Abstract
Inhibition of B-Raf/MEK/ERK signaling is an effective therapeutic strategy against certain types of cancers such as melanoma and thyroid cancer. While demonstrated to be effective anticancer agents, B-Raf or MEK inhibitors have also been associated with early tumor progression and development of secondary neoplasms. The ligation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) with its receptor, death receptor 5 (DR5), leading to induction of apoptosis, offers a promising anticancer strategy. Importantly, this is also a natural immunosurveillance mechanism against cancer development. We previously demonstrated that activated B-Raf/MEK/ERK signaling positively regulates DR5 expression. Hence, our current work sought to address whether B-Raf/MEK/ERK inhibition and the consequent suppression of DR5 expression impede cancer cell response to DR5 activation-induced apoptosis and activated immune cell-induced killing. We found that both B-Raf (e.g., PLX4032) and MEK inhibitors (e.g., AZD6244 and PD0325901) effectively inhibited ERK1/2 phosphorylation and reduced DR5 levels in both human thyroid cancer and melanoma cells. Similar to the observed effect of genetic knockdown of the B-Raf gene, pretreatment of cancer cell lines with either B-Raf or MEK inhibitors attenuated or abolished cellular apoptotic response induced by TRAIL or the DR5 agonistic antibody AMG655 or cell killing by activated T cells. Our findings clearly show that inhibition of B-Raf/MEK/ERK signaling suppresses DR5 expression and impairs DR5 activation-induced apoptosis and T cell-mediated killing of cancer cells. These findings suggest a potential negative impact of B-Raf or MEK inhibition on TRAIL- or DR5-mediated anticancer therapy and on TRAIL/DR5-mediated immune-clearance of cancer cells.
Keywords: B-Raf, MEK, DR5, TRAIL, apoptosis
Introduction
The MEK/ERK kinase cascade is known to be the predominant and best-studied effector pathway downstream of Ras and Raf. It is often hyper-activated in many types of cancers, particularly those with Ras or Raf mutations such as melanoma, thyroid and colon cancers, hence playing a critical role in supporting the survival and proliferation of cancer cells. As a result, great effort has been devoted to developing effective anticancer drugs targeting the Ras/Raf/MEK/ERK signaling pathway over the past decades. 1–3 The recent success of B-Raf and MEK inhibitors in the treatment of advanced melanoma represents a giant stride forward and has stimulated further research into the potential applications of this therapeutic strategy in other types of cancers. 2, 3 Despite the demonstrable efficacy in patients with melanoma harboring the B-Raf V600E mutation, resistance to B-Raf inhibitors emerges within months. Moreover, secondary tumors, most commonly cutaneous squamous-cell carcinomas and keratoacanthomas, develop following prolonged treatment with B-Raf inhibitors. 4, 5 Similarly, increased cutaneous squamous-cell carcinomas and keratoacanthomas are also seen in patients with advanced renal cell carcinoma or primary liver cancer in association with treatment with sorafenib.6–8 These drawbacks limit the clinical impact of these B-Raf inhibitors. Potential mechanisms mediating the increased incidence of secondary neoplasia are under active investigation. Ras (particularly H-Ras) mutation through paradoxical C-Raf-mediated activation of MEK/ERK signaling has been suggested as an important factor contributing to increased risk of secondary tumors.9 Consequently, the combination of B-Raf and MEK inhibitors was proposed as a strategy to delay or even prevent the onset of resistance without increasing the development of secondary cancers. Indeed, a recent phase II trial comparing the combination of B-Raf and MEK inhibitors with B-Raf inhibition alone showed increased progression-free survival and reduced incidence of secondary malignancies in patients with mutant B-Raf V600 melanoma.10 Nonetheless, the mechanisms underlying the development of rapid resistance and secondary cancers and even other potential risks with this targeted therapy remain to be fully elucidated.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) represents a tumor-selective apoptosis-inducing cytokine with potential as a cancer therapeutic agent. Its ligation with death receptor 5 (DR5; also called TRAIL-R2 or Killer/DR5) rapidly activates the extrinsic apoptotic pathway, leading to apoptotic cell death. This process involves trimerized DR5 interacting specifically with the adaptor protein Fas-associated death domain (FADD) via death domain interaction. The subsequent recruitment of caspase-8 through the death effector domain of FADD leads to caspase-8 activation and ultimately, apoptosis. 11 Additionally, induction of DR5 aggregation or trimerization, e.g., by a DR5 agonistic antibody, also triggers apoptosis. Consequently, pharmacological DR5 agonistic antibodies have been developed and tested in the clinic as potential cancer therapeutics.11 Thus, recombinant TRAIL and DR5 agonistic antibodies constitute a novel group of anticancer therapeutics with promising potential.
TRAIL can be expressed by various cells of the immune system, including natural killer (NK) cells, T cells, natural killer T cells (NKT cells), dendritic cells and macrophages.12 Induction of apoptosis by ligation of endogenous TRAIL with its receptors (e.g., TRAIL/DR5) has been recognized as a critical mechanism underlying the body’s immune surveillance against tumors and metastases. 12, 13 We demonstrated in our previous studies that Ras/Raf/MEK/ERK signaling positively regulates DR5 expression through enhanced CHOP and Elk-mediated transcription. 14, 15 Moreover, we showed that human cancer cell lines with Ras or Raf mutations were enriched in human cancer cell lines most responsive to the DR5 agonistic antibody, AMG655. 15 The current study aims to address whether inhibition, particularly pharmacological inhibition, of B-Raf/MEK/ERK signaling downregulates DR5 expression and impairs cancer cell response to DR5 activation-induced apoptosis including immune-killing induced by activated T cells.
