Abstract
Introduction
Patients with end stage renal disease(ESRD) develop hypocalcemia, resulting in secondary hyperparathyroidism(SHPT). No clear criterions exist to aid in surgical decision making for SHPT. The 2009 Kidney Disease: Improving Global Outcomes(KDIGO) guidelines provide target ranges for serum calcium, phosphate and parathyroid hormone(PTH) levels in patients with ESRD. Parathyroidectomy can help achieve these targets. The study purpose is to examine how parathyroidectomy for SHPT impacts KDIGO targets during immediate and long-term follow-up, and to evaluate KDIGO categorization with receipt of additional surgical intervention.
Methods
A retrospective review of a prospective parathyroidectomy database was performed. Included patients had SHPT, were on dialysis and underwent parathyroidectomy. Calcium, phosphate and PTH values were classified as below, within, or above KDIGO targets.
Results
Between 2000 and 2013, 36 patients with SHPT met criteria. Subtotal parathyroidectomy was performed in 89%, total parathyroidectomy in 11%. Follow-up time was 54±7 months. 8 patients(22%) required additional surgery. 28 patients(76%) were alive at last follow-up. At last follow up, patients had phosphate(46%), and PTH(17%) above KDIGO ranges.
Factors associated with re-operation were assessed. Patient PTH within or above target immediately post-operative had a higher rate of reoperation(p<0.01). At last follow-up, higher phosphate(p=0.054) and PTH(p<0.001) were associated with higher reoperation rates, but calcium(p=0.33) was not.
Conclusions
PTH and phosphate levels above KDIGO indices were associated with additional surgical intervention. Many patients had laboratory indices above range at last follow-up, suggesting more patients had persistent or recurrent disease than underwent reoperation. Patients may benefit from more aggressive medical and/or surgical management.
Keywords: Secondary hyperparathyroidism, Renal hyperparathyroidism, Parathyroidectomy, KDIGO, mineral and bone disorder
Introduction
Secondary hyperparathyroidism (SHPT) makes up a small fraction of patients undergoing parathyroidectomy.(1, 2) Underlying renal disease leads to calcium excretion and loss, inability to activate vitamin D, and hyperphosphatemia. This ultimately results in hypocalcemia, and ongoing parathyroid gland stimulation.(1, 3, 4) Parathyroidectomy under these circumstances can provide significant reductions in serum parathyroid hormone (PTH) and to a lesser degree phosphate, but can exacerbate issues with hypocalcemia.(5, 6) Often, patients with SHPT are only referred for surgical management after they are felt to be refractory to medical management. Nephrologists make this determination and historically only considered referral when PTH were >800 pg/mL despite medical treatment, although upper limits of normal vary.(7–9) Elevated calcium phosphate product (> 55 to 70) was another historic indication for parathyroidectomy, while the development of calciphylaxis or severe renal osteodystrophy are still considered indications for surgery.(8, 10–12) Indications for reoperation are equally ambiguous. At present there are no standardized criterions for parathyroidectomy indications, nor are success, persistence, or recurrence after parathyroidectomy for SHPT well defined.(8, 9, 11, 13–17)
In efforts to better standardize the management of mineral and bone disorders (MBD) in patients with chronic kidney disease (CKD), the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice guidelines were developed.(5) These guidelines established definitions for both MBD and renal osteodystrophy, and provided evidence-based recommendations regarding diagnosis, management and treatment of abnormal biochemistries, vascular calcification and bone disorders. Achievement of these parameters benefits not just bone health, but results in decreased cardiovascular morbidity and decreased mortality in hemodialysis patients.(18) Target ranges were provided for serum calcium, phosphate and PTH, which prompted intervention when targets were not attained.(5) Specifically, when PTH are unable to be maintained within two to nine times the upper limits of the assay with maximum medical therapy, parathyroidectomy is recommended.(5) These recommendations were subsequently supported by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) for use in the United States, and replaced older guidelines which had previously been developed by the National Kidney Foundation KDOQI in 2003.(6, 19)
The aim of this study was to describe the natural history of secondary hyperparathyroidism after parathyroidectomy with respect to alteration of the biochemical parameters set forth in the KDIGO guidelines. The hypothesis was that the KDIGO guidelines for CKD and MBD can be used to determine which patients require initial surgical referral for SHPT, and maybe used to assess need for further medical and/or surgical intervention after parathyroidectomy.
