Abstract
Objective
Unipolar psychotic depression (PD) is a severe and debilitating syndrome, which requires intensive monitoring. The objective of this study was to provide an overview of the rating scales used to assess illness severity in PD.
Method
Selective review of publications reporting results on non-self-rated, symptom-based rating scales utilized to measure symptom severity in PD. The clinical and psychometric validity of the identified rating scales was reviewed.
Results
A total of 14 rating scales meeting the predefined criteria were included in the review. These scales grouped into the following categories: I. Rating scales predominantly covering depressive symptoms, II. Rating scales predominantly covering psychotic symptoms, III. Rating scales covering delusions, and IV. Rating scales covering psychotic depression. For the vast majority of the scales, the clinical and psychometric validity had not been tested empirically. The only exception from this general tendency was the 11-item Psychotic Depression Assessment Scale (PDAS), which was developed specifically to assess the severity of PD.
Conclusion
In PD, the Psychotic Depression Assessment Scale (PDAS) represents the only empirically derived rating scale for the measurement of overall severity of illness. The PDAS should be considered in future studies of PD and in clinical practice.
Keywords: Affective disorders, Depression, Psychoses, Psychometrics
Introduction
The specification of unipolar psychotic depression (PD) as a distinct syndrome evolved out of antidepressant studies conducted in the 1960s and 1970s, which demonstrated that “delusional depression” was associated with a poorer response to antidepressants than non-delusional major depression as well as with both different clinical characteristics and a poorer course [1–3]. These differences resulted in the incorporation of PD as a distinct syndrome of major depression in the Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition (DSM-III) [4] and the International Classification of Disease, 10th Revision (ICD-10) [5].
In PD, delusions that are congruent with the depressed mood, such as “I will never ever recover”; “my carelessness has ruined my family”; “My intestines are rotting” are common and may be difficult to recognize (e.g. “My bad business decisions will bankrupt my family” or “my depression is hopeless and I will never recover”). Delusions that are incongruent with depression may also occur and are generally easier to recognize [6–8]. PD is a prevalent mental disorder with approximately 20–30% of severely depressed inpatients meeting diagnostic criteria [9–11]. The prevalence has been reported as even higher in samples of older depressed inpatients [12, 13]. Unfortunately, clinicians may not recognize the delusions associated with PD. Indeed, our group has demonstrated that PD documented by subsequent research interviews was not identified in 27% of patients admitted to four academic hospitals in North America [14].
As with other psychopathological syndromes [15], severity assessment is crucial in PD, both for the monitoring of the individual patient as well as for the classification of treatment outcome in clinical trials [16]. Because the recognition of PD as a distinct syndrome is a relatively recent development in psychiatric nosology [4], psychometric instruments (rating scales) specifically dedicated to the measurement of both the affective and psychotic domains of symptoms in PD have been unavailable. Therefore, in many PD studies, rating scales that have been traditionally used to assess either the severity of major depression that is not complicated by psychotic symptoms or schizophrenia have been applied to patients with PD [16]. By only assessing one of the two main domains of psychopathology (depression or psychosis), specific depression and psychosis scales are likely to have limited validity for assessing the severity of PD.
Aims of the study
The aim of the present study was to provide a focused and critical review of the rating scales, which have been or currently are used to evaluate the symptom severity of psychotic depression in clinical trials and in clinical practice. Specifically, we aimed at providing our colleagues with directions for the measurement of the severity of psychotic depression in future clinical practice and research studies.
Material and methods
Through a selective review of databases (PubMed, Embase and PsycINFO), we identified rating scales utilized to measure the severity of depressive and/or psychotic symptoms of PD in clinical studies. The search was restricted to symptom scales in the English language. Global outcome scales were not included since the use of these scales requires that the rater is a clinician with extensive experience in the assessment of the severity of the specific disorder. Less experienced raters will tend to overestimate the global severity because they have not seen the most ill patients [15]. In addition, self-rating scales were not included since the validity of self-ratings of psychotic symptoms in depressed patients is uncertain [17]. Strengths and weaknesses of the included rating scales are discussed in terms of clinical validity (Figure 1) - the extent to which a rating scale reflects the overall clinical severity of the syndrome, and unidimensionality (Figure 2) - the extent to which the symptoms represented by the items on a rating scale appear in an orderly fashion as the severity of PD increases, such that scoring on higher prevalence items (representing less severe symptoms) precedes scoring on lower prevalence items (representing more severe symptoms) [15]. When a rating scale is clinically valid and unidimensional, the individual item scores of the rating scale can be added to a total score, which reflects the overall severity of PD. Essentially, the concept of clinical validity encompasses construct validity (the extent to which a scale adequately assesses the theoretical concept that it is intended to assess), content validity (the extent to which a scale represents every single element of a construct) and criterion validity (the extent to which a scale predicts an outcome based on information another variable, ideally a global clinical assessment). Similarly, unidimensionality was chosen as a validity criterion over internal consistency (the extent to which items within a scale correlate with one another) since the latter, although often claimed, does not evaluate the measurement properties of a scale [15].
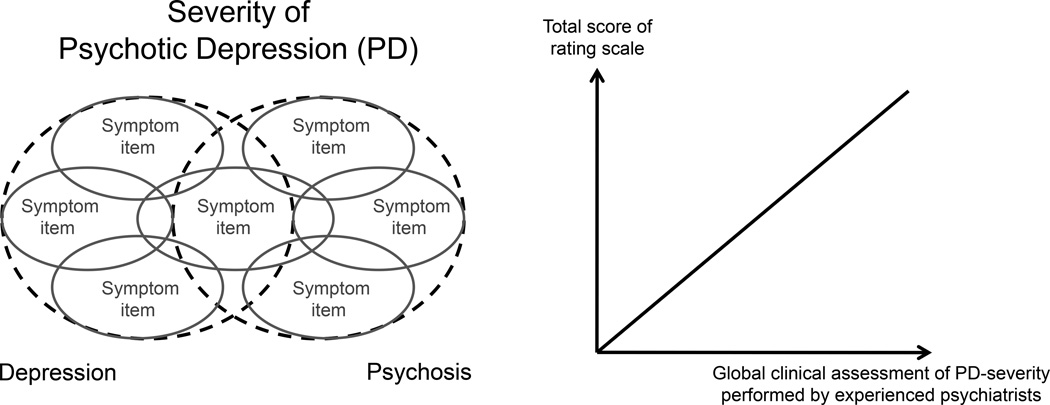
Figure 1. Clinical validity.
