Abstract
BACKGROUND
The aortic valve interstitial cell (AVIC) has been implicated in the pathogenesis of aortic stenosis. In response to pro-inflammatory stimulation, the AVIC undergoes a phenotypic change from that of a myofibroblast phenotype to that of osteoblast-like cell. Matrix gla-protein (MGP) has been identified as an important inhibitor of vascular calcification. We therefore hypothesized that MGP expression is reduced in diseased AVICs, and loss of this protective protein contributes to calcification of the aortic valve. Our purpose was to compare MGP expression in normal vs. diseased AVICs.
MATERIALS AND METHODS
Human AVICs were isolated from normal aortic valves from explanted hearts (n=6) at the time of heart transplantation. AVICs were also isolated from calcified, diseased valves of patients (n=6) undergoing aortic valve replacement. AVICs were grown in culture until they reached passages 2–6 prior to experimentation. Immunofluorescent staining (IF), RT-PCR, immunoblotting (IB), and ELISA were used to compare levels of MGP in normal and diseased AVICs. Statistics were by Mann Whitney U test (p<0.05).
RESULTS
MGP expression was significantly decreased in diseased AVICs relative to normal AVICs by IF, RT-PCR, IB, and ELISA.
CONCLUSIONS
An important anti-calcification defense mechanism is deficient in calcified aortic valves. MGP expression is significantly lower in diseased relative to normal AVICs. Lack of this important “anti-calcification” protein may contribute to calcification of the aortic valve.
Keywords: aortic stenosis, valve interstitial cell, matrix gla-protein
INTRODUCTION
Clinically significant calcification of the aortic valve affects 2–8% of the adult population over the age of 751. This disease is ultimately responsible for the majority of the 67,500 aortic valve replacement procedures performed annually in the United States2. Mechanisms of aortic valve calcification are poorly understood, and many cellular processes have been implicated. One critical cell type is the human aortic valve interstitial cell (AVIC), which has been found to adopt an osteogenic phenotype in response to various stimuli3,4. These cells are responsible for many of the cellular pro-inflammatory and pro-osteogenic responses occurring within the valve5.
Few anti-calcification mechanisms have been identified in the aortic valve6. However, several circulating inhibitors of calcification have been identified in vascular smooth muscle cells. One such inhibitor, Matrix Gla-Protein (MGP), has been shown to play a critical role in preventing aortic wall calcification in mice7. In calcifying vascular cells, MGP inhibited calcification when bone-morphogenetic protein-2 expression was relatively high8. In addition, MGP modulates osteogenic differentiation of C3H10T1/2 cells9. Interestingly, a clinical study found that circulating inactive MGP levels were significantly lower in patients with aortic valve disease10. These findings led us to hypothesize that MGP expression is reduced in diseased AVICs, and loss of this protective protein contributes to calcification of the aortic valve.
The purpose of this study was to compare MGP expression in AVICs from normal and calcified valves. We isolated human AVICs from both normal and stenotic aortic valves. Our results demonstrate that the expression of MGP is significantly lower in AVICs from calcified valves than in AVICs from normal valves.
MATERIALS AND METHODS
This project was approved by the Colorado multiple institutional review board at the University of Colorado School of Medicine.
Reagents
Donkey serum and fetal bovine serum (FBS) were purchased from Aleken chemicals (Nash, TX). Medium 199, penicillin G, streptomycin, amphotericin B, and Earle’s balanced Salt Solution (EBSS) were purchased from Lonza (Walkersville, MD). We obtained Laemmli sample buffer, nitrocellulose membranes, and 4–20% gradient polyacrylamide mini-protean TGX gels from Bio-Rad (Hercules, CA). Chemiluminescent substrate was obtained from Thermo Scienific (Rockford, IL). Rabbit-derived anti-human MGP antibody was obtained from Santa Cruz (Dallas, TX). This antibody detects the fully activated, phosphorylated, and gamma-carboxylated form of MGP. Rabbit-derived anti-human beta-actin was purchased from Cell Signaling (Danvers, MA). The MGP ELISA kit was purchased from MyBioSource Inc (San Diego, CA). All other chemicals were purchased from Sigma Chemicals (St. Louis, MO).