Materials and Methods
With IRB approval, we conducted a retrospective review of a prospective parathyroidectomy database at a large, tertiary referral center with both endocrine surgery and renal transplant services. All patients with SHPT due to end stage renal disease (ESRD) and on dialysis, who underwent parathyroidectomy, were identified. Patients with SHPT due to gastrointestinal malabsorption were excluded. A minimum of 6 months follow up after surgery was required for inclusion. Patients with a failed previous transplantation were still considered to have SHPT. Decision to operate was based on pre-operative laboratory values, and clinical symptomology (severity of bone disease, calciphylaxis, etc.)(5, 11, 12). Serum calcium, phosphate and PTH were noted pre-operative, at the time of postoperative visit (1 to 2 weeks), as well as 6 months and last available laboratory data within the electronic medical record (EMR). EMR were accessed to determine the need for subsequent surgery or clinical concern for disease recurrence or persistence based on physician notes.
Operative procedures were defined as a subtotal parathyroidectomy with a cervical remnant, a total parathyroidectomy with autotransplant to the forearm, or a minimally invasive parathyroidectomy ± autotransplant in cases of revision surgery.(11, 20) Additionally, operative reports were reviewed to determine if a cervical thymectomy was performed as unilateral, bilateral or not at all. Patients were classified as no further surgery or additional parathyroidectomy. Renal transplantation after parathyroidectomy was noted. Patients were classified as either having a successful renal transplant at time of last follow up, or no transplant and/or a failed transplant. Survival after parathyroidectomy was noted. As the EMR received information from the social security registry regarding mortality, to the best of our ability we assume those patients not otherwise designated as expired are still alive, even if no recent follow up laboratory data is available.
KDIGO target values based on the 2009 guidelines on CKD and MBD, were used to classify patients as normal, above or below target for serum calcium, phosphate and PTH.(5) Per these guidelines, calcium is to be maintained within the normal range (8.4 to 10.2 mg/dL), as is phosphate (2.5 to 4.6 mg/dL). PTH is to be maintained between two to nine times the upper limit of normal (130 to 600 pg/mL). These definitions were applied to all time points.
Statistical analysis was performed using IBM SPSS Statistics for Windows, version 22 (IBM Corporation, Armonk, NY, USA). Categorical variables were compared using χ2 and Fisher’s exact test. Independent t-test and one-way ANOVA were used for continuous variables. A Kaplan Meier analysis was performed to calculate estimated all-cause survival after parathyroidectomy. A p-value of <0.05 was determined to be significant. Data are expressed as mean ± standard error of the mean (SEM) for continuous variables, and as number and percentage of total for categorical variables, unless otherwise specified.
Results
Between 2000 and 2013, 44 patients with SHPT were identified, and 36 patients met inclusion criteria (Table 1). Mean patient age was 44 ± 2.1 years. 15 patients were female (42%). Pre-operative laboratory values included calcium of 8.8 ± 0.2 mg/dL (median 8.9 mg/dL, range 4.8), phosphate of 6.1 ± 0.3 mg/dL (5.9, 8.7) and PTH of 1171 ± 119 pg/mL (1040, 2780). Cinacalcet (Sensipar) use was noted in the pre-operative period in 18 patients (50%).
Table 1.
Cohort Demographics
| Study Population | 36 |
|---|---|
| Female | 15 (42%) |
| Male | 21 (58%) |
| Age | 44 ± 2.1 years |
| Race | |
| African American | 5 (14%) |
| Caucasian | 24 (67%) |
| Not Recorded | 8 (22%) |
| Use of Cinacalcet Pre-Operatively | 18 (50%) |
| Extent of initial Surgery | |
| Subtotal Parathyroidectomy | 33 (92%) |
| Total Parathyroidectomy | 3 (8%) |
| Extent of intial thymectomy | |
| None | 22 (61%) |
| Unilateral | 6 (17%) |
| Bilateral | 8 (22%) |
| Weight of largest excised gland | 1431 ± 240 mg |
Data expressed as number (percent) or as mean ± standard error of the mean.