Left: For a rating scale to be clinically valid, it must map the severity of the syndrome of interest. In the case of psychotic depression, both the psychopathological domains of depression and psychosis must therefore be covered by the items of the rating scale. Right: Furthermore, there must be a high correlation between the rating scale total score and a global evaluation of syndrome severity performed by experienced psychiatrists (“gold-standard”).
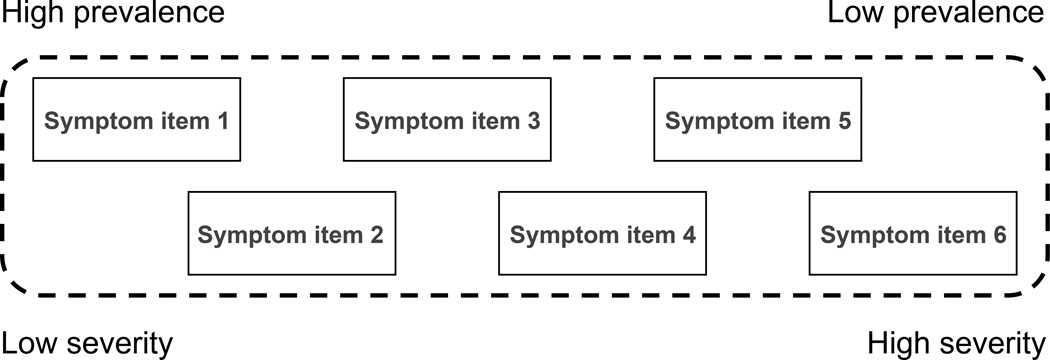
Figure 2. Unidimensionality.
Unidimensionality: This figure shows a hypothetical six-item rating scale, which is unidimensional because the symptoms represented by the items appear in an orderly fashion as the severity of the syndrome increases, such that scoring on higher prevalence items (less severe items) precedes scoring on lower prevalence items (more severe items). When a rating scale is unidimensional, each individual item adds unique information about the severity of the latent syndrome being rated (represented by the dashed frame) and the total score obtained by adding the individual item score is therefore a valid measure for the severity of the latent syndrome (for instance psychotic depression), provided that the scale is also clinically valid (see figure 1).
Results
A total of 14 rating scales meeting the predefined criteria for inclusion (Non-self-rated, symptom-based rating scales in the English language utilized to measure symptom severity of PD in clinical studies) were identified: The 17-item Hamilton Depression Rating Scale [18], The 21-item Hamilton Depression Rating Scale [18], The 24-item Hamilton Depression Rating Scale [19], The Modified Hamilton Rating Scale for Depression [20], The Montgomery–Asberg Depression Rating Scale [21], The Bech-Rafaelsen Melancholia Scale [22], The Calgary Depression Scale [23], The Brief Psychiatric Rating Scale [24], The Delusion Severity Item of the Schedule for Affective Disorders and Schizophrenia [25], The Spiker Psychoticism Scale [26], The Positive and Negative Symptom Scale [27], The Dimensions of Delusional Experience Rating Scale [28], The Delusion Assessment Scale [29], and The Psychotic Depression Assessment Scale [16]. These 14 scales can be divided into the following categories: I. Rating scales predominantly covering depressive symptoms, II. Rating scales predominantly covering psychotic symptoms, III. Rating scales covering delusions, and IV. Rating scales covering psychotic depression. Below, we evaluate the validity of the rating scales contained by these categories one by one.
Rating scales predominantly covering depressive symptoms
17-item Hamilton Depression Rating Scale (HAM-D17)
The HAM-D17 [30] is the most widely used rating scale in major depression and has been employed as outcome measure in several studies of PD [26, 31–35]. However, the content validity of the HAM-D17 for assessing the psychotic symptoms of PD is limited. There are no HAM-D17 items dedicated to rating hallucinations, and only two items that capture specific forms of delusions (delusions of guilt and somatic delusions). Therefore, in some PD studies, a continuous item or scale or global judgment has been used to define the presence or absence of delusions as part of the criteria for remission/response [31, 36]. However, this dichotomous approach is not ideal in light of the substantial body of literature indicating that psychotic symptoms are not categorical, but exist on a dimensional spectrum where even less severe presentations are associated with significant distress [29, 37–40] and resistance to antidepressant monotherapy [41]. This is not picked up by the categorical “all or nothing” approach to psychosis. Furthermore, the HAM-D17 was employed in PD without prior psychometric validation for this particular syndrome. The first psychometric evaluation of the HAM-D17 in PD was performed by our group and showed that the scale is not a unidimensional measure of PD [16], which entails that the validity of adding the individual 17 item scores to a total score is questionable from a measurement perspective [15].
21-item Hamilton Depression Rating Scale (HAM-D21)
The HAM-D21 [30] consists of the HAM-D17 plus four extra items: Diurnal variation; Depersonalization and derealization; Paranoid symptoms; Obsessional and compulsive symptoms and has been used as outcome measure in several studies of PD [42–45].
However, the HAM-D21 was never intended for severity measurement in depression. According to Hamilton, the four extra items were to be used to capture different forms of major depression and not to quantify severity: "The scale contains 21 items of which only 17 are used to give a total score of severity. Of the other items "diurnal variation" is recorded at the first interview it should not be altered at subsequent ones. It is one of the reasons why this item is not included in a total score of severity" [46]. This statement by the developer of the scale is of course, in itself, an argument against using the HAM-D21 as a measure of the severity of PD. Furthermore, the psychometric validity (clinical validity and unidimensionality) of the HAM-D21 has not been tested in relation to PD. Finally, since the HAM-D17 is not a unidimensional measure of PD [16], it seems highly unlikely that the addition of four items (three of which do not evaluate psychosis) will change this.
