 ml
Glutamine (100×) 0.5 ml
FGF-b (10 μg/ml) 50 μl
EGF (10 μg/ml) 100 μl
Heparin (0.2 %) 5 μl
- Enteric neural stem/progenitor cell proliferation medium:
- To make 50 ml of medium:
DMEM/F12 medium 44 ml
Heat inactivated Fetal Bovine Serum (HS-FBS) 1 ml
Chick Embryo Extract 2.5 ml
Penn/Strep (100×) 0.5 ml
Gentamicin (500×) 100 μl
Amphotericin (100×) 0.5 ml
N2 (100×) 0.5 ml
B27 (50×) 1 ml
- Digestion medium:
- Collagenase digestion medium: 1 mg/ml collagenase IV (from Worthington), 0.5 mM CaCl2, 10 mM HEPES in HBSS.
- Trypsin digestion medium: 0.05 % Trypsin with 0.53 mM EDTA in HBSS.
- Digestion neutralizing medium: 400 U DNase, 1 mg/ml BSA in DMEM/F12 medium.
Growth factor-reduced Matrigel.
3 Methods
3.1 Isolation of Smooth Muscle/Myenteric Plexus (SM/MP) Strips from Mouse Gut
(The whole procedure is done on ice, see Note 2)
Immediately prior to the experiment, euthanize the adult mouse using CO2.
Dissect out the whole small intestine (usually from duodenum to ileum or the regions of interest), and put into 10 cm petri dish filled with ice-cold HBSS (Ca2+ and Mg2+ free) solution.
Carefully remove mesenteries, fat tissue, and gut-associated organs free from the gut wall.
Flush the stool from ileum to duodenum three times using 10 ml syringe connected with a polyethylene tube.
Wash the gut with HBSS three times and transfer into a new sterile petri dish containing HBSS solution.
Cut the gut into 8 cm long pieces and use the scissors to open the lumen.
Transfer one piece into agar plate (see Note 1). Use forceps to unfold the gut with the lumen upward for SM/MP dissection. At one end of the gut, use one forceps to hold the gut and the other one to scratch off the mucosa layer followed by submucosa layer carefully under the stereomicroscope to make a window. When the submucosa layers are separated from SM/MP layers, use one forceps to hold the SM/MP layers and the other forceps to peel off the submucosa and mucosa layer (see Note 3).
Transfer the dissected SM/MP strips into new sterile petri dish containing HBSS.
Use the fine scissors to cut the layers/MP strips into about 1 cm long pieces and transfer all of them into 15 ml tube.
3.2 Enzymatic Dissociation of SM/MP Tissues
Centrifuge the collected tissues (200×g, 5 min at 4 °C) and aspirate the solution, add 3 ml prewarmed (37 °C) collagenase digestion medium to resuspend the tissue and incubate in 37 °C water bath shaker (40 rpm) for 15 min (see Note 4). For every 5 min, take the 15 ml tube out and invert several times to resuspend the tissues.
-
Release the smooth muscle cells from the myenteric plexuses (see Note 5):
Spin down the dissociated tissue (200×g, 5 min at 4 °C) and aspirate the solution. Add 2 ml HBSS and use a cut-off P1000 pipette tip to transfer the pellet into a 10 cm petri dish containing 10 ml HBSS. Shake softly (15 rpm) for 10 min at room temperature. Use the sterile forceps to transfer the tissues into a new 10 ml petri dish containing 10 ml HBSS and shake 10 min at room temperature.
Transfer all the tissues into 15 ml tube with 1 ml of Trypsin digestion medium, and incubate in 37 °C water bath shaker (40 rpm) for 10 min.
Add 2 ml digestion neutralizing medium into the tube and spin down at 200×g, 5 min, 4 °C. Aspirate the solution, add 1 ml digestion neutralizing medium and triturate vigorously using a P1000 pipette until no visible tissue clumps remain.
Add the HBSS solution to 10 ml, spin down the cells (200×g, 5 min at 4 °C), and resuspend the cells using 1 ml digestion neutralizing medium.
Count the cell number using standard Trypan blue staining and hemocytometry.
3.3 Culture of Primary Enteric Neurons
-
Matrigel coating of the culture plates (see Note 6):
Thaw the Matrigel on ice (If at room temperature, it will form the gel quickly).-
–Dilute the Matrigel (1:200) in ice cold DMEM/F12 medium, vortex 10 s. Precool the pipette tip before transferring the Matrigel
-
–Add the diluted Matrigel into the precooled culture plates and put into the incubator (37 °C) for 1–2 h. The coating volume used depends upon the experimental purpose: for 10 cm dish, 6 ml; 60 mm dish, 3 ml; 35 mm dish or 1 well of 6-well plate, 1.5 ml; 24-well plate, 0.5 ml/well; 96-well-plate or 8-well chamber slide, 100 μl/well. For immunocytochemistry and/or confocal imaging, sterile coverslips can be added into 6-well or 24-well plate.