Based on the KDIGO targets, pre-operative calcium was within target for 22 patients (61%), but below in 10 (28%) and above in only 4 patients (11%)(Table 2). Phosphate was mostly above KDIGO targets (27 patients, 77%), with only 7 patients (20%) within and 1 patient (3%) below target. Twenty seven (75%) of patients had PTH above target, while 9 patients (25%) were within target, and none were below. At the pre-operative time point, patients were further defined based on how many of the three KDIGO values were either within target or above target (Table 3). Only two patients (6%) were noted to have all three categories fall within target value pre-operatively; one with severe renal osteodystrophy and the other with calciphylaxis. Further distribution between having no values within range to two within range varied from 28 to 36%. Four patients (11%) had no values above KDIGO target in the pre-operative period; this includes the previous two patients as well as two others with severe renal osteodystrophy and pruritus. A majority of patients (56%) were noted to have 2 values above target in the pre-operative period.
Table 2.
Laboratory trends in calcium, phosphate and parathyroid hormone from pre-operative visit, immediately post-op and during follow-up.
| KDIGO Target | Calcium | Phosphate | Parathyroid hormone |
|---|---|---|---|
| Pre-Operative Labs | |||
| Below | 10 (28%) | 1 (3%) | 0 |
| Within | 22 (61%) | 7 (20%) | 9 (25%) |
| Above | 4 (11%) | 27 (77%) | 27 (75%) |
| Post-Operative Visit | |||
| Below | 17 (47%) | NA | 21 (67%) |
| Within | 19 (53%) | NA | 11 (33%) |
| Above | 0 | NA | 1 (3%) |
| 6 Months Post-Op* | |||
| Below | 8 (38%) | 2 (10%) | 10 (48%) |
| Within | 13 (62%) | 6 (30%) | 9 (43%) |
| Above | 0 | 12 (60%) | 2 (10%) |
| At Last Follow-Up | |||
| Below | 19 (53%) | 2 (6%) | 16 (44%) |
| Within | 13 (36%) | 16 (49%) | 14 (39%) |
| Above | 4 (11%) | 15 (46%) | 6 (17%) |
Data presented as number (percentage). NA – Not Available.
Data only available at this time point for 21 patients (58%).
Table 3.
Distribution (percentage) of patients with multiple values within and above KDIGO target range for each time point (pre-operative, 6 month follow up and time of last follow up).
| Time Point | Number of Values In Target Range | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Pre-operative visit | 31% | 36% | 28% | 6% |
| 6 months post-op | 19% | 38% | 33% | 10% |
| At last follow up | 19% | 42% | 33% | 6% |
| Number of Values Above Target Range | ||||
| Pre-operative visit | 11% | 25% | 56% | 8% |
| 6 months post-op | 43% | 48% | 10% | 0 |
| At last follow up | 50% | 33% | 14% | 3% |
Most patients underwent initial subtotal parathyroidectomy (n=33, 92%) versus total parathyroidectomy (n=3, 8%)(Table 1). Thymectomy was generally not performed (n=22, 61%), although unilateral (n=6, 17%) and bilateral thymectomy (n=8, 22%) were done occasionally. Subsequent procedures were required for 8 patients (22%). Four patients (11%) underwent 2 additional procedures, 1 patient (3%) underwent 3 additional procedures, and 3 patients (8%) required a single additional operation. Six patients (16%) went on to successful renal transplantation after parathyroidectomy. Two patients (5%) were noted to be taking Cinacalcet (Sensipar) at time of last follow up. Twenty eight patients (76%) were alive at last follow up, with an over-all follow up 54 ± 7 months. Estimated all-cause survival of the study population was 100% at 1 year, 95% at 2 years, 82% at 5 years and 65% at 10 years (Figure 1).
Figure 1.