24-item Hamilton Depression Rating Scale (HAM-D24)
The HAM-D24 [19] consists of the HAM-D21 plus three extra items: helplessness; hopelessness; worthlessness (reflecting Beck’s cognitive triad in depression [47]) and has been applied in several studies of PD [48–53]. It has been assumed that the addition of the items covering helplessness, hopelessness and worthlessness would increase the clinical validity of the HAM-D24 in relation to the mood-congruent psychotic symptoms of PD, as hopelessness and worthlessness can be rated as delusional. However, the psychopathology reflected by these three items is, as discussed by Hamilton [46], already covered by the HAM-D17 items of work and interests (helplessness), depressed mood (hopelessness), and guilt feelings (worthlessness), and the extra items are therefore somewhat redundant [54]. Furthermore, since the HAM-D24 contains both the HAM-D21 and the HAM-D17the psychometric reservations mentioned in relation to these two scales also apply for the HAM-D24which, like the HAM-D24has never been formally validated in PD.
Modified Hamilton Rating Scale for Depression (MHRSD)
The MHRSD [20] was constructed “to enable paraprofessional research assistants to make reliable and valid assessments of depressive symptoms” [20] and was used as an outcome measure in the study by Gaudiano et al. [55], analyzing the effect of combined pharmacotherapy and psychotherapy in PD compared to the effect in non-psychotic depression. MHRSD consists of a total of 25 items: the 17 items from the HAM-D17 plus the three “cognitive” HAM-D24 items (helplessness, hopelessness, worthlessness), the diurnal variation item from HAM-D21three items to assess melancholic symptoms (distinct quality of mood, reactivity of mood, loss of interest and pleasure), and finally one item assessing weight gain [20]. The motivation for selecting exactly these items is unclear. In relation to PD, the clinical validity and unidimensionality of the MHRSD have not been evaluated, and are likely to be less than optimal judged by the similarity between the MHRSD and the other versions of the HAM-D (HAM-D17HAM-D21and HAM-D24).
Montgomery–Asberg Depression Rating Scale (MADRS)
The 10-item MADRS was used as a secondary outcome measure by Kunzel et al. [50] in their study comparing treatment with trimipramine monotherapy to a combination of amitriptyline and haloperidol in PD. It was also used by Benazzi to investigate potential differences between unipolar and bipolar psychotic depression [56]. The MADRS was developed specifically to be sensitive to change in the severity of depression [21]. However, the inclusion of symptom-items, which tap into typical side effects of antidepressants (reduced sleep, reduced appetite), is likely to decrease the sensitivity of the scale in trials of such medications [15]. Furthermore, the MADRS has never been formally validated in PD. In that regard, the reported sadness item is likely to be problematic as some patients with severe PD may deny being depressed [6]. In contrast, among patients being aware of their depressed mood, the scores on the reported sadness and the apparent sadness items will tend to be highly correlated and result in redundancy / local dependency, which reduces the unidimensionality of the scale [15, 57].
Bech-Rafaelsen Melancholia Scale (MES)
The MES [22] consists of 11 items and has demonstrated high psychometric validity in relation to the measurement of severity of major depression [58–60]. In PD, the MES has been used as primary [61] and secondary [62] outcome measure in studies of psychopharmacological treatments. However, the clinical validity of the MES has not been established for PD.
Calgary Depression Scale (CDS)
The CDS [23] was developed specifically to assess symptoms of depression in patients with schizophrenia, but has also been used in studies including subjects with PD [50, 63]. Notably, out of the nine items of the CDS, three items cover aspects of guilt (guilty ideas of reference, pathological guilt, self depreciation,) and another three cover aspects of depression (depression, observed depression, hopelessness). This is likely to lead to significant redundancy and, as was the case for the MADRS, the self-rated depression item may have limited use in PD. Importantly, most of the Calgary items listed can take the form of mood-congruent delusions (e.g, of guilt or hopelessness), but delusional intensity is not considered in the anchoring of the items. Finally, like the majority of the other depression rating scales, the CDS has not been validated for use in PD.
Rating scales predominantly covering psychotic symptoms
Brief Psychiatric Rating Scale (BPRS)
The BPRS [24] consists of 18 items and has been used in many studies of PD [35, 49–51, 56, 64]. However, the clinical validity and the unidimensionality of the BPRS as a rating scale for PD has never been tested empirically. The 18 items included in the BPRS define a highly heterogenic syndrome and in relation to PD, the grandiosity item may be particularly problematic as it taps into the exact opposite of PD, namely (psychotic) mania. For this reason probably, both the “psychotic subscale” (items: unusual thought content (delusions), hallucinations, conceptual disorganization, and suspiciousness) and the “psychoticism subscale” (items: unusual thought content (delusions), hallucinations, conceptual disorganization, suspiciousness, somatic concern, anxiety, guilt feelings, and emotional withdrawal) have also been used in studies of PD [51–53, 64, 65]. However, neither the clinical validity nor the unidimensionality of these subscales has been tested. The first study to test the psychometric properties of the BPRS items in relation to the severity of PD was that of Oestergaard et al [16]. The results of this study showed that five items from the BPRS carried information regarding the severity of the defining psychotic symptoms of PD (delusions and hallucinations) in PD: hallucinatory behavior, unusual thought content (delusions), suspiciousness, emotional withdrawal and blunted affect. Notably, conceptual disorganization (a typical feature of schizophrenia) from the psychotic and psychoticism subscales of the BPRS was not among the informative items. The five BPRS items identified by Oestergaard et al [16] as being relevant for the measurement of PD are discussed in further detail below (under the paragraph covering the Psychotic Depression Assessment Scale).
Spiker Psychoticism Scale (SPS)
In order to “obtain a different perspective on manifestations of ‘psychoticism’” in their randomized controlled trial comparing the effect of monotherapy with amitriptyline to the combination of amitriptyline and perphenazine in patients with PD, Spiker et al [26] defined and used the SPS as outcome measure. The scale consists of the items work and activities and insight from the HAM-D17 and emotional withdrawal, conceptual disorganization, suspiciousness and unusual thought content from the BPRS. The authors do not provide further rationale for the selection of exactly those items over other items, and the clinical validity and unidimensionality of the SPS has not been tested.
Positive and Negative Symptom Scale (PANSS)
The 30-item PANSS [27] was created by merging the 18-item Brief Psychiatric Rating Scale with 12 items from the Psychopathology Rating Schedule [66]. The choice of these 30 items was made with the aim of obtaining content validity for both the positive and the negative symptoms of schizophrenia, as well as the associated general psychopathology [27]. The PANSS was used as an outcome measure in the study by Müller-Siecheneder et al [61], which included patients with both depressive and psychotic symptoms (schizophrenia, schizo-affective disorder, and PD). This heterogenic diagnostic composition of the sample is likely to be the reason for choosing the PANSS as an outcome measure, which is unusual in studies of PD. The same is likely to be the case for the study by Taiminen et al [63] in which habituation of the blink reflex was studied in patients with first-episode schizophrenia, non-psychotic depression, and PD. However, even in schizophrenia, the psychometric properties of the PANSS have been questioned [67–69], and the scale has never been formally validated in PD.
