Abstract
Adolescents are especially prone to risky behavior and to the emergence of psychological disorders like substance abuse, anxiety and depression. However, there is a sex (or gender) difference in this vulnerability, with females being more prone to developing internalizing disorders and males being more likely to engage in risky behavior and drug use. While several researchers have proposed that there is a relationship between corticolimbic circuit development and adolescent vulnerability, the current proposed models do not take sex differences into account. In this review, we explore recent findings from both human and rodent studies of sex differences during adolescence. In particular, we consider epidemiological studies on the factors that contribute to the development of substance abuse and internalizing disorders, laboratory studies on reward-related and decision-making behavior, and neuroanatomical studies on the development of several structures in the corticolimbic circuit (i.e., prefrontal cortex [PFC], amygdala and striatum). We then integrate these recent findings into models of adolescent vulnerability to substance use that have previously not addressed sex differences. Lastly, we discuss methodological considerations for the interpretation and design of studies on sex (or gender) differences during adolescence while highlighting some opportunities for future investigations.
0. Introduction
Adolescence, which is the period of pubertal, social and cognitive development occurring between childhood and adulthood, is a time of significant morbidity and mortality compared to other stages of life [1]. Adolescents appear to be especially vulnerable to accidental death and the emergence of disorders such as substance abuse, anxiety and depression [2]. Interestingly, some of these adolescent-onset disorders have substantial gender differences in their prevalence. For example, females are twice as likely as males to develop problems with depression and anxiety [3] and this gender difference is not evident until individuals are well into puberty [2].
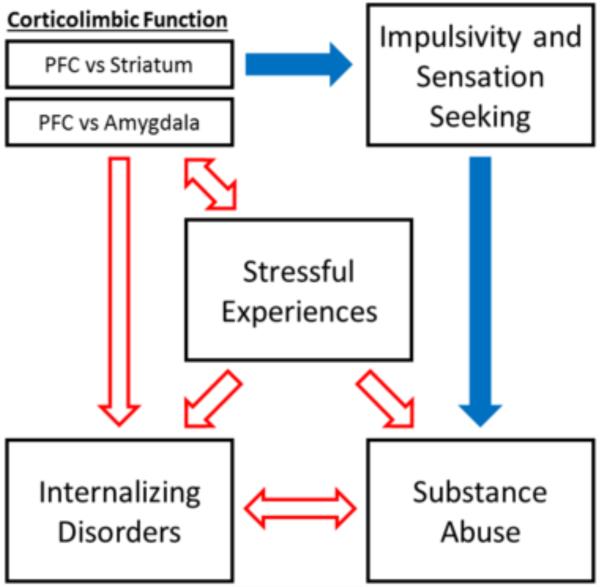
Although adolescence is inextricably tied to puberty and the process of sexual maturation, relatively few studies have focused on the role of sex in adolescent vulnerability to substance use disorders. This is unfortunate given the known differences between males and females in a number of developmental processes occurring during adolescence, including the timing of pubertal and neural changes [4-6]. Theories of adolescent vulnerability have proposed that the process of adolescent neural development results in behavioral changes and heightened sensitivity to stressors that make adolescents especially vulnerable to developing psychological disorders [2,7-11]. Thus, sex differences in adolescent neural development may give rise to sex differences in the nature of adolescent vulnerability. However, published reviews of the adolescent substance abuse vulnerability literature [2,7-11] do not consider the role of sex differences. Similarly, the reviews that have discussed sex differences in vulnerability to internalizing disorders [3] and substance abuse [12-15] almost exclusively focus on sex differences in adulthood. In this review, we discuss the growing body of literature examining sex differences in vulnerability, reward-related behavior, decision making, and neural development in adolescent humans and laboratory animals. We also discuss whether these sex differences are consistent with those predicted by influential theories of adolescent vulnerability to substance abuse [2,7,16-18]. We propose that males and females have different pathways of vulnerability to substance abuse: in adolescent boys sensation seeking and impulsivity may drive drug and alcohol use, while stressful experiences and comorbid internalizing disorders may mediate substance use in adolescent girls (Fig. 2).
Figure 2.
Sex differences in adolescent vulnerability to developing substance abuse. Though adolescents are thought to be especially vulnerable to each of these pathways, red unfilled arrows indicate most influential pathways for adolescent girls and blue filled arrows indicate the most influential pathways for adolescent boys. Not included here is the hypothesized balance between amygdala and striatum function that has been suggested to govern the choice of approach versus avoidance and affects both internalizing disorders and substance abuse [see for review: 98].
1. Sex differences in adolescent vulnerability
1.1. Epidemiological studies
Individuals seem to be at their most vulnerable during adolescence. Accidental injury, accidental death, suicide and homicide all increase sharply from childhood to adolescence [19], leading to high levels of mortality during a period when individuals tend to be otherwise at their healthiest [20]. Nonfatal injuries and suicide attempts, for example, peak around ages 15-19 before declining throughout adulthood [19]. The increase in accidental injury and death may be driven by behavioral changes that predispose adolescents toward riskier decision-making. For example, while sensation-seeking begins to increase at the beginning of adolescence [17,21], the ability to control behavior (i.e., inhibitory control) doesn’t increase until mid-adolescence [21]. This increase in sensation-seeking relative to inhibitory control is also thought to contribute to adolescent drug experimentation [7,20,22,23].
Adolescent-onset substance use can increase the risk that an individual will develop lasting problems with substance abuse and dependence while also increasing an individual’s vulnerability to developing other psychosocial problems. For example, an analysis that used DSM-IV criteria of problem alcohol use behaviors revealed that males and females are most likely to develop alcohol abuse and dependence if drinking begins around 13 to 14 years of age [24]. After this peak, the likelihood of an individual developing alcohol dependence falls by 13.2% and 14.7% per year for females and males, respectively; similarly, the likelihood of developing alcohol abuse falls by 9.1 and 7.0% for females and males [24]. In addition, adolescent-onset alcohol abuse may have lasting effects on the likelihood that an individual will experience legal troubles, engage in illicit drug use, or display antisocial behavior in adulthood [25,26], though these problems may merely be correlated with, rather than caused by, adolescent-onset alcohol use. Interestingly, there are gender differences in the likelihood that adults will display problems associated with adolescent alcohol use. Males that stop abusing alcohol after adolescence resemble men without any history of alcohol abuse [26]. However, females that stop abusing alcohol after adolescence continue to differ from controls in adulthood, particularly with regard to illegal drug use, antisocial behavior and social dysfunction [25]. If adolescent-onset drug use was, in fact, causing these problems, then the especially long-lasting effects in females may contribute to sex differences in the severity of problems associated with drug use.
Though males are more likely to use drugs [27], it has been suggested that female drug users exhibit “telescoping” - a more rapid and severe progression from experimentation to addiction [see for review: 24-27]. Describing drug use in females as less common but more severe is something of an oversimplification, however. First, gender differences in the rate of drug use seem to be age-dependent. While males tend to use drugs more frequently in general, adolescent girls are more likely to engage in alcohol and non-medical psychotherapeutic use compared to adolescent boys [27]. In fact, girls aged 12-17 exhibit a greater rate of dependence on psychotherapeutics along with equal rates of alcohol and cocaine abuse and dependence [27]. Second, it seems that gender differences in vulnerability to developing substance abuse are drug-dependent. Males are more likely to transition from casual use to dependence for cannabis and alcohol, whereas the likelihood of developing nicotine or cocaine dependence is similar if not greater in females [29-31]. Lastly, researchers have attempted to explain the seemingly paradoxical relationship between drug abuse prevalence and severity in women by proposing that women are less likely to use drugs because of cultural factors that inhibit drug use [12,13,28]. In this model, females are more vulnerable to drug abuse in general, which is an idea supported by preclinical studies showing that female rats are more sensitive to the rewarding properties of drugs [32]. It is simply the low rate of drug experimentation that makes them less likely to realize this vulnerability. However, it is also possible that the low prevalence of drug use in women is due to differences in the risk factors that influence drug use, along with how much risk they confer.
Gender differences in addiction risk factors, such as depression and sensation seeking, may contribute to some of the aforementioned gender differences in addiction vulnerability. For example, among Swedish 7th graders, the presence of a multiple risk factors (e.g., bullying or peer drug use) has a greater effect on drinking in females than in males [33]. Females that begin abusing alcohol during adolescence tend to have experienced a high-level of stressful life events [25], while this relationship is not seen in males [26]. Among 11 to 14 year olds, females were more likely to report using alcohol in order to cope with negative emotions, while males were more likely to engage in alcohol use as a result of sensation seeking (e.g., to find out what effect the drug would have) [34].