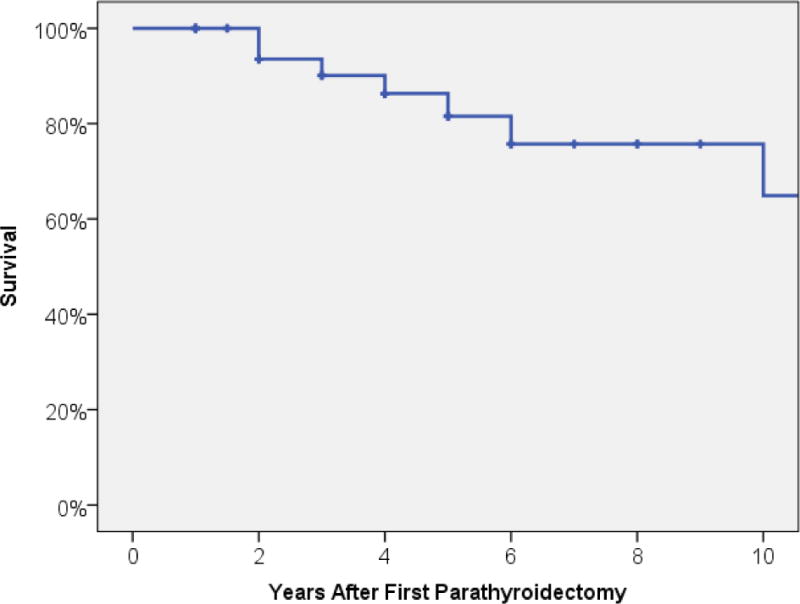
Estimated all-cause survival after parathyroidectomy for secondary hyperparathyroidism within study cohort.
At the post-operative visit, only calcium and PTH were monitored (Table 2). Calcium was below target for 17 patients (47%) and within target for 19 (53%); no patient was above the calcium target. PTH was below target for 21 patients (67%), within target for 11 (33%) and above target in 1 patient (3%). By 6 months, although no patients were above target for calcium, 60% were above target for phosphate, and 10% were above target for PTH. At time of last follow up, 11% of patients were above target for calcium, 46% for phosphate and 17% for PTH. All of the patients noted to have above target PTH, also had above target phosphate, but patients with above target phosphate had variable PTH levels. PTH and phosphate categories at last follow up were found to be associated with one another (p=0.03), whereas phosphate and calcium (p=0.95) and calcium and PTH (p=0.79) were not. Additionally, correlation between KDIGO target grouping at pre-operative and post-operative time points were not significant (Data not shown).
Factors associated with re-operation were assessed. Patient age (p=0.37), gender (p=1.0), pre-operative calcium (p=0.26), phosphate (p=0.56), PTH (p=0.20), initial operative procedure (p=0.54), pre-operative use of cinacalcet (Sensipar, p=1.0), weight of largest excised gland (p=0.18) and extent of thymectomy (p=0.93) were not associated with need for additional operative intervention (additional parathyroidectomy). However, above KDIGO target values were associated with re-operation. Patients with calcium below KDIGO targets at the post-operative visit had a re-operative rate of 6%, but those patients within the KDIGO range had a re-operative rate of 37% (p=0.04). PTH below target at the postoperative visit was associated with a 5% re-operation rate, while patients within target required re-operation 37% of the time, and those above target had a 100% re-operation rate (p<0.01). Six month follow up KDIGO targets for calcium (p=1.0), phosphate (p=0.76) and PTH (p=0.09) were not found to be associated with re-operation, although data was only available for 58% of patients as this time point. At last follow up, calcium was not associated with an increased rate of re-operation (p=0.33). Phosphate within or above KDIGO targets were suggestive of higher re-operative rates (6% and 40%, respectively), while patients below target had no re-operations (p=0.054). PTH above KDIGO target had high rates of re-operation (83%), compared to patients within (21%) or below target (0, p<0.001). Patients with one or more KDIGO value above target at last follow up had higher observed rates of re-operation (p<0.01). This was not seen at any other time point. Additional factors assessed for association with re-operation included: mortality (p=0.65), transplantation after parathyroidectomy (p=0.30), and use of Cinacalcet at last follow up (p=0.04).
Additional analyses were performed to evaluate the pre-operative, operative and post-operative values as listed above, and association with survival at time of last follow up. No association between any of these variables and survival at last follow-up were found (data not shown).
The number of KDIGO variables which were noted to be within target range and above target range at pre-op, 6 month follow up and at last follow up (Table 3). Gland weight was assessed and found to not be associated with KDIGO variables in target pre-op (p=0.21), at 6 months (p=0.19) and at last follow up (p=0.68). Additionally, the initial operation performed (subtotal versus total parathyroidectomy) was not associated with the number of KDIGO variables in target at last follow up (p=0.15), nor was the extent to thymectomy performed (p=0.30).