Results
Small interfering RNA (siRNA)-mediated suppression of B-Raf expression reduces DR5 levels and impedes cell response to TRAIL-induced apoptosis
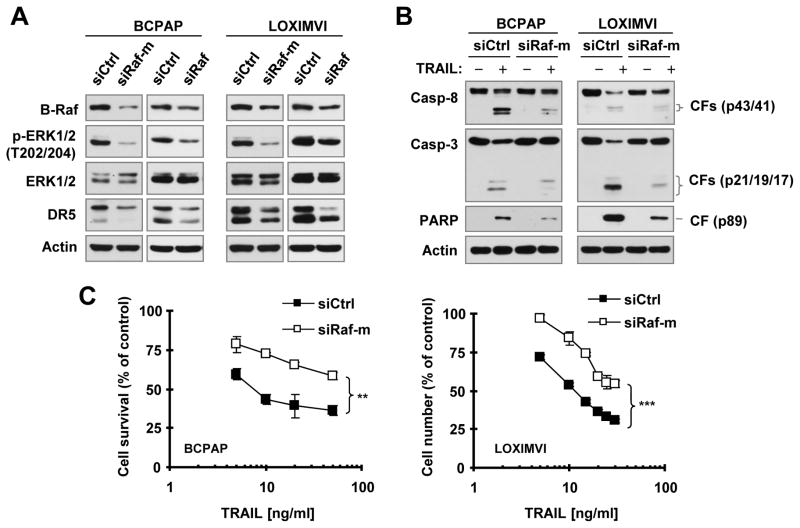
To determine whether downregulation of DR5 expression by B-Raf inhibition affects cancer cell response to DR5-mediated apoptosis, we used two different B-Raf siRNAs: one targets mutant B-Raf V600E (siRaf-m) only and another silences both wild-type and mutant B-Raf (siRaf). We knocked down B-Raf expression in two cancer cell lines with B-Raf V600E mutation and then evaluated cell responses to TRAIL-induced apoptosis. In both BCPAP and LOXIMVI cell lines, knockdown of B-Raf expression with either of the two B-Raf siRNAs reduced p-ERK1/2 levels accompanied by reduced levels of DR5 (Fig. 1A), hence confirming our previous finding that B-Raf positively regulates DR5 expression. 15 Following exposure to TRAIL, we detected much higher levels of cleaved PARP, capase-8 and caspase-3 in control siRNA-transfected cells than in B-Raf siRNA-transfected cells (Fig. 1B) as evaluated by Western blotting. In agreement, there were much more survival cells in B-Raf siRNA-transfected cells than in control siRNA-transfected cells upon TRAIL treatment (Fig. 1C). These results together clearly indicate that genetic inhibition of B-Raf confers resistance of these cancer cell lines to undergo TRAIL-induced apoptosis.
Fig. 1. Knockdown of B-Raf expression in B-Raf mutant cancer cell lines reduces DR5 expression (A) and attenuates cell responses to TRAIL-induced apoptosis (B and C).
BCPAP or LOXIMVI cells were transfected with the indicated siRNAs. After 62 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent Western blotting to detect the indicated proteins (A). The cells were also exposed to 25 ng/ml TRAIL (B) or different concentrations of TRAIL (C) for an additional 8 h. Protein cleavage in whole-cell protein lysates prepared from these cells was detected with Western blotting (B). Viable cell numbers were estimated with the SRB assay (C). The data are means ± SDs of four replicate determinations. CF, cleaved form. ** P < 0.001; *** P < 0.0001 between two transfected cells exposed to every tested concentration.
Pharmacological inhibition of B-Raf or MEK downregulates DR5 expression
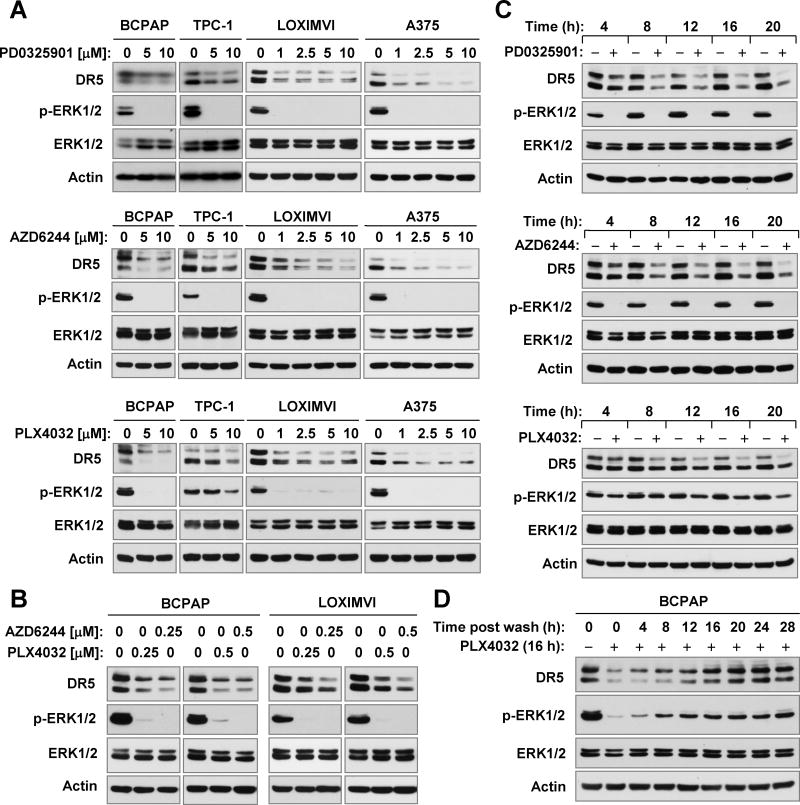
We then asked whether pharmacological inhibition of Raf/MEK/ERK signaling with either B-Raf or MEK inhibitors suppresses DR5 expression in cancer cell lines with activated Raf/MEK/ERK signaling. To this end, we chose three inhibitors in clinical development, PLX4032 (vemurafenib; a B-Raf V600E specific inhibitor), AZD6244 (selumetinib or ARRY-142886; a MEK inhibitor) and PD0325901 (a MEK inhibitor), and tested their effects on DR5 expression in two thyroid cancer cell lines (BCPAP and TPC-1) and two melanoma cell lines (LOXIMVI and A375). At the tested concentration ranges (1–10 μM), all three agents decreased the levels of p-ERK1/2 in the four cancer cell lines, indicating the effective suppression of the Raf/MEK/ERK signaling. Correspondingly, we observed reduction of DR5 levels in these cell lines post exposure to these inhibitors (Fig. 2A). Moreover we examined the effects of PLX4032 and AZD6244 at sub-micromolar concentrations on DR5 expression and found that both agents at 0.25 μM and 0.5 μM effectively reduced the levels of p-ERK1/2 and DR5 (Fig. 2B), indicating that these agents at low concentration ranges also suppress DR5 expression. Time-course analysis showed that the onset of DR5 reduction occurred as early as 4 h post treatment and was sustained for up to 20 h (Fig. 2C). Together these results clearly demonstrate that pharmacological inhibition of the Raf/MEK/ERK signaling pathway with either a B-Raf or a MEK inhibitor downregulates DR5 expression in cancer cells. We also looked at the effect of PLX4032 on DR4 expression and found that PLX4032 did not decrease the levels of DR4 in the tested cell lines (supplemental Fig. S1), suggesting that PLX4032 primarily decreases the expression of DR5, but not DR4.