Other gender differences that may contribute to differential vulnerability during adolescence are those for sensation seeking and inhibitory control. Males consistently exhibit higher levels of sensation seeking throughout development, whereas females seem to have greater inhibitory control [21]. In addition, males have dramatic increases in sensation-seeking while in females there is little [21] or no change [17] in this behavior. Females have higher levels of constraint, a personality trait associated with control and harm avoidance, despite this trait decreasing from mid to late puberty in females and remaining stable in males [35]. Given the putative role of sensation-seeking and inhibitory control in the initiation of drug use [36], it is unsurprising that males also tend to experiment with drugs to a greater degree than females [12]. In summary, females may use drugs as a coping strategy (i.e., self-medication) while males seem to be motivated to use drugs as a result of heightened sensation seeking [12].
The tendency to self-medicate may explain why females seem to be vulnerable to a more rapid and severe progression of addiction. The presence of comorbid disorders increases addiction vulnerability [37] and female drug users seem to be more likely than males to exhibit comorbid depression [38-40]. Indeed, among adolescent-onset drinkers, females who were unable to stop drinking in adulthood exhibited high levels of depression during adolescence relative to those that eventually stopped abusing alcohol [25]. In a separate study, there was no relationship between persistence of drinking and depression among adolescent men [26]. Gender differences in the effects of stress may be relevant, as women who have experienced childhood abuse or neglect are more likely to use drugs and this relationship is not as strong for men [12,41]. It therefore appears that female drug users are more likely to have been influenced by depression and stress, though this relationship may be driven by greater rates of depression and anxiety among females [3].
Females are much more likely than males to suffer from internalizing disorders, such as depression and anxiety. Women are roughly twice as likely as men to suffer from depression or anxiety at some point in their lives [3,42]. This sex difference in depression is tied to pubertal development, rather than age, and does not emerge until Tanner stage III (mid-puberty) when there is a sharper increase in the prevalence of depression in females [2,43]. This high prevalence in women may be due to greater vulnerability to stress, as women who have experienced abuse or early life stress are more likely to develop depression than men with similar experiences [42]. However, girls also experience higher levels of stress, particularly interpersonal stress, and this may contribute to the high rate of depression in females [44]. One study of 816 Australian youths found that the gender difference in experience of episodic, but not chronic, stress explained roughly 36% of the gender difference in the rate of depression [44]. Furthermore, the experience of episodic stress strongly predicted depression in females, but not in males [44]. Interestingly, females may actually be less impacted by acute stress with regard to stress hormone release. Numerous studies have shown that cortisol release in response to a social stressor increases in more in adolescent boys and is higher in men by adulthood [see for review: 45], but only adolescent girls demonstrate increases in cortisol release evoked by an injection of corticotropin-releasing hormone [46]. While there may be sex differences in the presence of risk factors for depression, it would seem that females are also more affected by these risk factors than males.
1.2. Laboratory studies
Studies of decision-making have been able to confirm some of the epidemiological and survey data about sex differences in risk taking during adolescence. In one study, for example, adolescent boys made riskier decisions than girls on the Cake Gambling Task, which tests probability estimation and reward evaluation [47]. In this study there was no effect of age on this sex difference, though boys and girls appeared to differ the most around ages 14-18. Another human study that used a mixed gambles task found no effect of sex on risk taking during adolescence or adulthood, despite there being sex differences in neural activation during the decision making process [48]. In a study looking at the response to unexpected information, which is a test of efficiency of processing, adolescent girls had shorter P300 latency and smaller P300 amplitude (i.e., more efficient information processing). Both of these measures decreased from adolescence to adulthood, suggesting that girls may have had more mature information processing throughout adolescence [49]. Enhanced processing of unexpected information may make girls better able to avoid reacting impulsively in emotionally charged situations. A recent study looking at the effect of viewing threatening faces on impulse control found that human males showed increased impulsivity when viewing threatening faces during adolescence, relative to childhood or adulthood [50]. However, this was not seen in human females, who displayed declining levels of threat-induced impulsivity from childhood to adulthood. Together, these studies in humans seem to support the notion that females are less likely to make risky decisions and better able to exert inhibitory control over their behavior, even in the presence of unexpected information.
Studies focusing on reward-related behavior in rodents have attempted to explain the adolescent tendency toward poor inhibitory control and heightened sensation-seeking. One plausible hypothesis is that adolescents are more motivated to engage in sensation seeking because they find rewards to be more salient. For example, rats are most sensitive to the reinforcing properties of palatable rewards during adolescence [51] and adolescents demonstrate greater motivation to obtain rewards [52]. In a study of cocaine self-administration in rats, adolescents responded more for cocaine than adults, even during periods of signaled non-availability. Sex differences in responding for cocaine emerged during adulthood, when females responded more than males [53]. However, when a separate group of rats was trained with food, rather than cocaine, experimenters found that adolescents had a lower level of responding for food and females responded less than males in adulthood [53]. A similar study examining operant responding for food or cocaine also found that sex differences were specific to cocaine; females exhibited faster acquisition, greater intake, and more progressive ratio responding – a measure of willingness to work for reward [54]. In a rodent study that used conditioned place preference (CPP) techniques to assess cocaine reward, female adults and adolescents of either sex had enhanced CPP for cocaine relative to adult males [55]. The greatest sensitivity to the rewarding properties of cocaine was exhibited by adolescent females [55], but a separate study showed no effect of sex on CPP for food in adolescent and adult rats [56]. Taken together, these rodent studies suggest that sex and age differences in conditioning seem to be dependent on the type of reinforcer (i.e., natural or drug reward).
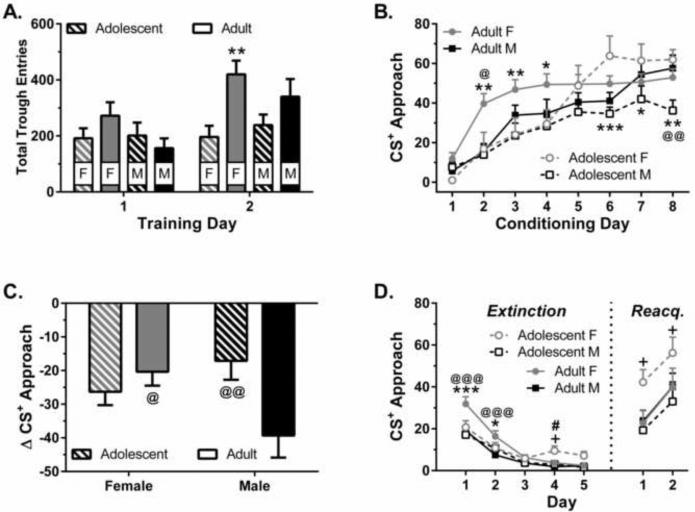
Though studies looking at the acquisition of conditioned behavior failed to show sex differences in natural reward behavior during adolescence, we have recently published data suggesting that sex may affect other aspects of learning about conditioned reinforcement, such as outcome devaluation and extinction [57]. Rats in our study first learned a Pavlovian association between a tone and the delivery of 20% sucrose solution; during these sessions female adults had the greatest level of unconditioned responding (Fig. 1A) and the fastest acquisition of a Pavlovian association (Fig. 1B). We next assessed habit formation using a sensory-specific satiety outcome devaluation procedure. During the outcome devaluation test animals received 60 minutes of access to a bottle filled with the 20% sucrose solution before a 10 minute extinction session, where we assessed whether the tone cue had been made less valuable (i.e., elicited fewer conditioned responses) by the satiety procedure. Though outcome devaluation led to reduced responding overall, adult females and adolescent males were less sensitive to the effects of outcome devaluation than adult males (Fig. 1C) [57]. Finally, we assessed extinction and reinstatement of the conditioned response. Female adults and female adolescents showed weaker extinction learning, responding more than males early or late in extinction training, respectively (Fig. 1D), but only female adolescents showed enhanced reacquisition of the conditioned response after extinction (Fig. 1D). Given the outcome devaluation results we suggest that adult females and adolescent males and females may be relatively insensitive to changes in the value of a reinforcer. In addition, females of either age seemed to be less sensitive to changes in contingency (as assessed through extinction responding) and learned associations seem to be especially persistent in adolescent females. Though these results suggest the possibility for an additive effect of adolescent age and female sex, there was no evidence for an interaction between these two factors with regard to outcome devaluation.
Figure 1.