Discussion
Herein, a series of SHPT patients managed with parathyroidectomy were followed for an average of 4.5 years. A majority of patients were noted to have at least one KDIGO variable above target pre-operatively, or in rare instances had other compelling clinical indications for parathyroidectomy (severe renal osteodystrophy or calciphylaxis). This suggests that the KDIGO guidelines when applied retrospectively to a surgical cohort, clearly aligned with surgeon decision making for initial parathyroidectomy. Additionally, during the course of follow up, PTH fell dramatically after surgery, while phosphate did not appear to be impacted much (Table 2) as a majority of patients continued to have difficulties with hyperphosphatemia. With respect to re-operation, the overall rate of re-operation for the cohort was 22%. Above target PTH was associated with higher rates of re-operation while above target phosphate was suggestive of higher re-operative rates but not statistically significant. Hypercalcemia was uncommon during follow up, and was not associated with a high rate of re-operation. These findings would suggest that the KDIGO guidelines for PTH, and to a less extent phosphate, also align with surgeon decision making for re-operative parathyroidectomy for SHPT. In this retrospective study, KDIGO targets for calcium did not influence re-operation rates. While not all patients who were above the suggested KDIGO target range for phosphate and PTH underwent a re-operation, the continued metabolic derangement suggests that more aggressive medical and/or surgical management may be indicated.
The management of SHPT, and MBD of CKD is mostly medical in nature, with parathyroidectomy reserved for the most severe cases, either unresponsive to or unable to tolerate medical therapy, and who are considered acceptable surgical risk.(17) The guidelines set forth first by the National Kidney Foundation KDOQI, and subsequently modified by the KDIGO, provide recommendations regarding the monitoring and medical management of SHPT.(5, 6, 19) With regards to the metabolic parameters, the changes found within KDIGO resulted in a more liberal control of calcium, but much tighter focus on phosphate as it were noted to be associated with increased mortality and cardiovascular disease.(21) Since calcium phosphate product was largely driven by the phosphate level, it was no longer required by KDIGO.(6) PTH range was also liberalized. As the KDIGO guidelines represent the most current clinical management guidelines for MBD of CKD, these were selected for the purposes of this study over the older and outdated KDOQI recommendations.
Mazzaferro et al first looked at parathyroidectomy as a means to achieve KDOQI guidelines.(9) They found that patients undergoing parathyroidectomy achieved the target KDOQI ranges between 14–43% for calcium and between 65–76% for phosphate. However, PTH levels were in target only 6–9%, with a majority of patients having below target. Kovacevic et al evaluated both the KDOQI and KDIGO target parameters after total parathyroidectomy with autotransplant.(22) They found that parathyroidectomy resulted in a greater percentage of patients meeting KDOQI targets than KDIGO. However, they did not go into detail as to which were above versus below target, and given the differences in the target ranges for each guideline, this variability is to be expected. In contrast to the data presented herein, these previous studies on KDOQI and/or KDIGO targets did not provide any long term follow data with respects to re-operation, transplantation and overall survival.
Multiple small, cohort studies have looked at medical versus surgical management of SHPT and consistently demonstrated a survival benefit with parathyroidectomy. Lin et al reviewed 53 patients, all of whom were recommended to undergo parathyroidectomy.(10) They compared those patients who consented to parathyroidectomy to those who refused. Patients undergoing parathyroidectomy had worse baseline labs, as well as a higher modified Charlson comorbidity index, but during the 72 month follow up, surgical patients had a lower rate of cardiovascular events than those on medical therapy alone.(10) Costa-Hong et al also compared outcomes between patients with medically resistant SHPT managed with parathyroidectomy versus continued medical management.(7) They also found that both cardiovascular morbidity and all-cause mortality were improved in patients who underwent parathyroidectomy. Our results also demonstrate favorable patient outcomes with excellent long term survival at both 5 and 10 years after surgery.
After intervention, ongoing surveillance is required.(5) Calcium and phosphate are to be measured every one to three months, and PTH every three to six months. Values falling outside of target must prompt additional intervention with either adjustment of medications or evaluation for further surgical resection. Within the study population, 22% of patients underwent additional operative intervention, presumably for persistent/recurrent disease. Even with that high rate of re-operation, 17% of patients had PTH above target, and 46% had elevated phosphate at time of last follow up. This suggests that more aggressive management both medically and surgically could have been considered.