Rating scales covering delusions
The Delusion Severity Item of the Schedule for Affective Disorders and Schizophrenia (SADS)
The delusion severity item from the SADS [25] has been used as outcome measure in two studies of PD [26, 31]. In the study by Spiker et al [26], the SADS delusion severity item was among the secondary outcome measures. In the study by Meyers et al. [31], the item-score was part of the definition of the primary outcome, namely remission, which required a HAM-D17 total score of 10 or lower at two consecutive ratings and a score of 1 on the SADS delusion severity item at the second remission of depression assessment. The potential implications of the categorical approach to delusions are mentioned in relation to the HAM-D17 earlier in this review. Furthermore, the SADS delusion severity item does not take the severity of hallucinations into account, so when using this item alone, the clinical validity in relation to overall psychotic symptom severity of PD (and not just “delusional depression”) is likely to be limited [16]. To our knowledge, the full SADS has not been used in studies of PD.
The Dimensions of Delusional Experience Rating Scale (DDERS)
The DDERS [28] was used to measure the severity of delusions in a series of studies of monotherapy with 2nd generation antidepressants (without placebo control) [42–45]. The DDERS consists of five items pertaining to the following aspects of delusions: conviction, extension, bizarreness, disorganization, and pressure. The DDERS was tested on a sample of 52 patients of which 42 (more than 80%) had either schizophrenia or schizoaffective disorder and only three had PD. Therefore the validity in PD is questionable and was not tested prior to the application of the scale in the antidepressant monotherapy studies [42–45]. Furthermore, the DDERS was not designed to measure the severity of hallucinations and the validity in measuring the overall severity of the psychotic symptoms in PD is therefore limited.
The Delusion Assessment Scale (DAS)
The DAS is an adaptation of the DDERS designed specifically for the assessment of delusions in PD [29] and was used to assure that irrational ideation met criteria for being delusional and to characterize the dimensions of delusions in PD among participants in the Study of Pharmacotherapy of Psychotic Depression (STOP-PD) trial. [31]. The DAS consists of 15 items: temporal pressure, acting on the belief, temporal pressure during interview, Acting irrationally distrustful during the interview, emotional pressure, cognitive integration, internal consistency, temporal continuity, accommodation, subjective feeling of certainty, relationship to cultural context, implausibility/bizarreness, places/situations involved, people/objects involved, and mood congruence. As for the DDERS, the DAS was not designed to measure the severity of hallucinations, but provides a systematic phenomenological description of individual delusions in PD [29]. In a recent study focusing on the implications of impaired insight into delusions in PD, four items from the DAS (subjective feeling of certainty, temporal pressure during the interview, acting irrationally distrustful during the interview, and accommodation) were used to quantify “delusional conviction” [70]. However, the validity of the DAS in relation to the measurement of the overall severity of psychotic symptoms in PD is unstudied.
Rating scales covering psychotic depression
The Psychotic Depression Assessment Scale (PDAS)
As the name implies, the PDAS was constructed specifically to assess the severity of PD [16]. The scale was developed through analysis of item-level ratings on the HAM-D17 and the BPRS. The PDAS consists of 11 items, the 6-item melancholia subscale (HAM-D6) [71], derived from the HAM-D17plus five psychosis items from the BPRS. The 11 items are: depressed mood, guilt feelings, work and activities, psychomotor retardation, psychic anxiety and somatic symptoms (general) from the HAM-D6 and hallucinatory behavior, unusual thought content (delusions), suspiciousness, emotional withdrawal and blunted affect from the BPRS. In order to obtain the same scaling (0–4) for all items for the analysis of the PDAS, ratings on the somatic symptoms (general) item (scored 0–2) was multiplied by 2 and the ratings on the five BPRS items (scored 1–7) were converted using this formula: (BPRS score − 1) × 2/3. When using Clinical Global Impressions [72] of severity (CGI-S) and improvement (CGI-I) as reference, the PDAS demonstrated clinical validity, responsiveness (sensitivity to change in illness severity) and unidimensionality [16]. Taken together, these findings are consistent with using the sum of the individual PDAS item scores (i.e., the total score) as a measure for the overall severity of depressive and psychotic symptoms in PD. The validity of the PDAS in the measurement of the overall severity of PD was recently further supported by a Danish multi-center study [73] of PD patients diagnosed according to the 10th edition of the International Classification of Disease (ICD-10) [5]. This Danish study did however use a different interview as well as slightly different item-definitions compared to the PDAS study based on data from STOP-PD (see Ostergaard et al [73] for further details). In a secondary analysis applying the PDAS as an outcome measure, it was shown that the scale was able to detect statistically significant differences in treatment effect between Olanzapine+Placebo and Olanzapine+Sertraline (the latter being superior) in patients with PD [74]. Finally, apart from its application as a rating scale measuring severity, the PDAS has also shown promising results as a tool to detect cases of PD among patients with depressive disorders in general [75, 76].
Discussion
In this paper, we have reviewed the symptom rating scales employed in clinical studies to measure the severity of unipolar psychotic depression (PD). Based on our assessment of the literature it has become evident that the vast majority of the rating scales used in PD has not been validated in terms of clinical validity and unidimensionality. In particular, it has been assumed that rating scales developed for major depression (without psychotic symptoms) and schizophrenia would also be valid for PD. However, these two disorders differ from PD in their overall phenomenology and in both the types and severity of associated psychopathology [11, 77–81], including the symptomatology [82–87]. The fact that rating scales valid in non-psychotic major depression and schizophrenia are not necessarily valid in PD points to a highly problematic psychometric and methodological deficiency that may undermine research into PD. As outlined in this review, the Psychotic Depression Assessment Scale (PDAS) offers a methodological procedure to fill this gap, since it represents the only empirically derived rating scale covering both the depressive and psychotic symptoms of PD. Another advantage of the PDAS is its brevity as it consists of only 11-items [88]. However, most studies using the PDAS have derived the item scores from the two “mother scales”, namely the HAM-D17 and the BPRS [16, 74–76]. Ideally, the PDAS could be administered without first rating the entire HAM-D17 and BPRS rating scales. To this end, a new version of the PDAS for clinical/research use, which includes a semi-structured interview focusing specifically on the 11 PDAS items, has been developed (available at: http://psychoticdepressionassessmentscale.com). For each of the 11 items, a detailed anchoring of scores from 0–4 is provided to ease rating for non-expert raters. This specific clinical/research version of the PDAS has however not been validated, as both the wording of the interview and the scoring of items differs from those used in the studies from which the PDAS was derived and validated. However, this limitation should be interpreted in the light of the almost complete lack of validation of other rating scales used in PD as demonstrated in this review. At present, the English original of the PDAS for clinical/research use is being translated to Danish, Dutch, Japanese, Korean, Tamil, and more translations are planned. Hopefully these scales will be subjected to further validation and be of use in future studies of PD as well as in clinical practice.