Fig. 2. Pharmacological inhibition of B-Raf (e.g., with PLX4032) or MEK (e.g., with AZD6244 or PD0325901) suppresses DR5 expression in cancer cells (A-C); this effect is reversible upon stopping of the treatment (D).
A and B, The indicated cancer cell lines were exposed to the given concentrations of the inhibitors as indicated for 16 h. C, TPC-1 cells were treated with 10 μM of the indicated inhibitors for different times as labeled. D, BCPAP cells were treated with 10 μM PLX4032 for 16 h. After removal of the medium, the cells were washed, re-fed with fresh medium and harvested at the indicated times. After these treatments, the cells were then harvested for preparation of whole-cell protein lysates and subsequent Western blotting to detect the indicated proteins.
We noted that PLX4032 had a weaker effect than AZD6244 and PD0325901on decreasing the levels of p-ERK1/2 in the TPC-1 cell line, which lacks an activating B-Raf mutation, indicating a limited effect on wild type B-Raf/MEK/ERK signaling. Accordingly, we observed a weaker effect of this agent on reducing DR5 levels in this cell line (Figs. 2A and 2C). Thus, it appears that the potency of suppression of MEK/ERK signaling is tightly associated with the degree of DR5 reduction.
We also examined the recovery rate of DR5 expression after stopping treatment. To this end, we treated BCPAP cells with PLX4032 for 16 h and then washed off the agent. Cells were re-fed with fresh complete medium and harvested at different time points post wash. As presented in Fig. 2D, recovery of DR5 expression was observed at 4 h which progressively increased until it reached almost normal levels at 16 h. In parallel, p-ERK1/2 levels showed a similar pattern of recovery. Similar results were also generated in BCPAP cells treated with AZD6244 (data not shown). These results indicate that the DR5 suppression by a B-Raf or MEK inhibitor is transient and its recovery is tightly associated with reactivation of MEK/ERK signaling.
Pharmacological inhibition of B-Raf decreases cell surface DR5 levels and suppresses DR5 transcription
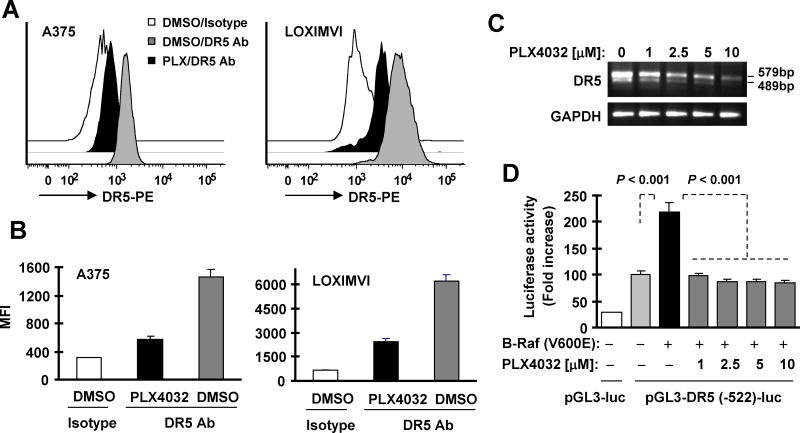
Since DR5 primarily function as a cell surface protein, we then further analyzed the effect of inhibition of B-Raf/MEK/ERK signaling on cell surface DR5 levels. As presented in Figs. 3A and 3B, PLX4032 substantially decreased the amounts of cell surface DR5 in both A375 and LOXIMVI cells. Thus, pharmacological inhibition of B-Raf/MEK/ERK signaling (e.g., with PLX4032) clearly reduces levels of functional cell surface DR5 in cancer cells.
Fig. 3. The B-Raf inhibitor PLX4032 decreases cell surface DR5 levels (A and B) and suppresses DR5 transcription (C and D).
A and B, A375 (A) or LOXIMVI (B) cells were exposed to 5 μM PL4032 (PLX) for 16 h and then harvested for detection of cell surface DR5 levels with flow cytometry. The data are means ± SDs of triplicate determinations. Ab, antibody. C, BCPAP cells were treated with the indicated concentrations of PLX4032 for 12 h and then harvested for extraction of cellular total RNA and subsequent RT-PCR to detection of DR5. D, 293T cells were co-transfected with DR5 reporter construct and B-Raf (V600E) expression plasmid for 23 h and then exposed to the indicated concentrations of PLX4032 for additional 10 h before the cells were harvested for luciferase assay. The data are means ± SDs of triplicate determinations
We previously demonstrated that Ras/Raf/MEK/ERK signaling positively regulates DR5 expression through enhancing its transcription. 14, 15 Thus we speculated that B-Raf inhibition will suppress DR5 transcription, leading to downregulation of DR5 expression. Indeed, we detected decreased DR5 mRNA levels in cells exposed to PLX4032 as evaluated with RT-PCR (Fig. 3C). Moreover, B-Raf (V600E)-induced DR5 promoter activity was substantially inhibited by PLX4032 in a DR5 promoter luciferase assay (Fig. 3D). Hence, it is clear that B-Raf inhibition (e.g., with PLX4032) suppresses DR5 expression at transcriptional level.