Values falling below target may also pose a challenge. Low PTH may contribute to, or worsen, adynamic bone disease. Adynamic bone disease is often multifactorial, and even with PTH within or above target ranges, skeletal resistance to PTH can be present.(23) In the previously discussed studies by Mazzaferro et al, and Kovacevic et al, patients were noted to fall below KDOQI and/or KDIGO target levels after parathyroidectomy but no suggestions were made as to how to manage those patients.(9, 22) Similarly, patients presented here were noted to have calcium and PTH levels below KDIGO targets roughly half the time during follow up. Phosphate was below KDIGO target in only 6 to 10% of patients. The consequences of these findings are still unclear.
This study has several limitations, in part due to the retrospective nature of its design. Many of the newer medications used for the management of SHPT became approved by the US Food and Drug Administration during the study period, resulting in continuous change in medical management throughout the study.(24) The KDIGO guidelines were released in 2009, and therefore were not available throughout the study period.(5) Prior to that, the KDOQI guidelines provided recommendations regarding the medical management of SHPT.(19) At present there is no consensus as to the best operation for the management of SHPT, nor established criteria for which parathyroidectomy should be performed.(6, 17) A vast majority of the study cohort underwent a subtotal parathyroidectomy without thymectomy, preventing comparisons between operative approaches and extent of thymectomy performed, with respect to need for re-operation and laboratory profile at last follow up. The population of patients with ESRD is very heterogeneous with respect to underlying etiology of kidney failure, age and additional co-morbidities, and this may influence decision making regarding referral and/or consideration of parathyroidectomy.(25, 26) For this reason, sicker patients with a greater degree of co-morbid disease may have not been considered for referral or declined surgical intervention, resulting in an inclusion bias. We cannot tell how many patients with above target KDIGO for calcium, phosphate and/or PTH were not referred for surgical consideration. Additionally, this patient cohort represents a large, endocrine surgery practice located at a tertiary referral center with a busy, well established renal transplant service.(27) It is possible that both the surgical and medical management of these ESRD patients, as well as their outcome, may not be representative of that found elsewhere in the country given the anticipated possibility of future renal transplantation.
Conclusion
The medical and surgical management of SHPT remains challenging. Ongoing refinement of medical and surgical management is needed to ensure optimal patient care. Use of the KDIGO guidelines for MBD of CKD appears to be a reasonable method for both determining need for initial parathyroidectomy, as well as defining persistent and/or recurrent disease. However, there are still many questions that must be addressed about how to achieve these guidelines with respect to medical management, as well as timing and extent of surgical excision. Only through collaboration with nephrologists can we prospectively study these issues, and determine the ideal overall management for these patients with SHPT.
Continued, frequent surveillance of patients with SHPT is mandatory. Further intervention, whether medical or surgical, should be considered if targets are not met. The consequence of falling below KDIGO guideline targets remains unclear, and requires further investigation.
Acknowledgments
This work was supported by NIH UL1TR000427 and NIH KL2TR000428.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Quick Shot Presentation at Academic Surgical Congress, Las Vegas, NV, Feb 2015.