Clinical Recommendations
Psychotic depression (PD) is a severe and debilitating syndrome, which requires intensive monitoring.
In PD, the Psychotic Depression Assessment Scale (PDAS) represents the only empirically derived rating scale for the measurement of overall severity of illness.
Additional Comments
A rating scale developed for one particular syndrome (e.g.. non-psychotic major depression or schizophrenia) must be validated specifically for other syndromes (e.g., psychotic depression), prior to being put into use in that other syndrome.
Acknowledgements
The STOP-PD received funding by the US Public Health Service - MH 62446, MH 62518, MH 62565, and MH 62624; National Center for Research Resources - M01-RR024153, RR000056, CTSC UL1RR024996; the National Institute of Mental Health (NIMH) - MH069430, MH067710, and P30 MH068368; The NIMH supported the STOP-PD and participated in its implementation through the UO1 mechanism. They did not participate in the collection, analysis, or interpretation of study data or in the preparation, review, or approval of this manuscript. A data safety monitoring board at the NIMH provided data and safety monitoring during the STOP-PD. Eli Lilly donated olanzapine and Pfizer donated sertraline and placebo/sertraline for the STOP-PD. Neither Eli Lilly nor Pfizer participated in the design, implementation, collection, analysis, or interpretation of data in the STOP-PD or in the preparation, review, or approval of this manuscript. S.D. Østergaard is supported by a grant from the Lundbeck Foundation.
Footnotes
Declaration of interest
A.J. Rothschild receives grant or research support from Alkermes, AssureRx, Cyberonics, the National Institute of Mental Health, and St Jude Medical, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Omnicare, and Pfizer Inc. Dr. Rothschild has received royalties for the Rothschild Scale for Antidepressant Tachyphylaxis (RSAT)®; Clinical Manual for the Diagnosis and Treatment of Psychotic Depression, American Psychiatric Press, 2009; The Evidence-Based Guide to Antipsychotic Medications, American Psychiatric Press, 2010; and The Evidence-Based Guide to Antidepressant Medications, American Psychiatric Press, 2012. A.J. Flint currently receives grant support from the NIMH, the Canadian Institutes of Health Research, Brain Canada, the Ontario Brain Institute, and Lundbeck and within the past three years has received honoraria from Pfizer Canada. B.H. Mulsant currently receives research funding from Brain Canada, the CAMH Foundation, the Canadian Institutes of Health Research, and the US National Institute of Health (NIH). During the last five years, he also received research support from Bristol-Myers Squibb (medications for a NIH-funded clinical trial), Eli-Lilly (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial). He directly own stocks of General Electric (less than $5,000). E.M. Whyte has received research support from the NIMH, the National Institute of Child Health and Human Development (NICHD), the Department of Defense (DOD) and through a Small Business Innovation Research (SBIR) grant from Fox Learning Systems / National Institute of Neurological Disorders and Stroke (NINDS). B. Meyers receives research support from the NIMH. He is receiving medication donated by Pfizer and Eli Lilly for his NIMH trial. During the last three years he has provided legal consultation to AstraZeneca and research consultation for Forest Laboratories. S.D. Østergaard, A.K. Leadholm and P. Bech declare no conflicts of interest.
References
- 1.Glassman AH, Roose SP. Delusional depression. A distinct clinical entity? Arch Gen Psychiatry. 1981;38:424–427. doi: 10.1001/archpsyc.1981.01780290058006. [DOI] [PubMed] [Google Scholar]
- 2.Charney DS, Nelson JC. Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry. 1981;138:328–333. doi: 10.1176/ajp.138.3.328. [DOI] [PubMed] [Google Scholar]
- 3.Kantor SJ, Glassman AH. Delusional depressions: natural history and response to treatment. Br J Psychiatry. 1977;131:351–360. doi: 10.1192/bjp.131.4.351. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: 1980. [Google Scholar]
- 5.World Health Organization. Diagnostic criteria for research. Geneva: WHO; 1993. The ICD-10 Classification of Mental and Behavioural Disorders. [Google Scholar]
- 6.Rothschild AJ. Clinical Manual for Diagnosis and Treatment of Psychotic Depression. Washington, DC, USA: American Psychiatric Publishing, Inc; 2009. [Google Scholar]
- 7.Flores BH, Schatzberg AF. Psychotic Depression. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. Textbook of Mood Disorders. 1st ed. Arlington: American Pychiatric Publishing; 2006. [Google Scholar]
- 8.Ostergaard SD, Leadholm AK, Rothschild AJ. Persistent delusional theme over 13 episodes of psychotic depression. Acta Neuropsychiatr. 2013;25 doi: 10.1017/neu.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coryell W, Pfohl B, Zimmerman M. The clinical and neuroendocrine features of psychotic depression. J Nerv Ment Dis. 1984;172:521–528. doi: 10.1097/00005053-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Ostergaard SD, Petrides G, Dinesen PT, Skadhede S, Bech P, Munk-Jorgensen P, et al. The Association between Physical Morbidity and Subtypes of Severe Depression. Psychother Psychosom. 2013;82:45–52. doi: 10.1159/000337746. [DOI] [PubMed] [Google Scholar]
- 11.Ostergaard SD, Bille J, Soltoft-Jensen H, Lauge N, Bech P. The validity of the severity-psychosis hypothesis in depression. J Affect Disord. 2012;140:48–56. doi: 10.1016/j.jad.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Gournellis R, Lykouras L. Psychotic (delusional) major depression in the elderly: a review. Curr Psychiatry Rev. 2006:235–244. [Google Scholar]