Pre-treatment of cancer cells with a B-Raf or MEK inhibitor or with their combination impairs cancer cell response to DR5 activation-induced apoptosis
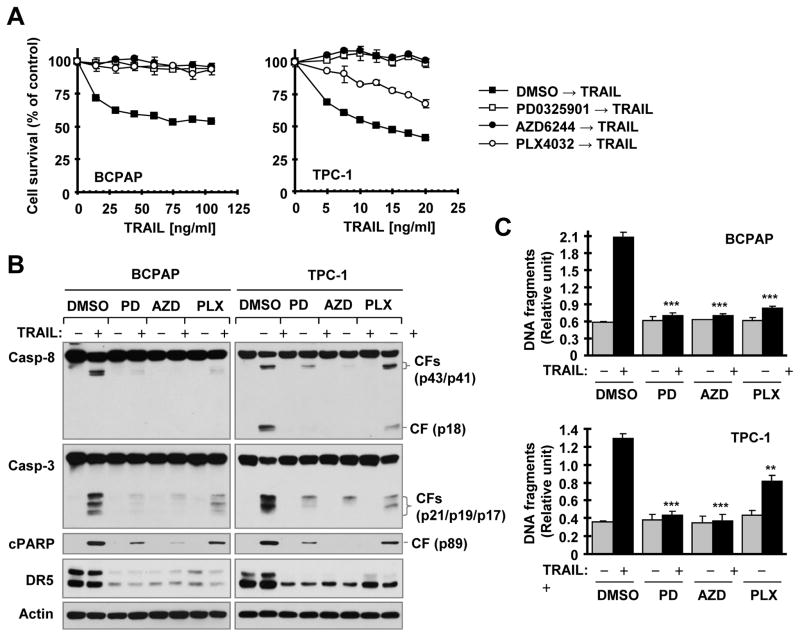
Following these findings, we further determined whether pharmacological inhibition of Raf/MEK/ERK signaling compromises cancer cell response to DR5 activation-induced apoptosis. The general strategy was to pre-expose cancer cell lines known to be sensitive to TRAIL or DR5 agonistic antibody to a B-Raf or MEK inhibitor for a given period of time and then switch to treatment with either TRAIL or a DR5 agonistic antibody (e.g., AMG655; conatumumab). Overnight pre-treatment (approximately 18 h) of BCPAP cells with PLX4032, AZD6244 or PD0325901 abolished the apoptosis-inducing effects of TRAIL as evidenced by increased survival of the tested cancer cells (Fig. 4A), decreased cleavage of caspase-8, caspase-3 and PARP (Fig. 4B), and decreased DNA fragmentation (Fig. 4C). In TPC-1 cells, we generated similar results with AZD6244 or PD0325901 pre-exposure. However, PLX4032 showed only partial inhibition of TRAIL effects on cell survival, protein cleavage and DNA fragmentation (Figs. 4A-C), likely due to its weaker suppression of MEK/ERK signaling and DR5 expression. Similar results were also generated in A375 cells pre-exposed to PLX4032 or in LOXIMVI cells pre-exposed to AZD6244 (supplemental Fig. S2). These data clearly show that pre-inhibition of Raf/MEK/ERK signaling indeed compromises or impairs cancer cell response to TRAIL-induced apoptosis.
Fig. 4. Pre-treatment of cancer cells with B-Raf or MEK inhibitors impedes cancer cell response to TRAIL-induced decrease in cell survival (A), cleavage of caspases and PARP (B) and increase in DNA fragmentation (C).
The given cell lines were pre-treated with 10 μM of the indicated inhibitors for 18 h. After removing medium with the inhibitors, the cells were washed 3 times with medium and then exposed to fresh medium containing different concentrations of TRAIL as indicated for another 15 h (A) or 100 ng/ml (BCPAP) or 25 ng/ml (TPC-1) of TRAIL for 4 h (B and C). The cell numbers were then estimated with the SRB assay (A). Protein cleavage was detected with Western blotting (B). DNA fragments were evaluated with the Cell Death Detection ELISAPlus kit (C). The data are means ± SDs of four replicate (A) or triplicate (C) determinations. CF, cleaved form. ** P < 0.001; *** P < 0.0001 in comparison with TRAIL alone treatment.
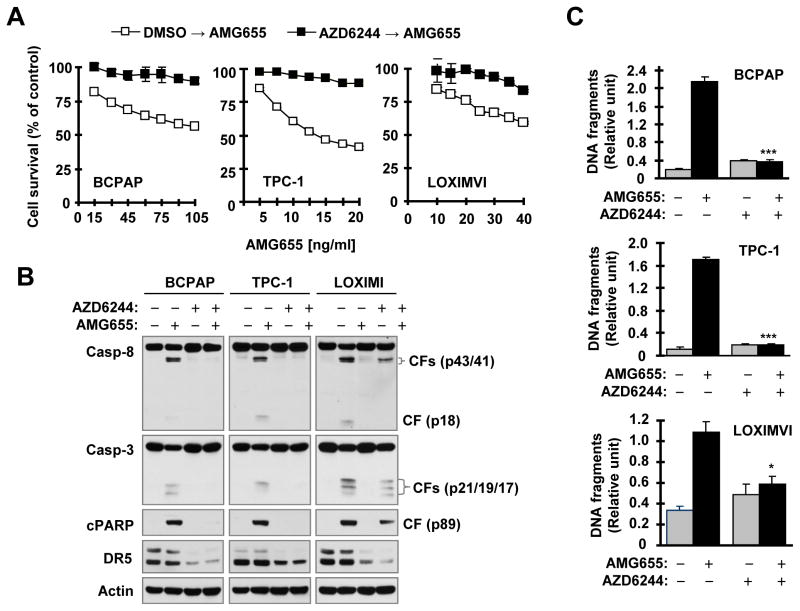
We also examined whether pre-treatment of cancer cells with these inhibitors impacts cancer cell response to AMG655-induced apoptosis. Using AZD6244 as a representative inhibitor, we found that AMG655 potently reduced cell survival, induced cleavage of caspase-8, caspase-3 and PARP and increased DNA fragmentation in BCPAP, TPC-1 and LOXIMVI cells pre-exposed to DMSO control solvent, whereas overnight pretreatment with AZD2644 abrogated these effects of AMG655 (Fig. 5). Therefore, pre-treatment of cancer cells with a MEK inhibitor also impedes cancer cell response to DR5 agonistic antibody-induced apoptosis.