Cell Isolation and culture
We procured normal valve leaflets in the operating room from the explanted hearts of four patients undergoing heart transplant at the University of Colorado Hospital. The leaflets were thin, pliable, and no nodules or fibrotic areas were identified. Stenotic, diseased aortic valve tissue was obtained at the time of aortic valve replacement from four separate donors. AVICs were isolated as described previously3. Briefly, valve tissue was transported from the operating room in sterile saline. The leaflets were washed with EBSS and then sectioned into small pieces. These were then placed in a 15-mL tube and exposed to collagenase (2.5mg/mL) for 30 minutes to remove endothelial cells. The supernatant was removed and the tissue was digested with 0.8mg/mL collagenase for 3 hours. The supernatant was removed and placed into a small 25cm^flask with cell culture medium. This cell culture medium was produced by filtering (0.2μm) a mixture of mixing medium 199, penicillin G, streptomycin, amphotericin B, and 10% FBS. Cells were stored in an incubator which remained at a temperature of 37 degrees C and 5% carbon dioxide. Cells in passages 2–6 were used for experiments.
Immunofluorescence
AVICs were plated onto 8-chamber slides. After three washes with phosphate-buffered saline (PBS), cells were permeabilized using 30% acetone and 70% methanol for 8 minutes. After another two washes with PBS, cells were fixed with 4% paraformaldehyde (PFA) for another 8 minutes. Following three washes with deionized (DI) water, cells were blocked with donkey serum for 45 minutes. Primary rabbit-derived antibody to MGP (diluted 1:100 in 1% bovine serum albumin (BSA) in PBS) or control rabbit IgG (equivalent concentration diluted in 1% BSA in PBS) was then applied to the cells overnight at 4 degrees Celsius. The chamber slides were then washed three times with DI water before incubation with the secondary antibody (Alexa-488 conjugated donkey anti-rabbit antibody). A nuclear counterstain for bisbenzimide was also applied over the same time period. The cells were then washed with DI water and covered with mounting medium and a cover glass which was sealed with nail polish. Images were taken with a Leica MDRXA confocal microscope (Leica, Wetzlar, Germany). We took images of the MGP and nuclear stain on the green and blue channels, respectively. Slidebook software was utilized to obtain images (Intelligent Imaging Innovations, Denver, CO).
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Cells were plated onto 12 well plates before isolating total RNA using a Qiagen RNeasy Mini Kit (Germantown, MD) per manufacturer protocol. Complementary DNA was synthesized using Bio-Rad iScript. Primers to MGP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were acquired from Integrated DNA Technologies (Coralville, IA). The primers utilized for MGP were 5’-CTTAGCGGTAGTAACTTTGTG-3’ (forward) and 5’-AGAGCCTTCTCGGATCCTCTC-3’ (reverse). Primers for GAPDH were 5’-TGTAGTTGAGGTCAATGAAGGG-3’ (forward) and 5’-ACATCGCTCAGACACCATG-3’ (reverse). For RT-PCR reactions, we used Bio-Rad Sybr Green Master Mix and a Bio-Rad iQ5 thermocycler. Denaturation was completed at 95 degrees C for 15 seconds, annealing was completed at 60 degrees C for 30 seconds, and elongation took place at 72 degrees C for 30 seconds. Forty cycles were completed per sample, and we used a melt curve analysis to ensure good primer specificity.
Immunoblotting
After plating cells onto 24-well plates, AVICs were lysed with 1X laemmli sample buffer with β-mercaptoethanol. We then loaded these lysates into 15-well 4–20% gradient mini-protean TGX gels (Biorad, Hercules, CA). Gels were run at 180V for 40 minutes. Protein transfer was performed onto nitrocellulose membranes at 100V for 70 minutes. A UV Stratalinker (Stratagene, La Jolla, CA) was then utilized to crosslink the membranes. Blocking was performed in 5% dry milk with 0.1% Tween in phosphate buffered saline (T-PBS) for 45 minutes. Membranes were then washed 3 times with T-PBS before incubation overnight at 4 degrees C with the primary antibody. Primary antibodies utilized were against human MGP (diluted 1:200 in 5% BSA in T-PBS) and β-actin (diluted 1:1000 in 5% BSA in T-PBS). Membranes were washed again with T-PBS before applying horseradish peroxidase-conjugated secondary antibody diluted in 5% dry milk in T-PBS for 1 hour. Membranes were washed with T-PBS again before being treated with ECL for 2 minutes at room temp. A bio-rad Chemi-Doc MP Imaging system (Hercules, CA) was used to expose and image the membranes. Image-J densitometry software was acquired from the National Institute of Health and used to perform image analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Cells were plated onto 24-well plates. After 48 hours, 100μL of cell culture media was removed and placed into two wells of the ELISA plate. We performed this assay in accordance with the published protocol provided by MyBioSource Inc. The average optical density of two wells was used for data analysis. Optical densities were determined using a Synergy H1 Hybrid Reader produced by BioTek (Winooski, VT).