- 13.Meyers BS, Greenberg R. Late-life delusional depression. J Affect Disord. 1986;11:133–137. doi: 10.1016/0165-0327(86)90019-4. [DOI] [PubMed] [Google Scholar]
- 14.Rothschild AJ, Winer J, Flint AJ, Mulsant BH, Whyte EM, Heo M, et al. Missed diagnosis of psychotic depression at 4 academic medical centers. J Clin Psychiatry. 2008;69:1293–1296. doi: 10.4088/jcp.v69n0813. [DOI] [PubMed] [Google Scholar]
- 15.Bech P. Clinical Psychometrics. Oxford, UK: Wiley-Blackwell; 2012. [Google Scholar]
- 16.Ostergaard SD, Meyers BS, Flint AJ, Mulsant BH, Whyte EM, Ulbricht CM, et al. Measuring psychotic depression. Acta Psychiatr Scand. 2014;129:211–220. doi: 10.1111/acps.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seemuller F, Riedel M, Obermeier M, Schennach-Wolff R, Spellmann I, Meyer S, et al. The validity of self-rated psychotic symptoms in depressed inpatients. Eur Psychiatry. 2012;27:547–552. doi: 10.1016/j.eurpsy.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guy W, Bonato ER. Second Revision (Chevy Chase) Maryland, USA: National Institute of Mental Health; 1970. Manual for the ECDEU Assessment Battery. [Google Scholar]
- 20.Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Res. 1985;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 22.Bech P, Gjerris A, Andersen J, Bojholm S, Kramp P, Bolwig TG, et al. The Melancholia Scale and the Newcastle Scales. Item-combinations and inter-observer reliability. Br J Psychiatry. 1983;143:58–63. doi: 10.1192/bjp.143.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- 24.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 25.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 26.Spiker DG, Weiss JC, Dealy RS, Griffin SJ, Hanin I, Neil JF, et al. The pharmacological treatment of delusional depression. Am J Psychiatry. 1985;142:430–436. doi: 10.1176/ajp.142.4.430. [DOI] [PubMed] [Google Scholar]
- 27.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Kendler KS, Glazer WM, Morgenstern H. Dimensions of delusional experience. Am J Psychiatry. 1983;140:466–469. doi: 10.1176/ajp.140.4.466. [DOI] [PubMed] [Google Scholar]
- 29.Meyers BS, English J, Gabriele M, Peasley-Miklus C, Heo M, Flint AJ, et al. A delusion assessment scale for psychotic major depression: Reliability, validity, and utility. Biol Psychiatry. 2006;60:1336–1342. doi: 10.1016/j.biopsych.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Meyers BS, Flint AJ, Rothschild AJ, Mulsant BH, Whyte EM, Peasley-Miklus C, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD) Arch Gen Psychiatry. 2009;66:838–847. doi: 10.1001/archgenpsychiatry.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wijkstra J, Burger H, van den Broek WW, Birkenhager TK, Janzing JG, Boks MP, et al. Treatment of unipolar psychotic depression: a randomized, double-blind study comparing imipramine, venlafaxine, and venlafaxine plus quetiapine. Acta Psychiatr Scand. 2010;121:190–200. doi: 10.1111/j.1600-0447.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- 33.Birkenhager TK, Pluijms EM, Lucius SA. ECT response in delusional versus non-delusional depressed inpatients. J Affect Disord. 2003;74:191–195. doi: 10.1016/s0165-0327(02)00005-8. [DOI] [PubMed] [Google Scholar]
- 34.Birkenhager TK, van den Broek WW, Mulder PG, Moleman P, Bruijn JA. Efficacy of imipramine in psychotic versus nonpsychotic depression. J Clin Psychopharmacol. 2008;28:166–170. doi: 10.1097/JCP.0b013e318166c51c. [DOI] [PubMed] [Google Scholar]
- 35.Anton RF, Jr, Burch EA., Jr Response of psychotic depression subtypes to pharmacotherapy. J Affect Disord. 1993;28:125–131. doi: 10.1016/0165-0327(93)90041-h. [DOI] [PubMed] [Google Scholar]
- 36.Wijkstra J, Lijmer J, Balk FJ, Geddes JR, Nolen WA. Pharmacological treatment for unipolar psychotic depression: Systematic review and meta-analysis. Br J Psychiatry. 2006;188:410–415. doi: 10.1192/bjp.bp.105.010470. [DOI] [PubMed] [Google Scholar]
- 37.Preti A, Bonventre E, Ledda V, Petretto DR, Masala C. Hallucinatory experiences, delusional thought proneness, and psychological distress in a nonclinical population. J Nerv Ment Dis. 2007;195:484–491. doi: 10.1097/NMD.0b013e31802f205e. [DOI] [PubMed] [Google Scholar]
- 38.Langer AI, Cangas AJ, Serper M. Analysis of the multidimensionality of hallucination-like experiences in clinical and nonclinical Spanish samples and their relation to clinical symptoms: implications for the model of continuity. Int J Psychol. 2011;46:46–54. doi: 10.1080/00207594.2010.503760. [DOI] [PubMed] [Google Scholar]
- 39.Johns LC. Hallucinations in the general population. Curr Psychiatry Rep. 2005;7:162–167. doi: 10.1007/s11920-005-0049-9. [DOI] [PubMed] [Google Scholar]
- 40.Wigman JT, van Nierop M, Vollebergh WA, Lieb R, Beesdo-Baum K, Wittchen HU, et al. Evidence that psychotic symptoms are prevalent in disorders of anxiety and depression, impacting on illness onset, risk, and severity--implications for diagnosis and ultra-high risk research. Schizophr Bull. 2012;38:247–257. doi: 10.1093/schbul/sbr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson JC, Mazure CM, Jatlow PI. Characteristics of desipramine-refractory depression. J Clin Psychiatry. 1994;55:12–19. [PubMed] [Google Scholar]
- 42.Zanardi R, Franchini L, Serretti A, Perez J, Smeraldi E. Venlafaxine versus fluvoxamine in the treatment of delusional depression: a pilot double-blind controlled study. J Clin Psychiatry. 2000;61:26–29. doi: 10.4088/jcp.v61n0107. [DOI] [PubMed] [Google Scholar]
- 43.Zanardi R, Franchini L, Gasperini M, Perez J, Smeraldi E. Double-blind controlled trial of sertraline versus paroxetine in the treatment of delusional depression. Am J Psychiatry. 1996;153:1631–1633. doi: 10.1176/ajp.153.12.1631. [DOI] [PubMed] [Google Scholar]