Fig. 5. Pre-treatment of cancer cells with the MEK inhibitor AZD6244 impairs cancer cell response to AMG655-induced decrease in cell survival (A), cleavage of caspases and PARP (B) and increase in DNA fragmentation (C).
The given cell lines were pre-treated with 10 μM of the indicated inhibitors for 18 h. After removing medium with the inhibitors, the cells were washed 3 times with medium and then exposed to fresh medium containing different concentrations of AMG655 as indicated (A) or 100 ng/ml (BCPAP and LOXIMVI) or 25 ng/ml (TPC-1) of AMG655 (B and C) for an additional 4 h (B and C) or 15 h (A). The cell numbers were estimated with the SRB assay (A). Protein cleavage was detected with Western blotting (B). DNA fragments were evaluated with Cell Death Detection ELISAPlus kit (C). The data are means of four replicate (A) or triplicate (C) determinations. CF, cleaved form. * P < 0.01; *** P < 0.0001 in comparison with AMG655 alone treatment.
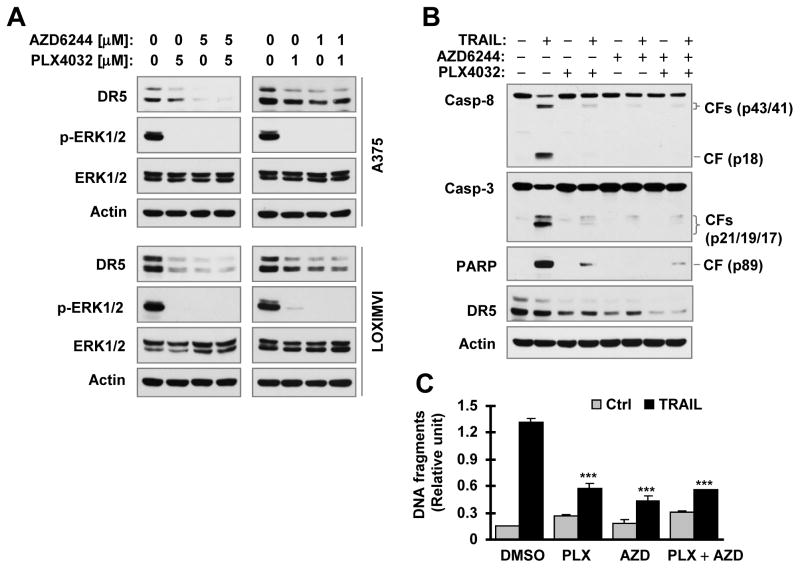
Given that the combination of a B-Raf inhibitor and a MEK inhibitor has been a common strategy for treatment of certain types of cancers (e.g., melanoma) in the clinic, we further examined the effects of the combination on DR5 expression and cell response to TRAIL-induced apoptosis. We found that the combination of PLX4032 and AZD6244 exerted similar results to PLX4032 or AZD6244 alone in suppressing ERK phosphorylation and in decreasing DR5 expression in both A375 and LOXMVI cells (Fig. 6A). In agreement, the combination was as effective as PLX4032 or AZD6244 alone in protecting the tested cancer cells from TRAIL-induced apoptosis as evaluate with detection of cleaved caspases and PARP (Fig. 6B) and DNA fragments (Fig. 6B) occurred during apoptosis. Collectively, we conclude that pre-treatment of cancer cells with a B-Raf or MEK inhibitor or with their combination impairs cancer cell responses to DR5 activation-induced apoptosis.
Fig. 6. The combination of PLX4032 and AZD6244 is as effective as PLX0432 or AZD6244 alone in suppressing DR5 expression (A) and attenuating TRAIL-induced apoptosis (B and C).
A, The indicated cancer cell lines were exposed to the given concentrations of the tested agent alone or their combination. After 16 h, the cells were harvested for preparation of whole-cell protein lysates and subsequent detection of the indicated proteins with Western blotting. B and C, A375 cells were pretreated with 5 μM PLX4032, AZD6244 or their combination for 12 h. After washing 3 times with the medium, the cells were re-fed with medium containing 100 ng/ml for additional 4 h. Protein cleavage was then detected with Western blotting (B) and DNA fragments were evaluated with the Cell Death Detection ELISAPlus kit (C) from these cells. The data are means ± SDs of triplicate determinations (C). CF, cleaved form. *** P < 0.0001 in comparison with TRAIL alone treatment.