Interaction between age and sex on natural reward behavior. (A) Number of trough entries during magazine training sessions for male (M) and female (F) adolescent and adult rats (n = 10-12/group). (B) Approach behavior during acquisition of Pavlovian approach behavior. (C) Approach behavior following reward devaluation, calculated as a difference score: devaluation session CS+ approach – final conditioning session CS+ approach. (D) CS+ approach during extinction (left) and reacquisition (right). * p < 0.05, ** p < 0.01 and *** p < 0.001, vs. adolescent females; + p < 0.05 vs. adolescent males; # p < 0.05 vs. adult females; @ p < 0.05, @@ p < 0.01 and @@@ p < 0.001 vs. adult males. Figure adapted from data published in [57]
If adolescents and females are insensitive to changes in the value of a reinforcer, they may also be less affected by negative experiences with that reinforcer. This behavior can be assessed with a conditioned taste aversion (CTA) paradigm. For example, a study of ethanol-induced CTA in rats found that both adolescents and female adults, relative to male adults, exhibited decreased CTA for a number of doses of ethanol [58]. Sex differences in CTA were limited to adulthood in the aforementioned study, but other studies have found sex differences in the effects of repeated exposure to ethanol during adolescence. One recent study showed that, over repeated sessions, CTA for 1.0 g/kg ethanol decreased in male adolescents but not in female adolescents [59]. Interestingly, female adults also demonstrated diminished CTA to 1.0 g/kg ethanol, relative to male adults, after repeated exposure [59]. Our lab has found that adolescent exposure to ethanol reduced CTA in adulthood for males, though females had lower levels of CTA in general [60]. In contrast, the effects of adolescent-exposure to ethanol on adult drinking behavior [61] do not seem to be sex specific [62]. Together, these studies suggest that though adolescents and females are relatively insensitive to CTA, there is no additive effect of female sex and adolescent age. Indeed, repeated experience with ethanol seems to have the greatest effect in male adolescents.
The presence of social partners during ethanol exposure may amplify the effects of sex on the response to ethanol. Male adolescents that had been intoxicated in a social context exhibited reduced CTA relative to both their female counterparts and to adolescent males that had been isolated while intoxicated; there was no effect of sex or social context on CTA for adult rats [63]. Interestingly, the effect of ethanol on social interaction predicts voluntary drinking in a sex-specific fashion. A recent study from this same research group found that adolescent males were likely to drink more if they responded to intoxication by increasing play behavior while adolescent females tended to drink more if they were socially anxious at baseline and preferred social contact while intoxicated [64]. These studies suggest that the social environment associated with drinking may influence male and female adolescents differently, with males being especially sensitive to the effects of social play on the reinforcing and aversive properties of ethanol.
2. Sex differences in neural development
Adolescent-typical behaviors, such as sensation seeking and risk taking, are thought to be tied to ongoing developmental changes in the brain during adolescence. The brain continues to develop after infancy and we now know that there is particularly dramatic remodeling in regions involved in decision making and reward behavior during adolescence [65-67]. The nature of this remodeling is region-specific: while the striatum and thalamus increase in volume during adolescence, the cortex follows an inverted U-shaped curve, reaching a peak volume during childhood before declining throughout adolescence [65,68]. In addition, the timing of adolescent development varies by region – the thalamus, for example, reaches a stable volume about 2 years later than the striatum [68].
Because adolescent development occurs in a heterogeneous fashion, with some regions developing earlier than others, functional connections between brain regions are in flux during this period. This could result in shifting balances in circuits with many adolescent-developing regions [2,7,69]. In the corticolimbic circuit, for example, the adolescent prefrontal cortex (PFC) is thought to contribute less than usual to the circuit’s output, while the adolescent striatum contributes more than usual, because the two regions develop at a different rate [7,16]. The PFC and striatum are involved in the control and performance of motivated behavior, respectively, and so adolescents may have less top-down inhibitory control of motivated behavior while these two regions are developmentally “off-balance” [7]. We will now review recent studies on sex differences in the development of regions involved in this circuit, specifically in the PFC, amygdala and striatum.
2.1. Prefrontal cortex
A number of human and rat studies have revealed sex differences in adolescent cortical remodeling. As a whole, the cortex develops in an inverted-U shaped pattern, with cortical volume increasing and then decreasing from childhood to adulthood [66]. The PFC seems to be the last of the cortical structures to mature [66,70]. Using human subjects, Giedd and his colleagues have repeatedly demonstrated that the cortex matures later in males [65,66,70,71], though it should be noted that several of these studies share subjects from an ongoing pediatric neuroimaging study conducted at the Child Psychiatry Branch of the National Institute of Mental Health [62,63,68; see for review: 69]. For example, males reach their peak cortical volume at 9.1 years while females reach theirs at 8.0 years [68]. In addition, males exhibit an increase in cortical surface area that is not seen in females [68] and males tend to have larger cortical volume throughout development [66,68].
The overall pattern of cortical development, where males have delayed maturation but consistently greater volume and thickness, may not hold true for all regions in the frontal cortex. In general, males start with thicker frontal cortical tissue at age 9, but undergo more rapid thinning compared to females [73]. Thus, males manage to “catch up” in many frontal regions by adulthood. But in late-developing frontal regions, such as the PFC and the orbitofrontal cortex, males do not catch up to females by adulthood [73]. In fact, females actually have faster thinning in the right lateral orbitofrontal cortex, which is involved in decision making, so that males and females become more different throughout adolescent development [73]. Meanwhile, males still have more rapid thinning in the dorsolateral PFC, which is a region important for inhibitory control and impulsivity [74], but do not achieve the same amount of thinning by adulthood [73]. These studies in humans suggest that there are some regional differences in the rate of PFC development that warrant further investigation. For example, it is still unclear if these sex differences in cortical volume are associated with sex differences in white matter; though there is some indication that males may have greater myelination within the cortex there is currently little consensus emerging from fractional anisotropy studies [see for review: 72,73]. It is difficult to interpret the significance of changes in cortical volume and thickness without more information about how white and gray matter are affected.
Studies of PFC maturation in rodents have been able to better explore the nature of the gross anatomical changes described in humans. These studies also show that males have greater PFC volume than females in adulthood [4,77], but they further clarify that this sex difference appears to emerge through programmed cell death (apoptosis) and synaptic pruning during adolescence. Using stereological techniques to quantify neuron number, Juraska and her colleagues have shown that there is a reduction in total neuron number within the rat ventral medial PFC from adolescence (postnatal day [P] 35) to adulthood (P90) in female rats, but not in males [77]. Interestingly, this sex difference was not present in the dorsal medial PFC and seemed to be specific to layer V/VI of the ventral medial PFC; both sexes experienced reductions in neuron number within layer II/III [77]. A more recent study from this lab revealed that there is also a reduction in dendritic complexity from adolescence to adulthood in females that is much smaller in males [78]. Taken together, these results suggest that females undergo more substantial pruning and apoptosis during adolescent development of the medial PFC relative to males [4]. Though these studies in rodents and the aforementioned human studies point toward sex influencing the structural development of the PFC, the significance of these changes cannot be interpreted without studying functional activity during development.
Just as there are sex differences in the volume and morphology of the PFC, studies of PFC activation in humans have revealed sex differences in function that go beyond what might be expected from the anatomical data. One measure that researchers have used is resting state functional connectivity (rsFC), which examines the degree of synchrony between two different brain regions or between nearby areas within a brain region. In the dorsolateral PFC, rsFC between the two hemispheres increases with age in males but decreases with age in females [79]. Though the precise significance of this is unclear, the researchers suggest that regions involved in higher order processing (i.e., the PFC), generally exhibit decreasing rsFC with age. This so-called “short-range” rsFC is thought to represent functional interactions within a brain region or even across hemispheres. Regions involved in sensory processing and motor control, in contrast, generally exhibit increasing short-range rsFC with age [79]. Greater dorsolateral PFC rsFC in males may, therefore, indicate that males have relatively less maturation of their PFC by adulthood, or perhaps that they follow a different trajectory of functional connectivity development [80].