- 44.Zanardi R, Franchini L, Gasperini M, Lucca A, Smeraldi E, Perez J. Faster onset of action of fluvoxamine in combination with pindolol in the treatment of delusional depression: a controlled study. J Clin Psychopharmacol. 1998;18:441–446. doi: 10.1097/00004714-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Gatti F, Bellini L, Gasperini M, Perez J, Zanardi R, Smeraldi E. Fluvoxamine alone in the treatment of delusional depression. Am J Psychiatry. 1996;153:414–416. doi: 10.1176/ajp.153.3.414. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M. The Hamilton Depression Scales. In: Sartorius N, Ban TA, editors. Assessment of Depression. Springer, Berlin, Germany: Berlin, Springer; 1986. [Google Scholar]
- 47.Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. New York, USA: Harper; 1967. [Google Scholar]
- 48.Petrides G, Fink M, Husain MM, Knapp RG, Rush AJ, Mueller M, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17:244–253. doi: 10.1097/00124509-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Rothschild AJ, Williamson DJ, Tohen MF, Schatzberg A, Andersen SW, Van Campen LE, et al. A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features. J Clin Psychopharmacol. 2004;24:365–373. doi: 10.1097/01.jcp.0000130557.08996.7a. [DOI] [PubMed] [Google Scholar]
- 50.Kunzel HE, Ackl N, Hatzinger M, Held K, Holsboer-Trachsler E, Ising M, et al. Outcome in delusional depression comparing trimipramine monotherapy with a combination of amitriptyline and haloperidol--a double-blind multicenter trial. J Psychiatr Res. 2009;43:702–710. doi: 10.1016/j.jpsychires.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 51.DeBattista C, Belanoff J, Glass S, Khan A, Horne RL, Blasey C, et al. Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry. 2006;60:1343–1349. doi: 10.1016/j.biopsych.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 52.Blasey CM, Block TS, Belanoff JK, Roe RL. Efficacy and safety of mifepristone for the treatment of psychotic depression. J Clin Psychopharmacol. 2011;31:436–440. doi: 10.1097/JCP.0b013e3182239191. [DOI] [PubMed] [Google Scholar]
- 53.Blasey CM, Debattista C, Roe R, Block T, Belanoff JK. A multisite trial of mifepristone for the treatment of psychotic depression: a site-by-treatment interaction. Contemp Clin Trials. 2009;30:284–288. doi: 10.1016/j.cct.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Bech P. Fifty years with the Hamilton scales for anxiety and depression. A tribute to Max Hamilton. Psychother Psychosom. 2009;78:202–211. doi: 10.1159/000214441. [DOI] [PubMed] [Google Scholar]
- 55.Gaudiano BA, Beevers CG, Miller IW. Differential response to combined treatment in patients with psychotic versus nonpsychotic major depression. J Nerv Ment Dis. 2005;193:625–628. doi: 10.1097/01.nmd.0000177791.33649.69. [DOI] [PubMed] [Google Scholar]
- 56.Benazzi F. Bipolar versus unipolar psychotic outpatient depression. J Affect Disord. 1999;55:63–66. doi: 10.1016/s0165-0327(98)00217-1. [DOI] [PubMed] [Google Scholar]
- 57.Adler M, Hetta J, Isacsson G, Brodin U. An item response theory evaluation of three depression assessment instruments in a clinical sample. BMC Med Res Methodol. 2012;12:84-2288-12-84. doi: 10.1186/1471-2288-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Licht RW, Qvitzau S, Allerup P, Bech P. Validation of the Bech-Rafaelsen Melancholia Scale and the Hamilton Depression Scale in patients with major depression; is the total score a valid measure of illness severity? Acta Psychiatr Scand. 2005;111:144–149. doi: 10.1111/j.1600-0447.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- 59.Bech P. The Bech-Rafaelsen Melancholia Scale (MES) in clinical trials of therapies in depressive disorders: a 20-year review of its use as outcome measure. Acta Psychiatr Scand. 2002;106:252–264. doi: 10.1034/j.1600-0447.2002.01404.x. [DOI] [PubMed] [Google Scholar]
- 60.Lauritzen L, Odgaard K, Clemmesen L, Lunde M, Ohrstrom J, Black C, et al. Relapse prevention by means of paroxetine in ECT-treated patients with major depression: a comparison with imipramine and placebo in medium-term continuation therapy. Acta Psychiatr Scand. 1996;94:241–251. doi: 10.1111/j.1600-0447.1996.tb09856.x. [DOI] [PubMed] [Google Scholar]
- 61.Muller-Siecheneder F, Muller MJ, Hillert A, Szegedi A, Wetzel H, Benkert O. Risperidone versus haloperidol and amitriptyline in the treatment of patients with a combined psychotic and depressive syndrome. J Clin Psychopharmacol. 1998;18:111–120. doi: 10.1097/00004714-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Adli M, Wiethoff K, Baethge C, Pfennig A, Stamm T, Bauer M. Olanzapine in the treatment of depression with psychotic features: A prospective open-label study. Int J Psychiatry Clin Pract. 2008;12:202–209. doi: 10.1080/13651500801911144. [DOI] [PubMed] [Google Scholar]
- 63.Taiminen T, Jaaskelainen S, Ilonen T, Meyer H, Karlsson H, Lauerma H, et al. Habituation of the blink reflex in first-episode schizophrenia, psychotic depression and non-psychotic depression. Schizophr Res. 2000;44:69–79. doi: 10.1016/s0920-9964(99)00140-1. [DOI] [PubMed] [Google Scholar]
- 64.Mulsant BH, Sweet RA, Rosen J, Pollock BG, Zubenko GS, Flynn T, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62:597–604. doi: 10.4088/jcp.v62n0804. [DOI] [PubMed] [Google Scholar]