Pharmacological inhibition of B-Raf signaling protects cancer cells from killing by activated human T cells
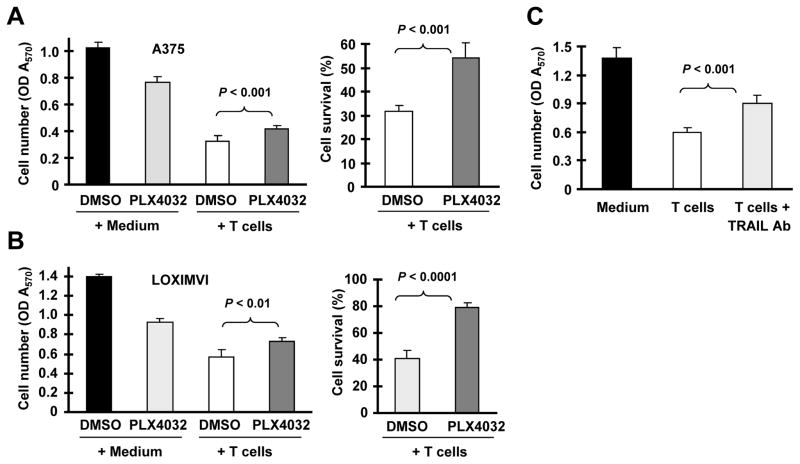
Lastly, we asked whether pharmacological inhibition of B-Raf/MEK/ERK signaling impairs cancer cell killing by immune cells. To this end, we used T cell-mediated killing of cancer cells as a model to address this question. In this particular study, we used A375 and LOXIMVI, which are sensitive to both TRAIL-induced apoptosis and T-cell-mediated killing. When comparing the responses of control and PLX4032-pretreated A375 or LOXIMVI cells to T cell-mediated killing following co-culture of activated human T cells and the tested cancer cells, we found a greater proportion of surviving cells with PLX4032-pretreated A375 or LOXIMVI cells than the matched control cells (Figs. 7A and 7B). In the presence of a neutralizing TRAIL antibody, T cell-mediated killing of A375 cells was significantly attenuated (Fig. 7C), indicating that TRAIL-mediated apoptosis is indeed involved in T cell-mediated killing of cancer cells. Together, our data suggest that B-Raf inhibition can protect sensitive cancer cells from activated T cell-mediated killing.
Fig. 7. Treatment of cancer cells with PLX4032 confers resistance to TRAIL-dependent killing by activated human T cells.
A and B, A375 or LOXIMVI cells pretreated with or without 5 μM (A375) or 2.5 μM (LOXIMVI) PLX4032 for 16 h were co-cultured with activated human T cells (cancer cells/T cells = 1:3). After 24 h, the T cells were gently removed from the culture system, and the adherent melanoma cells were subjected to MTT assay. Cell survival rates were also calculated in comparison with the control groups without T cells (right panels). Data are means ± SDs of four replicate determinations. C. A375 cells were co-cultured with activated human T cells pre-treated with or without an anti-TRAIL neutralizing antibody. After 24 h co-culture, T cells were gently removed and adherent A375 cells were subjected to MTT assay. Data are means ± SDs of four replicate determinations.
Discussion
The current study clearly shows that inhibition of B-Raf/MEK/ERK signaling, through suppression of mutant B-Raf expression with a B-Raf siRNA or through inhibition of B-Raf or MEK activity with a pharmacological inhibitor, downregulates the basal expression levels of DR5 including cell surface DR5 in the tested cancer cell lines (Figs. 1–3). The degree of DR5 reduction seems tightly associated with the potency of suppression of MEK/ERK signaling (e.g., the case of PLX4032 on DR5 suppression in TPC-1 cells). Hence these results reinforce our previously reported finding that Ras/Raf/MEK/ERK signaling positively regulates DR5 expression.14, 15 As demonstrated previously,14, 15 Ras/Raf/MEK/ERK signaling activates CHOP and Elk1-dependent DR5 transcription, resulting in elevated DR5 expression. In this study, inhibition of B-Raf with PLX4032 decreased DR5 mRNA levels and suppressed DR5 promoter activity (Figs. 3C and 3D), indicating that PLX4032 suppresses DR5 expression at transcriptional level. Hence, it is plausible to assume that inhibition of B-Raf or MEK (e.g., with a chemical inhibitor) suppresses the MEK/ERK signaling and CHOP/Elk1-dependent DR5 transcription, eventually resulting in downregulation of DR5 expression.
Induction of apoptosis with a recombinant TRAIL or an agonistic DR5 antibody has emerged as an attractive cancer therapeutic strategy, but has not been established to be successful in the clinic. In our previous study, we showed that cancer cell lines with Ras or Raf mutations were enriched in the category of cells most responsive or sensitive to AMG655. 15 In this study, we demonstrate that pre-treatment of the sensitive cancer cell lines (e.g., LOXIMVI, A375, TPC-1 and BCPAP) with a B-Raf or MEK inhibitor downregulates DR5 expression and impedes the response of these cancer cell lines to TRAIL- or AMG655-induced apoptosis, implying a potential negative impact of B-Raf- or MEK-targeted cancer therapy on the therapeutic efficacy of TRAIL- or DR5-based cancer therapy. Of the three tested inhibitors, PLX4032 is an FDA-approved anticancer, while AZD6244 and PD0325901 are under testing in the clinic. The clinically achievable concentration of PLX4032 in plasma is over 80 μM 16 and is 2–6 μM for AZD6244. 17, 18 We observed that these inhibitors effectively inhibit DR5 expression at these clinically relevant doses in our preclinical models (Fig. 2). Hence, it is safe to expect that sustained treatment of patients with these B-Raf or MEK inhibitors in the clinic may result in drastic downregulation or depletion of DR5 expression in cancer tissues. It also follows from our findings that cancer patients who have been exposed to B-Raf- or MEK-targeted therapy or other similar therapies that may inhibit the MEK/ERK signaling are less likely to respond to treatment strategies that depend on TRAIL- or DR5-mediated mechanisms. Thus, our findings are clearly of high translational significance.
Immunotherapy also represents an attractive strategy against cancers such as melanoma, and is believed to involve killing of cancer cells by induction of apoptosis. One of the primary underlying mechanisms involves death ligand-induced apoptotic signaling mainly by TRAIL from T cells, monocytes and dendritic cells. 19 Similar to TRAIL-based cancer therapy, our findings also raise important concerns for a potential negative impact of B-Raf or MEK-targeted pretreatment on the clinical efficacy of salvage treatment with immune-based cancer therapy. The most significant support for this concern comes from our data showing that human T cells, which cause TRAIL-mediated killing of cancer cells, were significantly less effective in killing cancer cells pretreated with the B-Raf inhibitor PLX4032 in comparison to the degree of cytotoxicity observed in untreated control cancer cells (Fig. 7).