Statistical Analysis
Results are presented as mean ± standard error of the mean. Mann-Whitney U test was used to perform statistical analysis, and p<0.05 was considered a significant difference.
RESULTS
Patient Information
Donor age ranged from 20–61 years for normal aortic valve donors, and 54–77 years for diseased aortic tissue donors. All donors were non-smoking males. Normal aortic valve tissue was obtained from the explanted hearts of six patients undergoing cardiac transplant. Echocardiogram prior to transplant demonstrated no significant valvular disease in any of these patients, and they all suffered from idiopathic dilated cardiomyopathy requiring transplant. Preoperative angiogram also demonstrated no significant coronary artery disease in heart transplant recipients prior to tissue procurement. Diseased aortic valves were procured from 4 patients undergoing aortic valve replacement surgery for aortic stenosis. Patients undergoing aortic valve replacement also demonstrated no significant coronary artery disease prior to tissue procurement. Additional Donor information is listed in Table 1.
Table 1.
Additional donor information
| Donor# | Type | Age | LVEF(%)* | Comorbidities† |
|---|---|---|---|---|
| 1 | normal | 49 | 20 | CKD, T2DM |
| 2 | normal | 61 | 15 | CKD, PHTN, OSA |
| 3 | normal | 54 | 35 | CKD |
| 4 | normal | 47 | 20 | CKD, OSA, PHTN |
| 5 | normal | 28 | 15 | Obesity, T2DM |
| 6 | normal | 20 | 20 | none |
| 7 | diseased | 62 | 70 | HTN, SLE |
| 8 | diseased | 77 | 60 | CKD, HTN |
| 9 | diseased | 72 | 55 | HTN |
| 10 | diseased | 70 | 45 | T2DM |
| 11 | diseased | 54 | 60 | Obesity, HTN, T2DM |
| 12 | diseased | 67 | 55 | none |
Left ventricular ejection fraction - estimation determined on preoperative echocardiography
CKD=chronic kidney disease, T2DM=type II diabetes mellitus, PHTN=pulmonary hypertension, OSA=obstructive sleep apnea, SLE=systemic lupus erythematosus, HTN=hypertension
Diseased AVICs express lower levels of MGP by Immunofluorescence
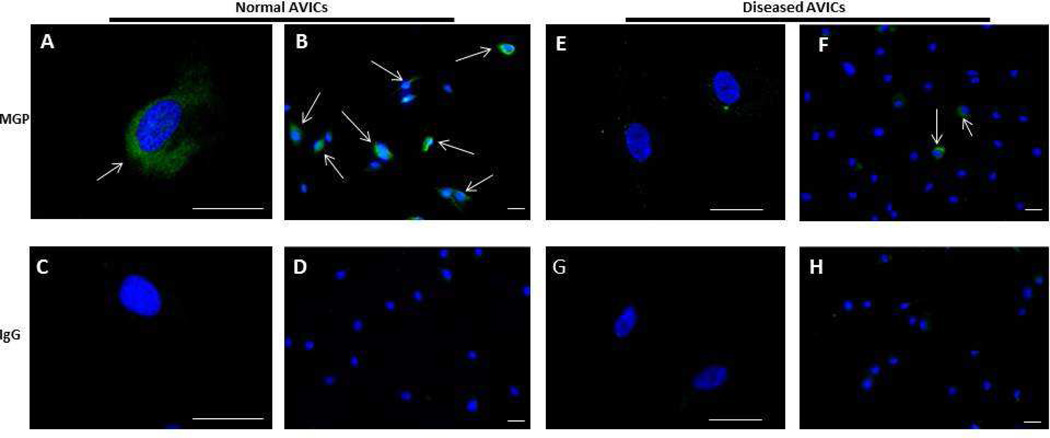
Using immunofluorescencent staining, we found that MGP was expressed in both diseased and normal AVICs. However, fewer diseased AVICs stained positively for MGP, as illustrated in Figure 1 (normal AVICs are shown in panels A–D, while diseased AVICs are illustrated in panels E–H). About 60–70% of the normal AVICs visualized stained positively for MGP (panels A, B), whereas only about 10–20% of the diseased AVICs stained positively for MGP (panels E, F). Cells treated with IgG served as negative controls for normal (panels C, D) and diseased AVICs (panels G,H) to ensure that our antibody was specifically binding MGP in the positively stained cells. No stain was identified in the negative controls, indicating minimal nonspecific binding of our primary or secondary antibodies.