Sex differences in functional connectivity may correspond to sex differences in neural activation during tasks that recruit the PFC. A study of neural activation during a risk-taking task found that men had greater activation of the frontal lobe when rejecting risky gambles, despite men and women having similar performance on the task and similar self-report of risky behavior [48]. Consistent with hypotheses about males having more adolescent-like PFC activity in adulthood, adolescents, relative to adults, also showed greater frontal lobe activation when rejecting risky gambles. However, this study did not examine whether sex affected age differences in the relationship between PFC activation and self-reported risk taking, presumably due to the sample size of their groups (n = 16-19). High risk taking was negatively associated with medial PFC activation when accepting risky gambles in adolescence, but this relationship was only present when rejecting risky gambles in adulthood [48]. Another study examining the neural response to emotional faces during an impulsivity task found that adolescent boys, who exhibited increased impulsivity in the presence of threatening faces, also had less left medial PFC activation, relative to adolescent girls. In fact, left medial PFC activity during the impulsivity task was especially high during adolescence, relative to childhood or adulthood, in females but not in males [50]. This high level of PFC activity in adolescent girls did not seem to be associated with less maturation of the medial PFC, however, as females had similar performance on the task in adolescence versus adulthood, while adolescent males performed worse than adults despite the two age groups having similar medial PFC activity. However, this study also had a relatively limited sample size, with just 8-10 subjects per age and sex, and so caution should be exercised when interpreting these results until they have been replicated. Therefore, sex differences in PFC activation during task performance are variable and more studies are needed to clarify the significance of these results.
2.2. Amygdala
The development of the amygdala resembles that of the PFC with regard to sex differences. Men have a greater peak volume, but they reach this peak later in puberty than women [81]. Despite this difference in the trajectory of volume changes, males and females may not differ in overall amygdala volume during adolescence. Specifically, boys and girls in mid-adolescence do not differ with regard to amygdala volume (as a percentage of total cranial volume), though the authors of this study controlled for age rather than pubertal stage and there may be volume differences when boys and girls are in the same stage of puberty [82]. Indeed, when Tanner stage is taken into account a volume difference does emerge in the left amygdala, which has a greater volume in boys [83]. In fact, there is an interaction between pubertal stage and sex, with males having increased, and females decreased, volume in left amygdala across pubertal stages [84]. Sex differences in the amygdala are not entirely puberty-dependent, however, because regardless of Tanner stage males have greater grey matter density in the left amygdala during adolescence [85].
Sex differences in rodent studies of the amygdala have not fully aligned with the human literature. The rat basolateral amygdala (BLA), a region involved in emotional and associative learning [86], loses neurons in a pattern that is similar to neuronal loss in the PFC, with females exhibiting a greater loss from adolescence to adulthood [87,88]. However, there is no sex difference in BLA volume by adulthood [87]. In addition, the rat BLA does not resemble the PFC with regard to changes in dendritic complexity across adolescence: neither males nor females exhibit reduced BLA dendritic complexity from P35 to P90 [78]. Sex differences in the rat medial amygdala (MeA), a region associated with social behavior, may be more in line with what would be expected from the human literature as males exhibit greater increases in MeA volume and have more cell proliferation from adolescence to adulthood, resulting in greater volume and cell number in the adult MeA [89]. Given the differences between the BLA and MeA, it is possible that sex has a region-specific effect on adolescent amygdala development in rodents.
Perhaps due in part to methodological limitations, studies of functional connectivity in humans have tended to focus on the amygdala as a whole rather than differentiating between the BLA and MeA. For example, women and girls have greater short-range (within-region) rsFC in the amygdala relative to males [90]. As mentioned previously, short-range rsFC generally declines with age in regions involved in higher-order processing [79] and a similar trend is found for rsFC within the amygdala [90]. Therefore, the higher level of amygdala rsFC in females may suggest that females actually lag behind males in the maturation of this region. Caution must be exercised, however, as it is difficult to interpret the significance of sex differences in amygdala rsFC without more information about activity within the BLA and MeA. If there are sex differences in rsFC between the BLA and MeA, this might have implications for the greater prevalence of anxiety in females: greater BLA-MeA rsFC is associated with anxiety disorder in adolescents [91].
When the amygdala is recruited for emotional processing, sex differences become more complex. One study has found that the extent to which the left amygdala is activated while viewing emotional faces decreases throughout adolescence for females, but not for males, though this study had only 9-10 participants per sex [92]. Meanwhile, a much larger (n = 235/sex) recent study has found that adolescent boys seem to exhibit heightened activation of the right amygdala while viewing angry or neutral faces [93]. These seemingly conflicting reports suggest a role for lateralization in interpreting sex differences in amygdala activation. Indeed, sex differences in rsFC between the amygdala and other regions are highly lateralized in adulthood, with men demonstrating substantially more connectivity between the right amygdala and other regions while women have more connectivity between the left amygdala and other regions [94].
2.3. Striatum
Striatal development occurs in a sex-dependent fashion. As with other regions, adolescent boys lag behind girls, reaching peak striatal volume at 14.7 and 12.1 years of age, respectively [68]. Striatal area and volume both increase from adolescence to adulthood in males, but not in females [68]. In addition, the trajectory of striatal development may differ between males and females in a region-specific fashion. For example, volumetric change in the ventral striatum (i.e., nucleus accumbens) follows sex-specific trends: cubic for males and linear for females. Therefore males exhibit an increase and decrease in ventral striatal volume while there is a steady decline in females. This is despite the absolute change in volume being similar in men (−8.7%) and women (−8.6%) by the end of puberty [81]. This change in volume may be hemisphere dependent, however. In a recent study of adolescent boys and girls, nucleus accumbens volume decreased from age 12.5 to age 16.5 in the right hemisphere but increased in the left [95]. Furthermore, a separate study found that, relative to total brain volume, left nucleus accumbens volume decreases across puberty for girls and increases for boys [96]. In the dorsal striatum (i.e., caudate and putamen) the trajectory of development is more similar between males and females [81,95]. Therefore it would seem that sex differences are most dramatic within the ventral striatum, where adolescent boys seem to follow a trend of expansion and contraction while in girls this region simply contracts.
While human studies have shown sex differences in the trajectory of volumetric changes, one rodent study has shown that males and females have very different trajectories for dopamine receptor changes in the striatum as well [97]. Male rats in this study had a substantial overproduction and pruning of striatal dopamine D1 and D2 receptors during adolescence, while females failed to show anything more than a modest increase early in adolescence. D1 and D2 receptor density was similar in males in females by adulthood for the dorsal striatum, but within the nucleus accumbens males maintained a greater density of D1 receptors well into adulthood [97]. As with volume changes in humans [68,81], dopamine receptor expression in the rodent striatum goes through greater increases and decreases in males and sex differences seem to be most pronounced within the ventral striatum.
Few functional imaging studies in humans have found sex differences in adolescent striatal activation. In the gambling task mentioned previously, males and females had similar levels of activation in the striatum during both adolescence and adulthood, though there was also no effect of age on striatal activation in this study [48]. An earlier study of neural activation during reward receipt and omission found that adolescents had more activation within the ventral striatum during reward receipt [98]. In that study, however, the authors suggested they were underpowered to determine if there were sex differences in this activation because they had only 7-10 subjects per sex and age [98]. Similarly, another study by Van Leijenhorst and colleagues demonstrated an inverted-U shaped curve for activation of the ventral striatum during risky decision making, but the sample size of 5-8 subjects per age and sex precluded analysis of sex differences [99]. Given that the only study examining sex differences was also the study that did not find any age-related differences, it is difficult to draw any conclusions about whether males and females would differ in striatal activation during adolescence. Nevertheless, a study conducted in adults demonstrated that women had more activation of the putamen during a reward-based decision task [100]. The finding of sex differences in dorsal striatum (e.g., putamen) activity may be significant as well, as Simon and Moghaddam have recently suggested [101] that the dorsal striatum, rather than the ventral striatum, may mediate risky decision making behavior during adolescence. Thus, it is reasonable to predict that adolescents may exhibit sex differences in striatal activation as well.
3. Role of sex differences in theories of adolescent vulnerability
To this point, we have highlighted a number of sex differences in risk profiles, decision making and reward-related behavior, and neural development during adolescence. We will now synthesize these findings by examining some of the leading theories of adolescent vulnerability to substance use. While there is emerging consensus on the role of corticolimbic development in risky decision making among these theories, none of them appear to incorporate or consider sex differences in adolescent vulnerability. Therefore, we will attempt to reinterpret these models with sex included as a factor.