While prior reports have shown that inhibition of B-Raf/MEK/ERK signaling with B-Raf inhibitors (e.g., Raf265, L-779450 and sorafenib) sensitizes certain types of cancer cells to TRAIL-induced apoptosis,20–22 these results were generated under conditions in which the cancer cells were co-treated with the combination of a given inhibitor and TRAIL. Our findings emphasize the negative impact of pretreatment of cancer cells with a B-Raf or MEK inhibitor on TRAIL-induced apoptosis and hence would suggest a potentially harmful effect of the sequence whereby patients are first treated with a pharmacological inhibitor of B-raf or MEK followed by an immune-based or TRAIL- or DR5-targeted cancer therapy.
Endogenous TRAIL and DR5 interaction leading to induction of apoptosis is recognized as a critical immunosurveillance mechanism against cancer cells. 12, 13 Hence defective death receptor signaling is a mechanism that may favor immune evasion and survival of cancer cells. Our demonstration of the downregulation of DR5 expression in cancer cells and impairment of cancer cell response to TRAIL-induced apoptosis or T cell-mediated killing by inhibition of Raf/MEK/ERK signaling may suggest that the long-term treatment of cancers with a B-RAF or MEK inhibitor may compromise immune surveillance of cancer cells or encourage their escape from immune surveillance. As a consequence, this may lead to increased metastasis or the appearance of secondary malignancies. Thus, further preclinical and clinical studies in this direction are warranted.
Our results clearly show that DR5 reduction due to pharmacological inhibition of the Raf/MEK/ERK signaling pathway is transient, with full repletion within 16 hours of stopping the treatment (Fig. 2). An intermittent treatment schedule rather than continuous administration may thus prevent sustained suppression of DR5 expression and the attendant negative consequences. Alternatively, a combination of Raf/MEK/ERK-targeted therapy with a biological agent that can prevent DR5 reduction without interfering with suppressive activity against Raf/MEK/ERK signaling should be explored.
Although the combination of a B-Raf and MEK inhibitor represents an effective strategy in preventing rebound activation of MEK/ERK signaling due to B-Raf inhibition, it does not eliminate the concerns we discussed above regarding suppression of DR5 expression and impairment of DR5 activation-induced apoptosis occurred during B-Ref- or MEK-targeted cancer therapy. This is simply because both inhibitors target the same signaling axis. Indeed, we found that the combination of PLX4032 and AZD6244 functioned just as PLX4302 or AZD6244 alone did in decreasing DR5 expression and protected cancer cells from TRAIL-induced apoptosis (Fig. 6). Hence the potential harmful effect of the combination regimen on DR5-targeted cancer therapy, immunotherapy and immune surveillance should be considered as well.
Material and Methods
Reagents
PLX4032, AZD6244 and PD0325901 were purchased from Selleckchem (Houston, TX). Human recombinant TRAIL was purchased from PeproTech, Inc. (Rocky Hill, NJ). AMG65 was supplied by Amgen Inc (Thousand Oaks, CA). B-Raf antibody was purchased from Cell Signaling Technology, Inc. (Beverly, MA). Human TRAIL neutralizing monoclonal antibody (2E5) was purchased from ENZO Life Science (Farmingdale, NY). Other antibodies were the same as described previously. 14
Cell lines
The melanoma cell lines, LOXIMVI and A375, with B-Raf V600E mutation were provided by Dr. P. Giannakakou (Weill Medical College of Cornell University, New York, NY) and by Dr. J. Arbiser (Emory University, Atlanta, GA), respectively, and not authenticated. The thyroid cancer cell lines, BCPAP (with B-Raf V600E mutation) and TPC-1 (with RET/PTC mutation), were authenticated and generously provided by Dr. R. Schweppes (University of Colorado School of Medicine, Aurora, CO).23 These cell lines were grown in RPMI 1640 medium supplemented with 5% fetal bovine serum at 37°C in a humidified atmosphere consisting of 5% CO2.
Cell survival assay
Cells were seeded in 96-well cell culture plates and treated the next day with the tested agents. Viable cell numbers were determined using sulforhodamine B (SRB) assay as described previously. 24
Detection of apoptosis
Apoptosis was evaluated with a Cell Death Detection ELISAPlus kit (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s instructions. Caspase and PARP cleavage were detected by Western blot analysis as additional indications of apoptosis.
T cell-mediated killing of cancer cells
Human T cells were purified from peripheral blood mononuclear cells of healthy donors with an EasySep™ Human T-cell Enrichment kit (STEMCELL Technologies Inc., Vancouver, BC). They were then activated by human T-Activator DanyBeads (Life Technologies, Grand Island, NY) and expanded in complete RPMI-1640 medium in 96-well plates (105 cells/ml) in the presence of recombinant human IL-2 (20 ng/ml) (PeproTech, Rocky Hill, NJ) for 4 days. After washing with RPMI-1640 medium, T cells were co-cultured at a 3:1 or 2:1 ratio with PLX4032- or DMSO-pretreated cancer cells (2×104 cells/well) in a 96-well plate for 24 h. Alternatively, activated T cells were pre-incubated with an anti-human TRAIL neutralizing antibody for 1 h at 4°C before being utilized for co-culture with the tested cancer cells. After gentle removal of T cells from the culture system, cancer cells were subjected to 3-(4,5- dimethylhiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described. 25 Cell survival was calculated by comparison to control cancer cells not exposed to T cells.
Western blot analysis
Whole-cell protein lysates were prepared and analyzed by Western blotting as described previously. 14
Detection of cell surface DR5
Cell surface DR5 expression was detected with flow cytometry as described previously. 26
Detection of DR5 mRNA expression
DR5 and GAPDH internal control mRNAs were detected with RT-PCR as described previously.26, 27
Detection of DR5 promoter activity
The reporter construct containing a 552-bp 5-flanking region of the DR5 gene, pGL3-DR5 (−522)-luc, plasmid transfection and subsequent luciferase assay was the same as described previously.26 B-Raf (V600E) expression plasmid used in the co-transfection was the same as previously described.15.