Figure 1.
Immunofluorescent staining of aortic valve interstitial cells (AVICs) for matrix glaprotein (MGP). MGP and the nuclei are stained green and blue, respectively. White arrows denote cells staining positive for MGP. Normal AVICs were stained for MGP and images were taken at high (A) and low (B) power. Normal AVICs were also stained with IgG as a control at high (C) and low (D) power. Diseased AVICs were stained with MGP and imaged at high (E) and low (F) power. Diseased cells were also stained with control IgG at high (G) and low (H) power. Disease AVICs express lower levels of MGP relative to normal AVICs. Scale bar = 50μm.
Diseased AVICs express lower levels of MGP mRNA relative to normal AVICs
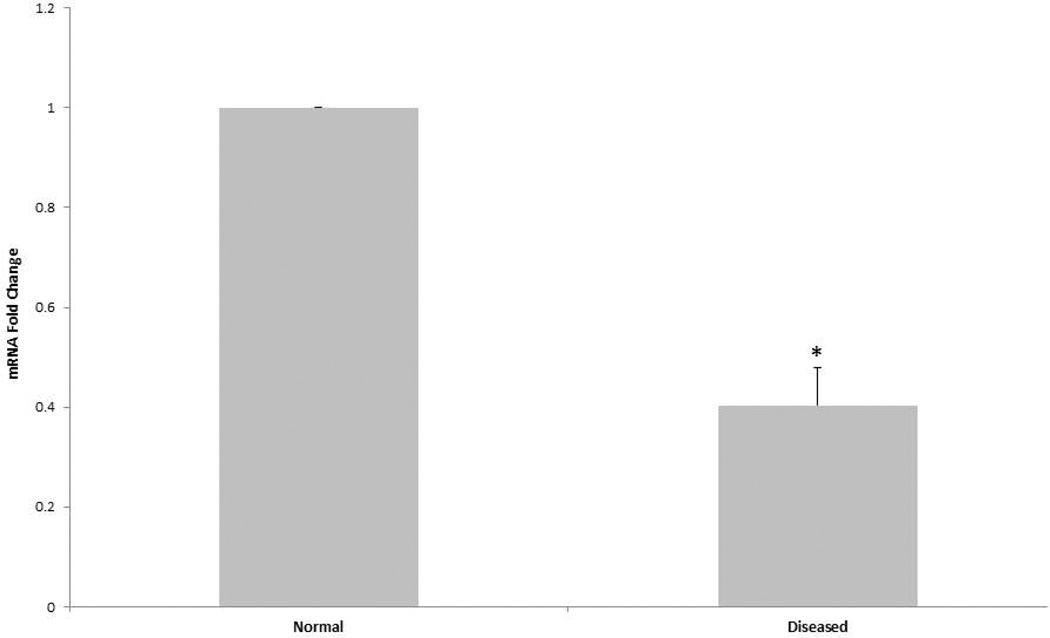
We utilized RT-PCR to compare diseased and normal AVIC baseline MGP mRNA expression. Figure 2 shows that diseased AVICs express significantly less MGP mRNA relative to normal AVICs.
Figure 2.
RT-PCR was performed on total mRNA isolated from both normal and diseased AVICs. Diseased AVICs express significantly less MGP mRNA relative to normal AVICs (*p<0.05). Data are normalized to GAPDH.