-
–Wash the coated wells once with HBSS, and add half volume of the culture medium immediately (the other half will be added together with the cells). Do not let the dish or plate dry after coating.
-
–
Seed the dissociated cells into Matrigel-coated dish or plate at the density of 1×105/cm2 with enteric neuronal culture medium, and culture at 37 °C with 5 % CO2, change medium every day.
The morphological changes of the cultured cells are observed daily under phase-contrast microscope. After 1–2 days in culture, enteric neurons attach to the coated surface and develop long processes. After 5–7 days in culture, some neurons attempt to form ganglion-like plexuses over a layer of the flatter, wider glial cells (Fig. 1a). Immunocytochemical staining using neuronal marker Tuj-1 (β tubulin III) validates the success of enteric neuronal culture (Fig. 1b).
Fig. 1.
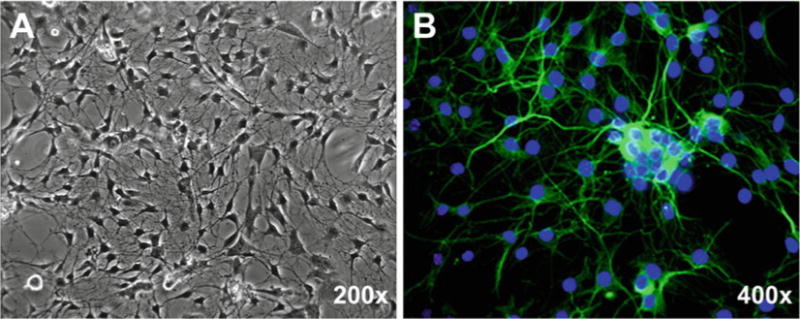
Representative phase-contrast (a) and immunofluorescent (b) micrographs of primary enteric neurons cultured from small intestinal myenteric plexuses of 8-week-old mouse. After 5 days in culture, cells were fixed with 4 % paraformaldehyde/PBS for 10 min and standard immunocytochemistry was performed using rabbit anti-Tuj-1 polyclonal antibody (1:5,000, Sigma) and Alexa Fluor® 488 (green) conjugated donkey anti-rabbit secondary antibody (1:400; Invitrogen). Hoechst 33258 was used for counterstaining of nuclei (Blue)
3.4 Culture and Differentiation of Enteric Neural Stem/Progenitor Cells (See Note 7)
-
Primary neurosphere culture of enteric stem/progenitor cells.
Seed the dissociated cells with the enteric neural stem/progenitor cell proliferation medium at the density of 1×105/cm2 in 60 mm sterile petri dish without any coating (5 ml culture medium per dish), and culture at 37 °C with 5 % CO2. After 6–7 days in culture, the enteric neurosphere will be ready for passage (Fig. 2a). Around 1,500–2,000 enteric neurospheres per adult mouse can be obtained.
- Dissociation of enteric neurospheres into single cells.
-
–Warm enteric neural stem/progenitor cell proliferation medium to 37 °C. Collect primary neurospheres to 15 ml tube, and spin down the neurospheres by centrifuge at 400 rpm (90×g) for 1 min.
-
–Aspirate the supernatant and add the Accutase solution to resuspend the neurospheres. Incubate at 37 °C for 15 min. Here is one example for neurospheres cultured in a 60-mm dish using 0.8 ml Accutase solution. Alternatively, the collagenase IV at final concentration of 1 mg/ml also works fine.
-
–Add 2× volumes of DMEM/F12 medium to 15 ml tube to dilute the enzyme. Centrifuge at 210×g for 4 min.
-
–Aspirate off all of the supernatant and resuspend cells in 0.2 ml neural stem/progenitor cell proliferation medium by gently pipetting the cell pellet using the 200 μl tip, and then add the medium to 1 ml. Count the cell number using standard Trypan blue staining and hemocytometry.
-
–
-
Expansion of enteric neural stem/progenitor cells (see Note 8).
Seed the dissociated single cells on the Matrigel-coated plate at the density of 2×104 cells/cm2 and culture with the enteric neural stem/progenitor cell proliferation medium at 37 °C with 5 % CO2. Change medium every day. At 90–95 % confluence (Fig. 2b), the cells can be dissociated into single cells and subculture again (1:3).
-
Neuronal differentiation of enteric neural stem/progenitor cells.