Gene silencing using siRNA
Gene silencing was achieved by transfecting siRNA using HiPerFect transfection reagent (Qiagen, Valencia, CA) following the manufacturer’s instruction. Control (siCtrl; i.e., non-silencing) and B-Raf (V600E) siRNA that targets the mutant B-Raf (siRaf-m) were described in our previous study. 15 B-Raf siRNA that targets both wild-type and mutant B-Raf (siRaf) was purchased from Cell Signaling (#12342; Danvers, MA). Gene silencing effects were evaluated by Western blot analysis as described above.
Supplementary Material
Acknowledgments
We are grateful to Drs. P. Giannakakou, J. Arbiser and R. Schweppes for providing cancer cell lines and Amgen Inc. for the generous provision of AMG655. We are also thankful to Dr. A. Hammond for editing the manuscript.
This study was supported by the NIH/NCI SPORE P50 grant CA128613 (to S-Y. S. for Project 2) and Emory Winship Cancer Institute Robbins Scholar awards (to Y-T. O. and to J. D.), Melanoma Research Fund (to J. D.) and Halpern Research Scholar award (to S-Y. S.).
FR Khuri, TK Owonikoko and S-Y Sun are Georgia Research Alliance Distinguished Cancer Scientists. S-Y Sun is a Halpern Research Scholar.
References
- 1.Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- 2.Huang T, Karsy M, Zhuge J, Zhong M, Liu D. B-Raf and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:30. doi: 10.1186/1756-8722-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinleye A, Furqan M, Mukhi N, Ravella P, Liu D. MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013;6:27. doi: 10.1186/1756-8722-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weeraratna AT. RAF around the edges--the paradox of BRAF inhibitors. N Engl J Med. 2012;366:271–273. doi: 10.1056/NEJMe1111636. [DOI] [PubMed] [Google Scholar]
- 5.Smalley KS, Sondak VK. Targeted therapy for melanoma: is double hitting a home run? Nat Rev Clin Oncol. 2013;10:5–6. doi: 10.1038/nrclinonc.2012.215. [DOI] [PubMed] [Google Scholar]
- 6.Arnault JP, Wechsler J, Escudier B, Spatz A, Tomasic G, Sibaud V, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59–61. doi: 10.1200/JCO.2009.23.4823. [DOI] [PubMed] [Google Scholar]
- 7.Breaker K, Naam M, La Rosa FG, Flaig IP, Flaig TW. Skin cancer associated with the use of sorafenib and sunitinib for renal cell carcinoma. Dermatol Surg. 2013;39:981–987. doi: 10.1111/dsu.12184. [DOI] [PubMed] [Google Scholar]
- 8.Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20–23. doi: 10.3816/CGC.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang A, Wilson NS, Ashkenazi A. Proapoptotic DR4 and DR5 signaling in cancer cells: toward clinical translation. Curr Opin Cell Biol. 2010;22:837–844. doi: 10.1016/j.ceb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Falschlehner C, Schaefer U, Walczak H. Following TRAIL’s path in the immune system. Immunology. 2009;127:145–154. doi: 10.1111/j.1365-2567.2009.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 14.Oh YT, Liu X, Yue P, Kang S, Chen J, Taunton J, et al. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J Biol Chem. 2010;285:41310–41319. doi: 10.1074/jbc.M110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh YT, Yue P, Zhou W, Balko JM, Black EP, Owonikoko TK, et al. Oncogenic Ras and B-Raf proteins positively regulate death receptor 5 expression through co-activation of ERK and JNK signaling. The Journal of biological chemistry. 2012;287:257–267. doi: 10.1074/jbc.M111.304006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, et al. The first-inhuman study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 18.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersey P, Zhang XD. Treatment combinations targeting apoptosis to improve immunotherapy of melanoma. Cancer Immunol Immunother. 2009;58:1749–1759. doi: 10.1007/s00262-009-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitzmann K, de Toni E, von Ruden J, Brand S, Goke B, Laubender RP, et al. The novel Raf inhibitor Raf265 decreases Bcl-2 levels and confers TRAIL-sensitivity to neuroendocrine tumour cells. Endocr Relat Cancer. 2011;18:277–285. doi: 10.1530/ERC-10-0108. [DOI] [PubMed] [Google Scholar]
- 21.Berger A, Quast SA, Plotz M, Kuhn NF, Trefzer U, Eberle J. RAF inhibition overcomes resistance to TRAIL-induced apoptosis in melanoma cells. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.347. [DOI] [PubMed] [Google Scholar]
- 22.Llobet D, Eritja N, Yeramian A, Pallares J, Sorolla A, Domingo M, et al. The multikinase inhibitor Sorafenib induces apoptosis and sensitises endometrial cancer cells to TRAIL by different mechanisms. Eur J Cancer. 2010;46:836–850. doi: 10.1016/j.ejca.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. The Journal of clinical endocrinology and metabolism. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57:4931–4939. [PubMed] [Google Scholar]
- 25.Rafei M, Deng J, Boivin MN, Williams P, Matulis SM, Yuan S, et al. A MCP1 fusokine with CCR2-specific tumoricidal activity. Mol Cancer. 2011;10:121. doi: 10.1186/1476-4598-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun SY, Liu X, Zou W, Yue P, Marcus AI, Khuri FR. The Farnesyltransferase Inhibitor Lonafarnib Induces CCAAT/Enhancer-binding Protein Homologous Protein-dependent Expression of Death Receptor 5, Leading to Induction of Apoptosis in Human Cancer Cells. J Biol Chem. 2007;282:18800–18809. doi: 10.1074/jbc.M611438200. [DOI] [PubMed] [Google Scholar]
- 27.Dhandapani L, Yue P, Ramalingam SS, Khuri FR, Sun SY. Retinoic acid enhances TRAIL-induced apoptosis in cancer cells by upregulating TRAIL receptor 1 expression. Cancer Res. 2011;71:5245–5254. doi: 10.1158/0008-5472.CAN-10-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.