Diseased AVICs express lower levels of MGP protein relative to normal AVICs
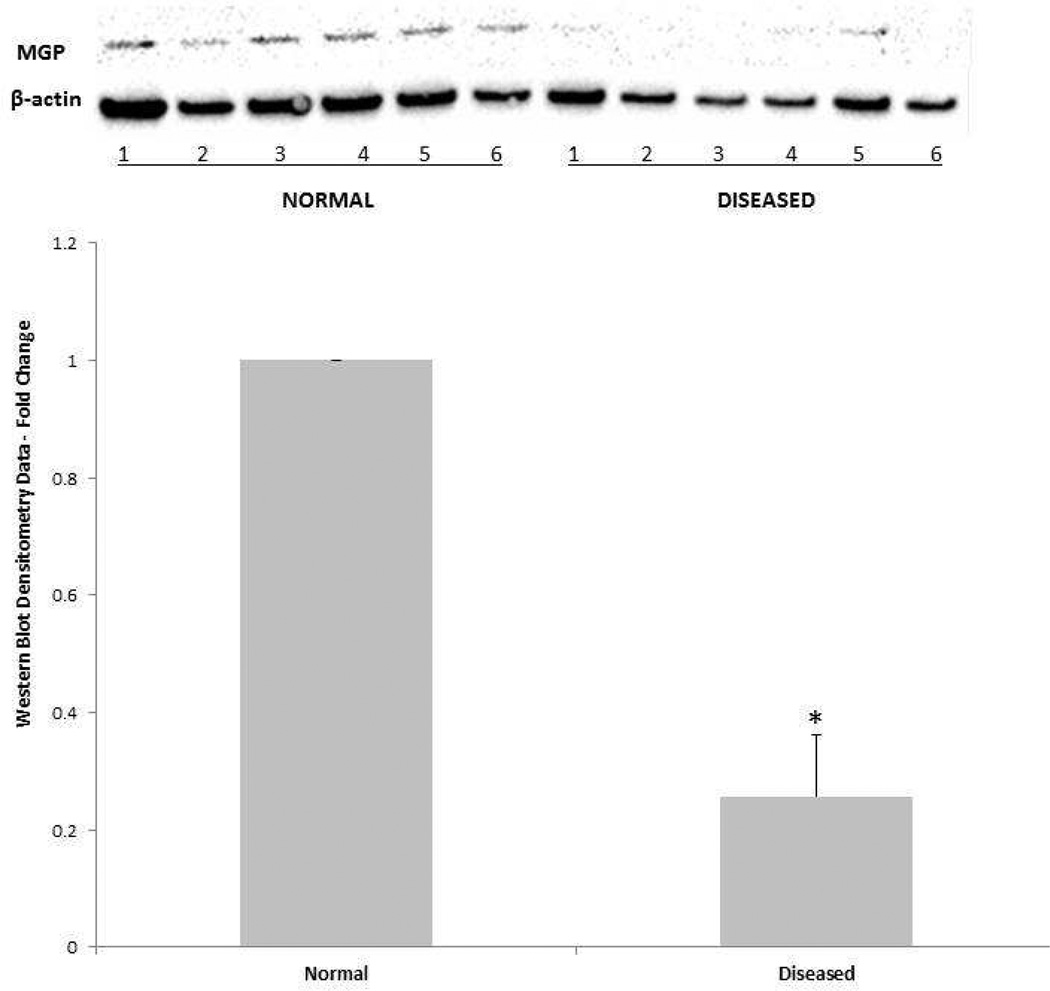
AVICs from diseased and normal valves were lysed and immunoblotting was performed to compare protein expression of the fully activated form (both phosphorylated and gammacarboxylated) of MGP. β-actin served as loading control. Immunoblot images and representative densitometry data are displayed in Figure 3. MGP protein expression was significantly lower in diseased AVICs relative to normal AVICs (*p<0.05).
Figure 3.
Normal and diseased cells from six normal and six diseased donors were lysed and run in a single gel. Immunoblotting was performed for proteins MGP and loading control β-actin. Immunoblot images are displayed at the top of the figure (NORMAL donors 1–6 and DISEASED donors 1–6), while densitometry data is illustrated in the graph below. Diseased AVICs expressed significantly less MGP protein relative to normal AVICs (*p<0.05).