Seed the dissociated single cells on the Matrigel-coated plate or coverslips at the density of 2×104 cells/cm2 and culture with enteric neuronal culture medium at 37 °C with 5 % CO2. Change medium every day. After 3–4 days in culture, the enteric neurons enriched with neurites will be observed under phase-contrast microscope (Fig. 2c). Immunocytochemical staining using neuronal marker Tuj-1 (β-tubulin III) validate the successful culture of enteric neurons differentiated from enteric neural stem/progenitor cells (Fig. 2d).
Fig. 2.
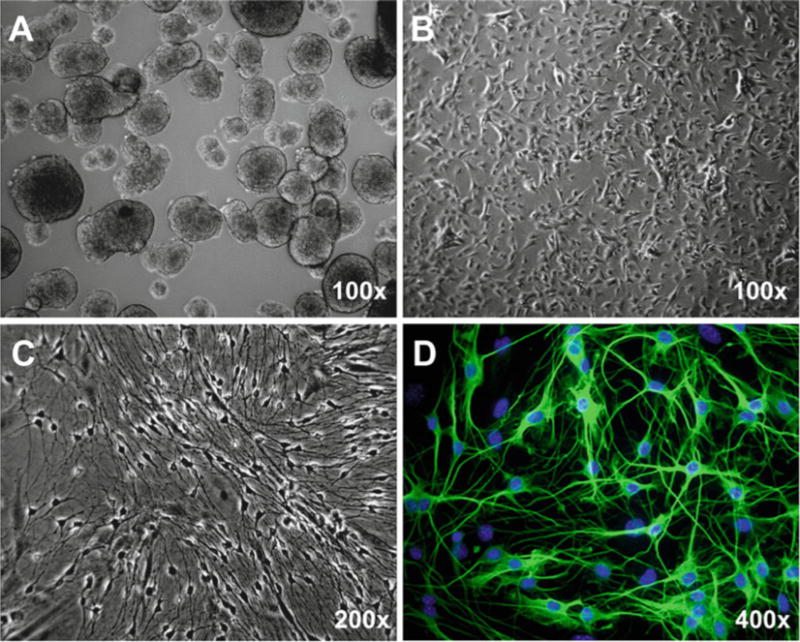
Culture and differentiation of enteric neural stem/progenitor cells from 8-week-old mouse. (a) Neurosphere culture for 7 days. (b) Monolayer culture. (c, d) Differentiation for 4 days. Representative phase-contrast (a–c) and immunofluorescent (d) micrographs are shown. Standard immunocytochemistry was performed using rabbit anti-Tuji1 polyclonal antibody (1:5,000, Sigma) and Alexa Fluor® 488 (green) conjugated donkey anti-rabbit secondary antibody (1:400; Invitrogen). Hoechst 33258 was used for counterstaining of nuclei (Blue)
Footnotes
Smooth muscle/myenteric plexus layer is a thin out layer of gut. It is very soft and easily damaged during the dissection. Agar plate is soft and will avoid damage to tissue during the dissection
There are a lot of enzymes in the gut, and the enteric neurons will be easily damaged. So every step of the dissection must be performed on ice. When dissected under the stereomicro-scope, solution should be changed every 5 min to keep lower temperature.
The myenteric plexus lies between the circular and longitudinal muscle, so we first peel off the mucosa and sub-mucosa layers to reduce the contamination from the contents in the lumen.
The time of digestion with collagenase is very critical, and different for individual animals. We checked the digestion every 5 min. When the color of tissue begins to change into white, stop immediately.
Smooth muscle/myenteric plexus layer contains a lot of smooth muscle cells. We first used the collagenase to separate the myenteric plexus from two smooth muscle layers and then treated the myenteric plexus with trypsin. After digestion of the smooth muscle/myenteric plexus, the cell suspension is composed of several cell types including neurons, glia cells, smooth muscle cells, fibroblast, and neural stem/progenitor cells. During the culture, smooth muscle cells will die off and the neuron and glial cells will survive due to the culture conditions.
We tested different coating matrixes. A few neurons will attach on the poly-D-lysine coated plate. Better adherence can be reached with the poly-D-lysine/fibronectin or poly-D-lysine/laminin coating. However, double coating costs a lot and needs 1–2 days for the coating process. BD Matrigel™ matrix is a reconstituted basement membrane preparation from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma. This material is comprised of approximately 60 % laminin, 30 % collagen IV, and 8 % entactin. We found that Matrigel-coated dishes or coverslips efficiently support the attachment and growth of primary neurons, differentiating neurons and neural stem/progenitor cells. The coating procedure takes less than 1 h.