- 65.Keller J, Gomez RG, Kenna HA, Poesner J, DeBattista C, Flores B, et al. Detecting psychotic major depression using psychiatric rating scales. J Psychiatr Res. 2006;40:22–29. doi: 10.1016/j.jpsychires.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 66.Singh MM, Kay SR. A comparative study of haloperidol and chlorpromazine in terms of clinical effects and therapeutic reversal with benztropine in schizophrenia. Theoretical implications for potency differences among neuroleptics. Psychopharmacologia. 1975;43:103–113. doi: 10.1007/BF00421012. [DOI] [PubMed] [Google Scholar]
- 67.Santor DA, Ascher-Svanum H, Lindenmayer JP, Obenchain RL. Item response analysis of the Positive and Negative Syndrome Scale. BMC Psychiatry. 2007;7:66. doi: 10.1186/1471-244X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Levine SZ, Rabinowitz J, Rizopoulos D. Recommendations to improve the positive and negative syndrome scale (PANSS) based on item response theory. Psychiatry Res. 2011;188:446–452. doi: 10.1016/j.psychres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Gerretsen P, Flint AJ, Whyte EM, Rothschild AJ, Meyers BS, Mulsant BH. Impaired Insight Into Delusions Predicts Treatment Outcome During a Randomized Controlled Trial for Psychotic Depression (STOP-PD Study) J Clin Psychiatry. 2015 doi: 10.4088/JCP.14m09003. (in press); [DOI] [PubMed] [Google Scholar]
- 71.Bech P, Gram LF, Dein E, Jacobsen O, Vitger J, Bolwig TG. Quantitative rating of depressive states. Acta Psychiatr Scand. 1975;51:161–170. doi: 10.1111/j.1600-0447.1975.tb00002.x. [DOI] [PubMed] [Google Scholar]
- 72.Guy W. Clinical Global Impressions Scale. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD, USA: US Department of Health, Education and Welfare pub no (AMD) 76-338, NIMH; 1976. [Google Scholar]
- 73.Ostergaard SD, Pedersen CH, Uggerby P, Munk-Jorgensen P, Rothschild AJ, Larsen JI, et al. Clinical and psychometric validation of the psychotic depression assessment scale. J Affect Disord. 2015;173:261–268. doi: 10.1016/j.jad.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 74.Ostergaard SD, Meyers BS, Flint AJ, Mulsant BH, Whyte EM, Ulbricht CM, et al. Measuring treatment response in psychotic depression: The Psychotic Depression Assessment Scale (PDAS) takes both depressive and psychotic symptoms into account. J Affect Disord. 2014 doi: 10.1016/j.jad.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park SC, Choi J, Kim JM, Jun TY, Lee MS, Kim JB, et al. Is the Psychotic Depression Assessment Scale a useful diagnostic tool? The CRESCEND study. J Affect Disord. 2014;166:79–85. doi: 10.1016/j.jad.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Park SC, Ostergaard SD, Choi J, Kim JM, Jun TY, Lee MS, et al. Is the BPRS-5 subscale of the psychotic depression assessment scale a reliable screening tool for psychotic depression?: Results from the CRESCEND Study. J Affect Disord. 2014;174C:188–191. doi: 10.1016/j.jad.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 77.Tsuang D, Coryell W. An 8-year follow-up of patients with DSM-III-R psychotic depression, schizoaffective disorder, and schizophrenia. Am J Psychiatry. 1993;150:1182–1188. doi: 10.1176/ajp.150.8.1182. [DOI] [PubMed] [Google Scholar]
- 78.Maj M, Starace F, Pirozzi R. A family study of DSM-III-R schizoaffective disorder, depressive type, compared with schizophrenia and psychotic and nonpsychotic major depression. Am J Psychiatry. 1991;148:612–616. doi: 10.1176/ajp.148.5.612. [DOI] [PubMed] [Google Scholar]
- 79.Coryell W, Winokur G, Shea T, Maser JD, Endicott J, Akiskal HS. The long-term stability of depressive subtypes. Am J Psychiatry. 1994;151:199–204. doi: 10.1176/ajp.151.2.199. [DOI] [PubMed] [Google Scholar]
- 80.Cubells JF, Price LH, Meyers BS, Anderson GM, Zabetian CP, Alexopoulos GS, et al. Genotype-controlled analysis of plasma dopamine beta-hydroxylase activity in psychotic unipolar major depression. Biol Psychiatry. 2002;51:358–364. doi: 10.1016/s0006-3223(01)01349-x. [DOI] [PubMed] [Google Scholar]
- 81.Meyers BS, Alexopoulos GS, Kakuma T, Tirumalasetti F, Gabriele M, Alpert S, et al. Decreased dopamine beta-hydroxylase activity in unipolar geriatric delusional depression. Biol Psychiatry. 1999;45:448–452. doi: 10.1016/s0006-3223(98)00085-7. [DOI] [PubMed] [Google Scholar]
- 82.Maj M, Pirozzi R, Magliano L, Fiorillo A, Bartoli L. Phenomenology and prognostic significance of delusions in major depressive disorder: a 10-year prospective follow-up study. J Clin Psychiatry. 2007;68:1411–1417. doi: 10.4088/jcp.v68n0913. [DOI] [PubMed] [Google Scholar]
- 83.Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, Shear PK. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry. 2000;157:1095–1100. doi: 10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- 84.Schatzberg AF, Rothschild AJ. Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry. 1992;149:733–745. doi: 10.1176/ajp.149.6.733. [DOI] [PubMed] [Google Scholar]
- 85.Gaudiano BA, Young D, Chelminski I, Zimmerman M. Depressive symptom profiles and severity patterns in outpatients with psychotic vs nonpsychotic major depression. Compr Psychiatry. 2008;49:421–429. doi: 10.1016/j.comppsych.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lykouras E, Malliaras D, Christodoulou GN, Papakostas Y, Voulgari A, Tzonou A, et al. Delusional depression: phenomenology and response to treatment. A prospective study. Acta Psychiatr Scand. 1986;73:324–329. doi: 10.1111/j.1600-0447.1986.tb02692.x. [DOI] [PubMed] [Google Scholar]
- 87.Frances A, Brown RP, Kocsis JH, Mann JJ. Psychotic depression: a separate entity? Am J Psychiatry. 1981;138:831–833. doi: 10.1176/ajp.138.6.831. [DOI] [PubMed] [Google Scholar]
- 88.Fava M. How can we make measures of psychotic depression symptoms more clinically useful? Acta Psychiatr Scand. 2014;129:161–162. doi: 10.1111/acps.12177. [DOI] [PubMed] [Google Scholar]