MGP secretion is decreased in diseased AVICs relative to normal AVICs
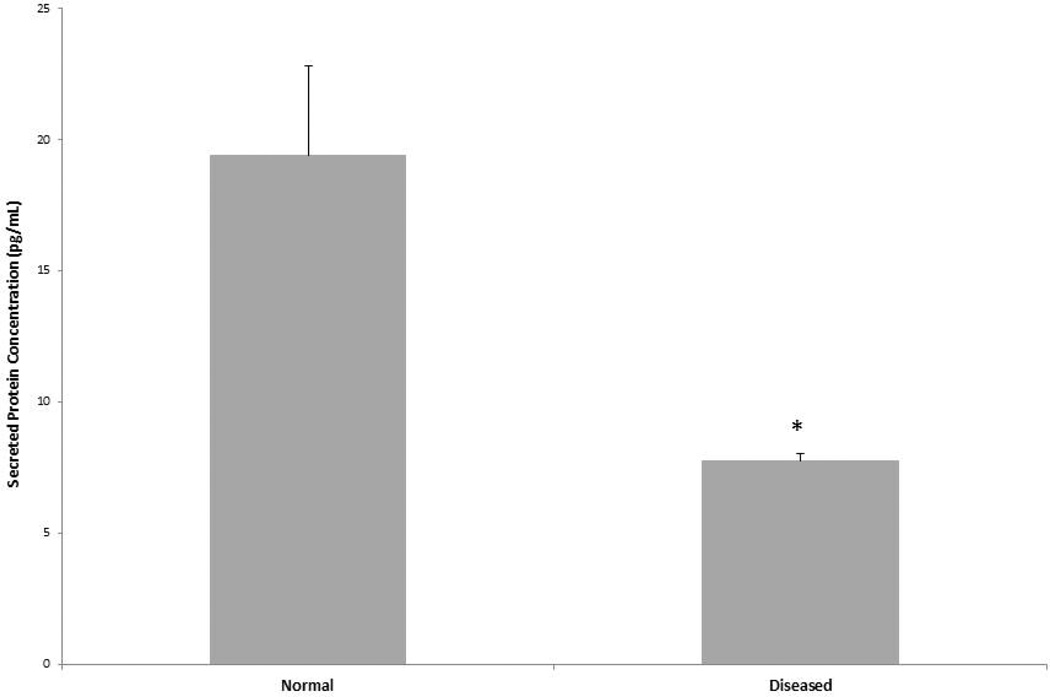
Cell culture media was removed and analyzed for secreted levels of MGP protein using the ELISA kit. Results are displayed in Figure 4. Secreted levels of this protein were low in both diseased and normal cells. However, diseased AVICs did secrete significantly lower levels of MGP relative to normal AVICs (*p<0.05).
Figure 4.
ELISA was utilized to quantify the amount of secreted MGP protein in cell culture media. Diseased AVICs secrete significantly lower levels of MGP relative to normal AVICs (*p<0.05).
DISCUSSION
The results of the present study demonstrate that diseased AVICs express significantly lower levels of MGP relative to normal AVICs. We determined that this difference holds true for multiple levels of expression, from mRNA to the fully formed secreted protein. These results suggest that an important anti-calcification protein is lacking in diseased AVICs.
As with any in vitro study, the conditions in cell culture plates do not fully mimic those found in vivo. Despite this limitation, we have previously demonstrated that AVICs in passages 2–6 display similar behavior to those isolated directly from the donors3. Aortic valve endothelial cells were not isolated, so we could not assess differences in MGP expression in these cells. Our ELISA results demonstrate that MGP secretion was low (on the order of 5–20pg/mL). However, these levels were within the detection range of the kit, and we also used 500μL of media per well for culture in a 24-well plate. Thus, a large cell culture media volume was used, decreasing the concentration of the detected MGP significantly. Finally, our normal (or control) AVICs were isolated from patients with non-ischemic cardiomyopathy, which means that they are not taken from truly normal hearts. However, the leaflets were normal appearing on preoperative echo and found to be thin, pliable, and grossly normal-appearing prior to digestion and AVIC isolation. Yet these findings do not rule out the potential for changes that might occur at a cellular level which may impact the comparison between normal and diseased cells.
Other studies of this MGP in AVICs or similar cells are sparse. MGP may contribute to calcification in patients with pseudoxanthoma elasticum in dermal fibroblasts11, which are similar to aortic valve interstitial cells. Bouchard-Martel and colleagues reported that MGP mRNA levels were lower in valve interstitial cells isolated from left-sided compared to right-sided heart valves, which is interesting since the left-sided heart valves more commonly calcify12. To our knowledge this is the first study to specifically examine MGP expression in aortic valve interstitial cells from normal and calcified valves. The results of the present study implicate MGP in the pathogenesis of calcific aortic stenosis.