The enteric neuron is a type of terminally differentiated cell. Direct culture of primary enteric neurons is often difficult to obtain large number of enteric neurons. In vitro culture of enteric neural stem/progenitor cells from embryonic, neonatal, and adult gut tissues has been established [16–20]. Differentiation of cultured enteric neural stem/progenitor cells provides an easy approach to obtain a larger number of enteric neurons which are usually more pure.
The enteric neural stem/progenitor cells can be expanded through either monolayer or neurosphere culture. We prefer the monolayer culture because of its higher efficiency of expansion, easy dissociation into single cells, and even exposure to nutrients.
References
- 1.Wood JD. Enteric nervous system: sensory physiology, diarrhea and constipation. Curr Opin Gastroenterol. 2010;26(2):102–108. doi: 10.1097/MOG.0b013e328334df4f. [DOI] [PubMed] [Google Scholar]
- 2.Benarroch EE. Enteric nervous system: functional organization and neurologic implications. Neurology. 2007;69(20):1953–1957. doi: 10.1212/01.wnl.0000281999.56102.b5. [DOI] [PubMed] [Google Scholar]
- 3.Brehmer A. Structure of enteric neurons. Adv Anat Embryol Cell Biol. 2006;186:1–91. [PubMed] [Google Scholar]
- 4.Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2007;23(2):121–126. doi: 10.1097/MOG.0b013e3280287a23. [DOI] [PubMed] [Google Scholar]
- 5.Sternini C. Structural and chemical organization of the myenteric plexus. Annu Rev Physiol. 1988;50:81–93. doi: 10.1146/annurev.ph.50.030188.000501. [DOI] [PubMed] [Google Scholar]
- 6.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136(4):1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 7.Mazzuoli G, Schemann M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One. 2012;7(7):e39887. doi: 10.1371/journal.pone.0039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saffrey MJ, Bailey DJ, Burnstock G. Growth of enteric neurones from isolated myenteric ganglia in dissociated cell culture. Cell Tissue Res. 1991;265(3):527–534. doi: 10.1007/BF00340876. [DOI] [PubMed] [Google Scholar]
- 9.Jessen KR, Saffrey MJ, Burnstock G. The enteric nervous system in tissue culture. I. Cell types and their interactions in explants of the myenteric and submucous plexuses from guinea pig, rabbit and rat. Brain Res. 1983;262(1):17–35. doi: 10.1016/0006-8993(83)90466-3. [DOI] [PubMed] [Google Scholar]
- 10.Hanani M, Xia Y, Wood JD. Myenteric ganglia from the adult guinea-pig small-intestine in tissue-culture. Neurogastroenterol Motil. 1994;6(2):103–118. doi: 10.1111/j.1365-2982.1994.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 11.Pouokam E, Rehn M, Diener M. Effects of H2O2 at rat myenteric neurones in culture. Eur J Pharmacol. 2009;615(1–3):40–49. doi: 10.1016/j.ejphar.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 12.Kugler EM, et al. Activity of protease-activated receptors in primary cultured human myenteric neurons. Front Neurosci. 2012;6:133. doi: 10.3389/fnins.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willard AL, Nishi R. Neurons dissociated from rat myenteric plexus retain differentiated properties when grown in cell culture. II. Electrophysiological properties and responses to neurotransmitter candidates. Neuroscience. 1985;16(1):201–211. doi: 10.1016/0306-4522(85)90057-0. [DOI] [PubMed] [Google Scholar]
- 14.Kang SH, Vanden P. Berghe, and T.K. Smith, Ca2+-activated Cl-current in cultured myenteric neurons from murine proximal colon. Am J Physiol Cell Physiol. 2003;284(4):C839–C847. doi: 10.1152/ajpcell.00437.2002. [DOI] [PubMed] [Google Scholar]
- 15.Hagl CI, et al. Enteric neurons from postnatal Fgf2 knockout mice differ in neurite outgrowth responses. Auton Neurosci. 2012;170(1–2):56–61. doi: 10.1016/j.autneu.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Lindley RM, et al. Properties of secondary and tertiary human enteric nervous system neurospheres. J Pediatr Surg. 2009;44(6):1249–1255. doi: 10.1016/j.jpedsurg.2009.02.048. discussion 1255–1256. [DOI] [PubMed] [Google Scholar]
- 17.Metzger M, et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137(6):2063–2073, e4. doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Almond S, et al. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56(4):489–496. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva AT, et al. Neural progenitors from isolated postnatal rat myenteric ganglia: expansion as neurospheres and differentiation in vitro. Brain Res. 2008;1218:47–53. doi: 10.1016/j.brainres.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 20.Schafer KH, Micci MA, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterol Motil. 2009;21(2):103–112. doi: 10.1111/j.1365-2982.2008.01257.x. [DOI] [PubMed] [Google Scholar]