3.1. Triadic and dual-systems models
One influential theory that has attempted to explain the high levels of risk taking and sensation seeking among adolescents is the triadic model. Proposed by Ernst and colleagues, this theory suggests that risky behavior and poor emotional control are caused by developmental imbalances between the PFC, amygdala, and striatum [7,9]. In this framework, the corticolimbic circuit is balanced differently during adolescence: the PFC does a poorer job of regulating signals from the striatum and amygdala, while the contributions of the striatum outweigh those of the amygdala. The result is a tendency toward reward seeking rather than harm avoidance. Another theory, the dual systems model proposed by Steinberg and colleagues, suggests that risk taking arises because the development of dopaminergic systems projecting from the striatum precedes the development of inhibitory control systems in the PFC and this results in sensation seeking rising before inhibitory control [16,17]. The triadic model differs from the dual systems model in that it includes a focus on motivated behavior (including approach and avoidance), while both models attempt to provide a framework to explain adolescent risk-taking [102]. These two theories, along with similar theories proposed by other investigators [18,103], suggest that developmental imbalances between the PFC and other regions in the corticolimbic circuit are responsible for adolescent behavioral tendencies. If these developmental imbalances are related to risky behavior and drug use, sex differences in the development of corticolimbic regions may explain why males and females differ in adolescent reward-related behavior and in vulnerability to psychological disorders that emerge during adolescence.
Sex differences in behavior during adolescence are consistent with the dual systems model. In addition to its focus on the striatum and PFC, the dual system model proposes that it is the misalignment of changes in behaviors associated with these regions, sensation seeking and inhibitory control, that leads to risk taking [16]. Females, who are less likely to engage in adolescent risk taking or use drugs, have lower peak sensation seeking and higher levels of inhibitory control [21]. As a result, females have a much smaller deficit of inhibitory control, relative to sensation seeking, during adolescent development. Therefore, sex differences in risk taking do seem to be consistent with what would be expected from the dual systems model. Because this consistency arises from females never reaching the same magnitude of difference between inhibitory control and sensation seeking, it may not necessarily be related to sex differences in the timing of PFC and striatal development.
While males and females do differ in the timing of PFC and striatal development, these sex differences are not consistent with what would be predicted by the dual systems model. It is thought that the period between striatal maturation and PFC maturation, when the PFC is not mature enough to inhibit activity within the relatively more mature striatum, is the most vulnerable [8,16]. But the extent to which females mature faster than males is region dependent, with peak striatal volume occurring 2.6 years earlier in females but peak PFC volume occurring 1.1 years earlier [68]. Thus, sex differences in the timing of development actually suggest that in females the striatum matures more quickly (relative to the PFC) than it does in males. This relatively faster maturation of the striatum would be expected to result in greater risk taking among females, yet females exhibit a lower level of risk taking during adolescence. However, both rodent [4,78] and human [48,68,104] studies have suggested that females may undergo greater PFC maturation, while also undergoing less dramatic striatal remodeling [68,97]. Therefore it may be that the trajectory and outcome of adolescent development, rather than the timing of changes, is responsible for sex differences in adolescent and lifelong risk taking and drug experimentation.
The triadic model is similar to the dual systems model with regard to motivated behavior, but includes some consideration of how developmental changes in the amygdala can also contribute to adolescent vulnerability. During adolescence, the amygdala and PFC are less able to work together to control and regulate emotional behavior [7]. Sex differences in the development of these regions, therefore, may affect vulnerability to developing problems with anxiety and depression. Indeed, amygdala development seems to have a larger effect on emotional behavior in females. A study looking at amygdala volume found that larger amygdala volume was correlated with poorer emotional control in female, but not male, adolescents [82]. It is unlikely that the amygdala is alone in contributing to sex differences in emotional control, given that males have larger amygdala volume in general and yet are less vulnerable to developing depression and anxiety.
While the triadic model proposes that adolescents have less emotional control because of reduced PFC control over the amygdala, there is some evidence that females may actually have greater PFC control over the amygdala. A study examining the effects of threatening faces on impulsivity found that females were less affected by viewing a threatening face, with regard to impulsivity, and also had substantially more activation in the medial PFC during the task relative to adult females and adolescent males [50]. Therefore they may have relied more heavily on the PFC to moderate their emotional response to threat than males. Given that the triadic model would predict greater emotional control with increased PFC control over the amygdala, it is possible PFC-amygdala connectivity, like amygdala volume [82], has sex-specific effects on vulnerability. In support of a role for PFC-amygdala connectivity in vulnerability, ventromedial PFC to amygdala rsFC strongly predicts symptoms of anxiety and depression in females, but not in males [69]. Rodent studies have not yet examined whether sex affects the development of connections between the PFC and amygdala [88], but the presence of sex differences in development of both regions suggests that there may be sex differences in how connectivity develops between these regions.
3.2. Vulnerability to disruption of development by stressors
While the corticolimbic circuit may have altered functioning during adolescence, which in turn could lead to riskier behavior and increased drug use during this time, many theories of adolescent vulnerability have focused on whether the normal development of this circuit may be vulnerable to disruption by stress [105,106] and drug exposure [88]. This is because the corticolimbic circuit is critically involved in modulating the stress response and is also particularly sensitive to the effects of stress [11,105]. Male and female rats differ in their vulnerability to exhibiting anxiety and depression-like behavior following adolescent stress [107]. For example, adolescent stress leads to depressive-like behavior (e.g., a reduction in sucrose preference) in female rats, but not in male rats [105]. The results are similar in humans, where episodes of interpersonal stress [44] and the cortisol response to this stress [108] are more strongly linked to internalizing symptoms and depression in adolescent girls.
Just as females are more vulnerable to stress during adolescence, they may also be more likely to exhibit lasting effects of early life stress. For example, females who had experienced early life stress had greater levels of cortisol during childhood (4.5 years old) and less rsFC between the ventromedial PFC and the left amygdala during adolescence [69]. This reduced ventromedial PFC-amygdala rsFC was associated with an increased number of anxious symptoms, particularly in females [69]. Therefore, females may be more vulnerable to the effects of early life stress on anxiety because of vulnerability within the left ventromedial PFC-amygdala pathway. It is not clear whether these results are limited to the left amygdala, as this study did not report data from the right hemisphere. Intriguingly, increased ventromedial PFC-amygdala rsFC was associated with an increased number of depressive symptoms, regardless of gender [69]. This suggests that there may be individual differences mediating how the adolescent ventromedial PFC-amygdala pathway responds to early life stress, with a decrease in rsFC leading to anxious symptoms in females and an increase in rsFC leading to depressive symptoms in both males and females. However, it is clear that regardless of the effects of ventromedial PFC-amygdala disruption on internalizing disorders, this pathway is more sensitive to disruption by stress in females.
The finding that the female PFC is more affected by stress has been corroborated in rodents. For example, female adults exhibited greater dendritic length and density on neurons projecting from the medial PFC to the BLA following repeated restraint stress [109]. Males, however, did not exhibit any changes along this pathway [110]. Moreover, this effect is mediated by hormones [11], as the effects of stress on medial PFC-BLA dendrites were attenuated when females in this study, all of whom had been ovariectomized, were not treated with estrogen [109]. It is likely that this sex difference in the effect of stress on PFC-amygdala connections may be mediated by adolescent development, given the numerous sex differences in the development of both regions [4] and in the effects of stress on rsFC in humans [69]. Within the rodent PFC, the effect of prenatal stress on adolescent development differs between males and females. Prenatally stressed female rats exhibited less maturation of PFC dendrites by adulthood (no increase in dendritic complexity from P30/P56 to P90) but did not seem to be affected during adolescence [111]. Meanwhile, prenatally stressed males had a slight reduction in dendritic complexity during early adolescence (P30) that faded by adulthood [111]. Taken together, these findings suggest that stress may have larger and more enduring effects on the PFC and its projections in females, though there is a need for additional studies.
Drugs of abuse, like stress, may have lasting effects on behaviors associated with the corticolimbic circuit [88]. Our lab has found that exposure to amphetamine during adolescence or adulthood, at doses beyond what typically would be used to treat attention deficit hyperactivity disorder, can have sex-specific effects on impulsivity later in life. In a recent study we trained rats that had been exposed to relatively high doses (3.0mg/kg) of amphetamine to withhold responding until a go-signal in order to obtain food. We found that males who had been exposed to amphetamine during adolescence, but not those exposed during adulthood, had modest impairments in inhibitory control [112], consistent with previous studies of PFC-related behavior from our lab [113-115], Meanwhile females that had been exposed to amphetamine during adulthood, but not during adolescence, had substantial impairments in inhibitory control [112]. Inhibitory control is tied to the function of the PFC, ventral striatum, and amygdala [36], so impairments in this measure may indicate that something in the corticolimbic circuit may have been affected by amphetamine. Therefore it would seem that adolescent exposure (P27-45) to drugs of abuse may have a greater effect on corticolimbic function and inhibitory control in males, despite females showing a much greater impairment following adult exposure. The lack of effect of amphetamine on inhibitory control in females exposed during adolescence is not consistent with what would be expected based on the epidemiological studies, where females are more likely to develop problems associated with adolescent drug use [26,116]. In addition, a recent human study has found that binge-drinking adolescent girls have greater reductions in neural activity and performance during a spatial working memory task, while boys were relatively resilient to the effects of ethanol exposure [117]. Therefore, females may be particularly vulnerable to disruption of development following alcohol use, but less vulnerable to amphetamine exposure.