The present study serves to further define and characterize the role that MGP plays at a cellular level, since growing clinical evidence suggests that circulating forms of this protein in patient’s plasma may be important in aortic stenosis13,14. Plasma levels of the inactive form of this protein have also been correlated with progression of aortic stenosis15. Most importantly, results from the present study serve as a foundation for further study of the role of MGP in valve interstitial cells. This becomes especially important since MGP expression and activation can be modulated by commonly used drugs and vitamins including warfarin and vitamin K16. By identifying anti-calcification mechanisms in these cells, we may identify targets for pharmacologic manipulation in the future. We strongly suspect that this protein plays an active role in these cells as well, but this investigation was beyond the scope of the present study.
In summary, the results of the present study demonstrate differences in MGP expression in AVICs isolated from diseased vs. normal valve tissue. From mRNA to the secreted protein, diseased AVICs seem to be incapable of expressing adequate amounts of MGP. These data suggest that a critical anti-calcification protein, MGP, may be deficient in patients with calcific aortic valve disease.
Acknowledgments
Funded by grants from the American Heart Association (AHA: 11GRNT7900016) and the National Institutes of Health (NIH RO1 HL106582-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 10th Annual Meeting of the Academic Surgical Congress, San Diego, California, February 3–5, 2015.
Contributions: Neil Venardos (conception, design, analysis, interpretation, data collection, writing the article), Daine Bennett (design, data collection), Michael J Weyant (design, analysis, interpretation), T. Brett Reece (conception, design, analysis), Xianzhong Meng (design, analysis, interpretation, critical revision of article, obtaining funding), David A Fullerton (design, analysis, interpretation, critical revision of article, writing article, obtaining funding)
DISCLOSULRE STATEMENT
The authors have nothing to disclose.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Clark MA, Duhay FG, Keyes MJ, et al. Clinical and economic outcomes after surgical aortic valve replacement in Medicare patients. 2012:117–126. doi: 10.2147/RMHP.S34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng X, Ao L, Song Y, et al. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294(1):C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 4.López J, Fernández-Pisonero I, Dueñas AI, et al. Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol. 2012;158(1):18–25. doi: 10.1016/j.ijcard.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 5.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leopold Ja. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv. 2012;5(4):605–614. doi: 10.1161/CIRCINTERVENTIONS.112.971028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386(6620):78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 8.Zebboudj AF, Shin V, Boström K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90(4):756–765. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 9.Boström K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276(17):14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 10.Koos R, Krueger T, Westenfeld R, et al. Relation of circulating matrix Gla-protein and anticoagulation status in patients with aortic valve calcification. Thromb Haemost. 2009:706–713. [PubMed] [Google Scholar]
- 11.Boraldi F, Annovi G, Vermeer C, et al. Matrix gla protein and alkaline phosphatase are differently modulated in human dermal fibroblasts from PXE patients and controls. J Invest Dermatol. 2013;133(4):946–954. doi: 10.1038/jid.2012.460. [DOI] [PubMed] [Google Scholar]
- 12.Bouchard-Martel J, Roussel E, Drolet M-C, Arsenault M, Couet J. Interstitial cells from left-sided heart valves display more calcification potential than from right-sided valves: an in-vitro study of porcine valves. J Heart Valve Dis. 2009;18(4):421–428. [PubMed] [Google Scholar]
- 13.Koos R, Mahnken AH, Mühlenbruch G, et al. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96(6):747–749. doi: 10.1016/j.amjcard.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Ueland T, Gullestad L, Dahl CP, et al. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 2010;268(5):483–492. doi: 10.1111/j.1365-2796.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- 15.Capoulade R, Côté N, Mathieu P, et al. Circulating Levels of Matrix Gla Protein and Progression of Aortic Stenosis: A Substudy of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) Trial. Can J Cardiol. 2014;30(9):1088–1095. doi: 10.1016/j.cjca.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med. 2013;19(4):217–226. doi: 10.1016/j.molmed.2012.12.008. [DOI] [PubMed] [Google Scholar]