Another study examined the effects of self-administering cocaine on operant responding for food or cocaine later in life. In this study, rats that had self-administered cocaine during adolescence, but not during adulthood, made more operant responses for food than rats who had learned to respond for food during adolescence, but this was not affected by sex [53]. The same was true of operant responding for cocaine later in life, as rats that had self-administered cocaine during adolescence responded more for cocaine in adulthood than rats who had first received access to cocaine as adults. However, adolescent cocaine self-administration had no effect on adult responding during periods of signaled non-availability for food or cocaine, suggesting that cocaine affected only reinforced responses. Though there is some evidence for sex-specific effects of early-life exposure to stimulants on inhibitory control, the evidence is lacking for sex-specific effects on perseverative responding and more work should be done to characterize sex differences in the response to other drugs of abuse, such as ethanol.
4.1. Challenges and opportunities in studying adolescent sex differences
Careful consideration should be given to task design when studying sex differences in adolescent behavior, as males and females may differ in their response to different behavioral tasks and manipulations. Among humans, it has been proposed that males could be more interested in videogame-based tasks while females are more interested in social-based tasks [118]. If this is true it would be difficult to know if sex differences in activation during a videogame-based task are genuine, or whether they are confounded by sex differences in the salience of the task. However, the lab that advanced this theory recently published a study looking at the effect of sex on feedback-related negativity (FRN) throughout adolescence (using a simple choice videogame) and found that the tendency toward greater FRN in males may actually reflect delayed maturation, rather than a sex difference in salience. This is because they found that FRN amplitude declines as children and adolescents mature [119]. Given the role of salience and task engagement in neural activation, future studies on sex differences in the salience of common elements of tasks, such as the use of monetary, food or social rewards, will help refine the assessment of sex differences in behavior and neural activation. In addition, researchers should consider using longitudinal, rather than cross-sectional, study designs as sex differences in task salience will have less of an effect in a within-subjects design.
Rodent studies should also consider whether there are sex differences in the motivational value of reinforcers. In particular, the effects of food restriction should be carefully considered. One study looking at extinction learning has demonstrated that adolescents responded more during extinction while food restricted, while adults were unaffected by food restriction [120]. This may be affected by sex, as well. While we have previously shown that food-restricted females (approximately 15% body weight loss) were substantially less impulsive than males food restricted to the same level [112], we have recently found that females made more premature responses than males when a milder food restriction method (no weight loss) is employed (Hammerslag and Gulley, unpublished results). We have also shown that female rats were less sensitive to the effects of satiety on extinction responding [57]. Therefore the method of food restriction used may have a large impact on behavioral findings in rodent studies with adolescent age increasing sensitivity, and female sex decreasing sensitivity, to the effects of food deprivation. However, a study on the effects of a cannabinoid on food-reinforced responding did not find any sex differences in the effect of food, though control females seemed to be less affected by food restriction than control males [121]. Another rat study has found that food restriction-induced increases in heroin intake are similar in males and females [122]. As there is not yet a consensus on whether and how sex and adolescence may interact to influence the response to food deprivation, researchers should take note of the method of food restriction employed in different studies, (e.g., body weight loss [57] or mild deprivation [123]) as this factor may influence results.
Another factor that needs to be considered is the influence of sex hormones. Researchers have noted that some of the regions with the largest sex differences in volume and gray matter in human studies, such as the amygdala and PFC, are also regions with high levels of sex steroid receptor expression in rodent studies [75,124,125]. In addition, a number of studies have shown a relationship between sex hormone levels and adolescent brain development [see for review: 123]. For example, one study in adolescent boys and girls found that high circulating testosterone levels predicted greater gray matter volume in the left amygdala, independent of Tanner stage [83]. Therefore, sex hormones may have an organizational effect on corticolimbic circuit development and this may be particularly pronounced in areas with high levels of sex steroid receptors. This organizational effect is in addition to well documented activational effects of sex hormones on corticolimbic function and reward-related behavior in adult humans and rodents [see for review: 12,14,32]. One way to separate the organizational and activational effects of sex hormones is to compare animals that are gonadectomized during adulthood to those that are gonadectomized before puberty [13,127]. Using this approach, adult gonadectomy reveals the activational effects of sex hormones while differences between adult gonadectomy and pre-pubertal gonadectomy represent the organizational effects of hormones during puberty and adolescence. While some studies have examined subjects both pre- and post-puberty with regard to reward-related behavior, few have examined pre-pubertal gonadectomy with regard to corticolimbic development [128,129]. Thus, opportunities exist to expand this field substantially in the coming years.
4.2. Need for future studies on sex differences in adolescent vulnerability
The studies we have reviewed present a compelling case for increasing research into the effects of sex on adolescent behavior and development. In addition to increasing the number of studies including females, efforts must be taken to examine the effects of sex in studies that include male and female subjects, even if this means increasing the number of subjects [98,99]. Indeed, a recent review of fMRI studies comparing adolescents and adults has demonstrated that the majority of studies on reward-related activation lack the power to examine sex differences [130]. Not only is it likely that sex differences in behavior and neural activation may be influencing error within mixed-sex groups, failing to design studies so that sex is included as a factor in analysis deprives us of valuable data that may help refine our theories of vulnerability. Thus, human and rodent studies that do not include sex as a factor may present results that are incomplete and care should be taken not to over-generalize these studies.
4.3. Conclusion
In this review, we have highlighted a number of recent studies demonstrating sex differences in adolescent vulnerability, behavior, and development. Just as females are less likely to engage in risk taking or experiment with drugs, they are also more likely to develop problems with anxiety and depression and to develop problem drug use after experimentation. Current theories of adolescent vulnerability have proposed that adolescents are especially vulnerable to risky behavior, drug use, and internalizing disorders as a result of adolescent corticolimbic circuit development. Just as females differ from males in these behaviors, they also differ in the timing and trajectory of adolescent changes within the corticolimbic circuit. Therefore there may be a complex relationship between sex and the factors (e.g., developmental and behavioral) that contribute to adolescent vulnerability to substance use (Fig. 2). In adolescent boys, corticolimbic development predisposes them to risky, sensation-seeking behavior that then leads to increased drug experimentation and abuse (Fig. 2, blue filled arrows). In adolescent girls, stress has a greater effect on corticolimbic function and on the risk for developing internalizing disorders, this leads to greater vulnerability to problems associated with drug use (Fig. 2, red unfilled arrows). Thus, sex differences in behavior and corticolimbic development are often, but not always, consistent with the dual systems and triadic theories of adolescent vulnerability to substance use.
The sex differences highlighted in this review suggest that future research into the factors influencing adolescent vulnerability to substance use must include some consideration of differences between males and females. Not only will these future studies help to expand our knowledge of gender-related disease vulnerability, they will also provide data that can be used to test and refine our models of adolescent vulnerability. This is because sex may affect both the inputs (e.g., corticolimbic circuit function and development) and the outputs (e.g., risk-taking behavior and disease vulnerability) of these models. We should therefore take advantage of the rapid expansion of research into sex differences as an opportunity to reexamine a number of models that were developed in male subjects.
Highlights.
Male and female adolescents differ in depression and substance use vulnerability.
There are also adolescent sex differences in risk-taking and sensation seeking.
Corticolimbic (e.g., PFC, striatum, amygdala) development is sex-dependent.
Sex differences in development and behavior may inform theories of vulnerability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Spear LP. The Behavioral Neuroscience of Adolescence. 1st W. W. Norton & Company, Inc.; New York, NY: 2009. [Google Scholar]
- [2].Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. doi:10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19. doi: 10.1016/j.yfrne.2014.03.008. doi:10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Juraska JM, Sisk CL, DonCarlos LL. Sexual differentiation of the adolescent rodent brain: Hormonal influences and developmental mechanisms. Horm Behav. 2013;64:203–10. doi: 10.1016/j.yhbeh.2013.05.010. doi:10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- [5].Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol. 2014;35:89–110. doi: 10.1016/j.yfrne.2013.10.004. doi:10.1016/j.yfrne.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. doi:10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ernst M, Phillips AG, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. doi:10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20:236–41. doi: 10.1016/j.conb.2010.01.006. doi:10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacol Biochem Behav. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. doi:10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- [10].Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–14. doi: 10.1196/annals.1376.022. doi:10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- [11].McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. doi:10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. doi:10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kuhn CM, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, et al. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58:122–37. doi: 10.1016/j.yhbeh.2009.10.015. doi:10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. doi:10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- [15].Fattore L, Melis M, Fadda P, Fratta W. Sex differences in addictive disorders. Front Neuroendocrinol. 2014;35:272–84. doi: 10.1016/j.yfrne.2014.04.003. doi:10.1016/j.yfrne.2014.04.003. [DOI] [PubMed] [Google Scholar]
- [16].Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–24. doi: 10.1002/dev.20445. doi:10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- [17].Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764. doi: 10.1037/a0012955. doi:10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- [18].Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–33. doi: 10.1016/j.bandc.2009.07.003. doi:10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Willoughby T, Good M, Adachi PJC, Hamza C, Tavernier R. Examining the link between adolescent brain development and risk taking from a social-developmental perspective. Brain Cogn. 2013;83:315–23. doi: 10.1016/j.bandc.2013.09.008. doi:10.1016/j.bandc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- [20].Casey BJ. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annu Rev Psychol. 2014:1–25. doi: 10.1146/annurev-psych-010814-015156. doi:10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- [21].Shulman EP, Harden KP, Chein JM, Steinberg L. Sex Differences in the Developmental Trajectories of Impulse Control and Sensation-Seeking from Early Adolescence to Early Adulthood. J Youth Adolesc. 2014 doi: 10.1007/s10964-014-0116-9. doi:10.1007/s10964-014-0116-9. [DOI] [PubMed] [Google Scholar]
- [22].Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–23. doi: 10.1016/j.bandc.2009.08.008. doi:10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [24].Grant B, Dawson D. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–10. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- [25].Foster KT, Hicks BM, Iacono WG, McGue M. Alcohol use disorder in women: Risks and consequences of an adolescent onset and persistent course. Psychol Addict Behav. 2014;28:322–35. doi: 10.1037/a0035488. doi:10.1037/a0035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: adolescent risk factors and adult outcomes. Alcohol Clin Exp Res. 2010;34:819–33. doi: 10.1111/j.1530-0277.2010.01154.x. doi:10.1111/j.1530-0277.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend Med. 2010;7:402–13. doi: 10.1016/j.genm.2010.09.004. doi:10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [28].Fattore L, Melis M, Fadda P, Fratta W. Sex differences in addictive disorders. Front Neuroendocrinol. 2014;35:272–84. doi: 10.1016/j.yfrne.2014.04.003. doi:10.1016/j.yfrne.2014.04.003. [DOI] [PubMed] [Google Scholar]
- [29].Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–30. doi: 10.1016/j.drugalcdep.2010.11.004. doi:10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend. 2007;86:191–8. doi: 10.1016/j.drugalcdep.2006.06.003. doi:10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [31].Lukas S, Wetherington CL. Sex- and gender-related differences in the neurobiology of drug abuse. Clin Neurosci Res. 2005;5:75–87. doi:10.1016/j.cnr.2005.08.004. [Google Scholar]
- [32].Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. doi:10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- [33].Danielsson A-K, Romelsjö A, Tengström A. Heavy episodic drinking in early adolescence: gender-specific risk and protective factors. Subst Use Misuse. 2011;46:633–43. doi: 10.3109/10826084.2010.528120. doi:10.3109/10826084.2010.528120. [DOI] [PubMed] [Google Scholar]
- [34].Kuntsche E, Müller S. Why do young people start drinking? Motives for first-time alcohol consumption and links to risky drinking in early adolescence. Eur Addict Res. 2012;18:34–9. doi: 10.1159/000333036. doi:10.1159/000333036. [DOI] [PubMed] [Google Scholar]
- [35].Schissel AM, Olson EA, Collins PF, Luciana M. Age-independent effects of pubertal status on behavioral constraint in healthy adolescents. Pers Individ Dif. 2011;51:975–80. doi: 10.1016/j.paid.2011.08.001. doi:10.1016/j.paid.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. doi:10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- [37].Kreek MJ, Nielsen DA, Butelman ER, Laforge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. doi:10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- [38].Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. doi:10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- [39].Waller MW, Hallfors DD, Halpern CT, Iritani BJ, Ford CA, Guo G. Gender differences in associations between depressive symptoms and patterns of substance use and risky sexual behavior among a nationally representative sample of U.S. adolescents. Arch Womens Ment Health. 2006;9:139–50. doi: 10.1007/s00737-006-0121-4. doi:10.1007/s00737-006-0121-4. [DOI] [PubMed] [Google Scholar]
- [40].Hallfors DD, Waller MW, Ford CA, Halpern CT, Brodish PH, Iritani B. Adolescent depression and suicide risk: association with sex and drug behavior. Am J Prev Med. 2004;27:224–31. doi: 10.1016/j.amepre.2004.06.001. doi:10.1016/j.amepre.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [41].Wilson HW, Widom CS. A prospective examination of the path from child abuse and neglect to illicit drug use in middle adulthood: the potential mediating role of four risk factors. J Youth Adolesc. 2009;38:340–54. doi: 10.1007/s10964-008-9331-6. doi:10.1007/s10964-008-9331-6. [DOI] [PubMed] [Google Scholar]
- [42].Kessler R. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. doi:10.1016/S0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- [43].Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- [44].Shih J, Eberhart N. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. J Clin Child Adolesc Psychol. 2006;35:103–15. doi: 10.1207/s15374424jccp3501_9. doi:10.1207/s15374424jccp3501. [DOI] [PubMed] [Google Scholar]
- [45].Ordaz SJ, Luna B. Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology. 2012;37:1135–57. doi: 10.1016/j.psyneuen.2012.01.002. doi:10.1016/j.psyneuen.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stroud LR, Papandonatos GD, Williamson DE, Dahl RE. Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology. 2011;36:1226–38. doi: 10.1016/j.psyneuen.2011.02.017. doi:10.1016/j.psyneuen.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Dev Neuropsychol. 2008;33:179–96. doi: 10.1080/87565640701884287. doi:10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- [48].Barkley-Levenson EE, Van Leijenhorst L, Galván A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev Cogn Neurosci. 2013;3:72–83. doi: 10.1016/j.dcn.2012.09.007. doi:10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Brumback TY, Arbel Y, Donchin E, Goldman MS. Efficiency of responding to unexpected information varies with sex, age, and pubertal development in early adolescence. Psychophysiology. 2012;49:1330–9. doi: 10.1111/j.1469-8986.2012.01444.x. doi:10.1111/j.1469-8986.2012.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dreyfuss M, Caudle K, Drysdale AT, Johnston NE, Cohen AO, Somerville LH, et al. Teens impulsively react rather than retreat from threat. Dev Neurosci. 2014;36:220–7. doi: 10.1159/000357755. doi:10.1159/000357755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Friemel CM, Spanagel R, Schneider M. Reward sensitivity for a palatable food reward peaks during pubertal developmental in rats. Behav Neurosci. 2010;4:1–10. doi: 10.3389/fnbeh.2010.00039. doi:10.3389/fnbeh.2010.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 2011;125:93–105. doi: 10.1037/a0022038. doi:10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- [53].Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology (Berl) 2011;215:785–99. doi: 10.1007/s00213-011-2181-z. doi:10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–46. doi: 10.1007/s00213-007-1028-0. doi:10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- [55].Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–4. doi: 10.1016/j.pbb.2008.11.002. doi:10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rubinow MJ, Hagerbaumer D, Juraska JM. The food-conditioned place preference task in adolescent, adult and aged rats of both sexes. Behav Brain Res. 2009;198:263–6. doi: 10.1016/j.bbr.2008.11.024. doi:10.1016/j.bbr.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hammerslag LR, Gulley JM. Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol. 2013;56:611–21. doi: 10.1002/dev.21127. doi:10.1002/dev.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schramm-Sapyta NL, Francis R, MacDonald A, Keistler C, O’Neill L, Kuhn CM. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl) 2014;231:1831–9. doi: 10.1007/s00213-013-3319-y. doi:10.1007/s00213-013-3319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: impact of age and sex. Behav Brain Res. 2014;261:323–7. doi: 10.1016/j.bbr.2013.12.048. doi:10.1016/j.bbr.2013.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225:104–9. doi: 10.1016/j.bbr.2011.07.003. doi:10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. doi:10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216:569–75. doi: 10.1016/j.bbr.2010.08.048. doi:10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Vetter-O’Hagen C, Varlinskaya EI, Spear LP. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. doi:10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Varlinskaya EI, Truxell EM, Spear LP. Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behav Brain Res. 2015;282:6–13. doi: 10.1016/j.bbr.2014.12.054. doi:10.1016/j.bbr.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Giedd J, Blumenthal J. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- [66].Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- [67].Ernst M, Mueller SC. The adolescent brain: insights from functional neuroimaging research. Dev Neurobiol. 2008;68:729–43. doi: 10.1002/dneu.20615. doi:10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci. 2014;111:1592–7. doi: 10.1073/pnas.1316911111. doi:10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–41. doi: 10.1038/nn.3257. doi:10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzisa C, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. doi:10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–73. doi: 10.1016/j.neuroimage.2007.03.053. doi:10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child Psychiatry Branch of the National Institute of Mental Health Longitudinal Structural Magnetic Resonance Imaging Study of Human Brain Development. Neuropsychopharmacology. 2014;40:43–9. doi: 10.1038/npp.2014.236. doi:10.1038/npp.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Raznahan A, Lee Y, Stidd R. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci. 2010;107:16988–93. doi: 10.1073/pnas.1006025107. doi:10.1073/pnas.1006025107/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. doi:10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3:19. doi: 10.1186/2042-6410-3-19. doi:10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. doi:10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- [77].Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–8. doi: 10.1016/j.neuroscience.2006.10.015. doi:10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- [78].Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. doi:10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- [79].Zuo X-N, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. doi:10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Douet V, Chang L, Cloak C, Ernst T. Genetic influences on brain developmental trajectories on neuroimaging studies: from infancy to young adulthood. Brain Imaging Behav. 2014;8:234–50. doi: 10.1007/s11682-013-9260-1. doi:10.1007/s11682-013-9260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Goddings A-L, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore S-J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–51. doi: 10.1016/j.neuroimage.2013.09.073. doi:10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Blanton RE, Chaplin TM, Sinha R. Sex differences in the correlation of emotional control and amygdala volumes in adolescents. Neuroreport. 2010;21:953–7. doi: 10.1097/WNR.0b013e32833e7866. doi:10.1097/WNR.0b013e32833e7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–73. doi: 10.1093/cercor/bhn100. doi:10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- [84].Bramen JE, Hranilovich J, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21:636–46. doi: 10.1093/cercor/bhq137. doi:10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–42. doi: 10.1016/j.psyneuen.2008.09.012. doi:10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- [86].Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- [87].Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–25. doi: 10.1002/cne.21924. doi:10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Gulley JM, Juraska JM. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 2013;249:3–20. doi: 10.1016/j.neuroscience.2013.05.026. doi:10.1016/j.neuroscience.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–7. doi: 10.1038/nn.2178. doi:10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lopez-Larson MP, Anderson JS, Ferguson M, Yurgelun-Todd D. Local brain connectivity and associations with gender and age. Dev Cogn Neurosci. 2011;1:187–97. doi: 10.1016/j.dcn.2010.10.001. doi:10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–9. doi: 10.1016/j.jaac.2012.12.010. e2. doi:10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Killgore W, Oki M, Yurgelun-Todd D. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427–33. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- [93].Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, et al. Boys do it the right way: sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56:1847–53. doi: 10.1016/j.neuroimage.2011.02.019. doi:10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- [94].Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–61. doi: 10.1016/j.neuroimage.2005.09.065. doi:10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- [95].Dennison M, Whittle S, Yücel M, Vijayakumar N, Kline A, Simmons J, et al. Mapping subcortical brain maturation during adolescence: evidence of hemisphereand sex-specific longitudinal changes. Dev Sci. 2013;16:772–91. doi: 10.1111/desc.12057. doi:10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- [96].Urošević S, Collins P, Muetzel R, Lim KO, Luciana M. Pubertal status associations with reward and threat sensitivities and subcortical brain volumes during adolescence. Brain Cogn. 2014;89:15–26. doi: 10.1016/j.bandc.2014.01.007. doi:10.1016/j.bandc.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- [98].Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–9. doi: 10.1093/cercor/bhp078. doi:10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- [99].Van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts S a RB, Westenberg PM, Crone E a. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–55. doi: 10.1016/j.neuroimage.2010.02.038. doi:10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- [100].Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, et al. Gender differences in reward-related decision processing under stress. Soc Cogn Affect Neurosci. 2012;7:476–84. doi: 10.1093/scan/nsr026. doi:10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Simon NW, Moghaddam B. Reward processing in adolescent rodents. Dev Cogn Neurosci. 2014:1–10. doi: 10.1016/j.dcn.2014.11.001. doi:10.1016/j.dcn.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: Anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. doi:10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. doi:10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zuo X-N, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. doi:10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2013;249:162–71. doi: 10.1016/j.neuroscience.2012.10.048. doi:10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Brown GR, Spencer KA. Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience. 2013;249:115–28. doi: 10.1016/j.neuroscience.2012.12.016. doi:10.1016/j.neuroscience.2012.12.016. [DOI] [PubMed] [Google Scholar]
- [107].McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: investigations in rodent models. Neuroscience. 2013;249:242–57. doi: 10.1016/j.neuroscience.2012.08.063. doi:10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- [108].Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: sex differences in the role of cortisol reactivity to interpersonal stress. J Clin Child Adolesc Psychol. 2009;38:513–24. doi: 10.1080/15374410902976320. doi:10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. doi:10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–84. doi: 10.1093/cercor/bhp003. doi:10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Markham JA, Mullins SE, Koenig JI. Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. J Comp Neurol. 2013;521:1828–43. doi: 10.1002/cne.23262. doi:10.1002/cne.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hammerslag LR, Waldman AJ, Gulley JM. Effects of amphetamine exposure in adolescence or young adulthood on inhibitory control in adult male and female rats. Behav Brain Res. 2014;263:22–33. doi: 10.1016/j.bbr.2014.01.015. doi:10.1016/j.bbr.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hankosky E, Gulley J. Performance on an impulse control task is altered in adult rats exposed to amphetamine during adolescence. Dev Psychobiol. 2012;55:733–44. doi: 10.1002/dev.21067. doi:10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hankosky E, Kofsky N, Gulley J. Age of exposure-dependent effects of amphetamine on behavioral flexibility. Behav Brain Res. 2013;252:117–25. doi: 10.1016/j.bbr.2013.06.002. doi:10.1016/j.bbr.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. doi:10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Foster KT, Hicks BM, Iacono WG, McGue M. Alcohol use disorder in women: Risks and consequences of an adolescent onset and persistent course. Psychol Addict Behav. 2014;28:322–35. doi: 10.1037/a0035488. doi:10.1037/a0035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35:1831–41. doi: 10.1111/j.1530-0277.2011.01527.x. doi:10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Crowley MJ, Wu J, Crutcher C. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Dev Neurosci. 2009;31:137–48. doi: 10.1159/000207501. doi:10.1159/000207501.Risk-Taking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Crowley MJ, Wu J, Hommer RE, South M, Molfese PJ, Fearon RMP, et al. A developmental study of the feedback-related negativity from 10-17 years: age and sex effects for reward versus non-reward. Dev Neuropsychol. 2013;38:595–612. doi: 10.1080/87565641.2012.694512. doi:10.1080/87565641.2012.694512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124:16–25. doi: 10.1037/a0018463. doi:10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguéns M, Torres I, Vaquero JJ, et al. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–13. doi: 10.1038/sj.npp.1301467. doi:10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- [122].Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: Sex differences. Exp Clin Psychopharmacol. 2001;9:307–16. doi: 10.1037//1064-1297.9.3.307. doi:10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- [123].Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: The role of dopamine and glutamate. Behav Brain Res. 2012;230:21–33. doi: 10.1016/j.bbr.2012.01.046. doi:10.1016/j.bbr.2012.01.046. [DOI] [PubMed] [Google Scholar]
- [124].Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. doi:10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- [125].Peper JS, van den Heuvel MP, Mandl RCW, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36:1101–13. doi: 10.1016/j.psyneuen.2011.05.004. doi:10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- [126].Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. doi:10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- [127].Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35:2039–49. doi: 10.1111/j.1530-0277.2011.01555.x. doi:10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–91. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- [129].Cooke BM, Woolley CS. Effects of prepubertal gonadectomy on a male-typical behavior and excitatory synaptic transmission in the amygdala. Dev Neurobiol. 2009;69:141–52. doi: 10.1002/dneu.20688. doi:10.1002/dneu.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 2013;37:976–91. doi: 10.1016/j.neubiorev.2013.03.004. doi:10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]