Author Contributions:
Sarah C. Oltmann participated in the creation of study design and concept, acquisition of data, analysis and interpretation of data, and preparation of the manuscript. Tariq M. Madkhali participated in the acquisition of data. Rebecca S. Sippel participated in the analysis and interpretation of the data and the preparation of the manuscript. Herbert Chen participated in the creation of the study design and concept, analysis and interpretation of data and the preparation of the manuscript. David F. Schneider participated in the creation of the study design and concept, analysis and interpretation of data and the preparation of the manuscript.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Adler JT, Sippel RS, Chen H. New trends in parathyroid surgery. Current problems in surgery. 2010;47:958–1017. doi: 10.1067/j.cpsurg.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Riss P, Asari R, Scheuba C, Niederle B. Current trends in surgery for renal hyperparathyroidism (RHPT)–an international survey. Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2013;398:121–130. doi: 10.1007/s00423-012-1025-6. [DOI] [PubMed] [Google Scholar]
- 3.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, et al. Recent Changes in Therapeutic Approaches and Association with Outcomes among Patients with Secondary Hyperparathyroidism on Chronic Hemodialysis: The DOPPS Study. Clinical journal of the American Society of Nephrology : CJASN. 2014 doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkhalili E, Tasci Y, Aksoy E, Aliyev S, Soundararajan S, et al. The Utility of Neck Ultrasound and Sestamibi Scans in Patients with Secondary and Tertiary Hyperparathyroidism. World journal of surgery. 2014 doi: 10.1007/s00268-014-2878-3. [DOI] [PubMed] [Google Scholar]
- 5.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney international Supplement. 2009:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 6.Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, et al. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55:773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 7.Costa-Hong V, Jorgetti V, Gowdak LH, Moyses RM, Krieger EM, et al. Parathyroidectomy reduces cardiovascular events and mortality in renal hyperparathyroidism. Surgery. 2007;142:699–703. doi: 10.1016/j.surg.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Kara M, Tellioglu G, Bugan U, Krand O, Berber I, et al. Evaluation of intraoperative parathormone measurement for predicting successful surgery in patients undergoing subtotal/total parathyroidectomy due to secondary hyperparathyroidism. The Laryngoscope. 2010;120:1538–1544. doi: 10.1002/lary.21023. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro S, Pasquali M, Farcomeni A, Vestri AR, Filippini A, et al. Parathyroidectomy as a therapeutic tool for targeting the recommended NKF-K/DOQI ranges for serum calcium, phosphate and parathyroid hormone in dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association. 2008;23:2319–2323. doi: 10.1093/ndt/gfm931. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Chen CL, Lin HS, Chou KJ, Fang HC, et al. Parathyroidectomy improves cardiovascular outcome in nondiabetic dialysis patients with secondary hyperparathyroidism. Clinical endocrinology. 2014;80:508–515. doi: 10.1111/cen.12333. [DOI] [PubMed] [Google Scholar]
- 11.Schneider R, Slater EP, Karakas E, Bartsch DK, Schlosser K. Initial parathyroid surgery in 606 patients with renal hyperparathyroidism. World journal of surgery. 2012;36:318–326. doi: 10.1007/s00268-011-1392-0. [DOI] [PubMed] [Google Scholar]
- 12.Girotto JA, Harmon JW, Ratner LE, Nicol TL, Wong L, et al. Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery. 2001;130:645–650. doi: 10.1067/msy.2001.117101. discussion 650–641. [DOI] [PubMed] [Google Scholar]
- 13.Agha A, Loss M, Schlitt HJ, Scherer MN. Recurrence of secondary hyperparathyroidism in patients after total parathyroidectomy with autotransplantation: technical and therapeutic aspects. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2012;269:1519–1525. doi: 10.1007/s00405-011-1776-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen CL, Lin SH, Yu JC, Shih ML. Persistent renal hyperparathyroidism caused by intrathyroidal parathyroid glands. Journal of the Chinese Medical Association : JCMA. 2014;77:492–495. doi: 10.1016/j.jcma.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Moor JW, Roberts S, Atkin SL, England RJ. Intraoperative parathyroid hormone monitoring to determine long-term success of total parathyroidectomy for secondary hyperparathyroidism. Head & neck. 2011;33:293–296. doi: 10.1002/hed.21441. [DOI] [PubMed] [Google Scholar]
- 16.Zitt E, Rix M, Urena Torres P, Fouque D, Jacobson SH, et al. Effectiveness of cinacalcet in patients with recurrent/persistent secondary hyperparathyroidism following parathyroidectomy: results of the ECHO study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association – European Renal Association. 2011;26:1956–1961. doi: 10.1093/ndt/gfq641. [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. The New England journal of medicine. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 18.Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, et al. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;46:925–932. doi: 10.1053/j.ajkd.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 19.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42:S1–201. [PubMed] [Google Scholar]
- 20.Pitt SC, Sippel RS, Chen H. Secondary and tertiary hyperparathyroidism, state of the art surgical management. The Surgical clinics of North America. 2009;89:1227–1239. doi: 10.1016/j.suc.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 22.Kovacevic B, Ignjatovic M, Zivaljevic V, Cuk V, Scepanovic M, et al. Parathyroidectomy for the attainment of NKF-K/DOQI and KDIGO recommended values for bone and mineral metabolism in dialysis patients with uncontrollable secondary hyperparathyroidism. Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2012;397:413–420. doi: 10.1007/s00423-011-0901-9. [DOI] [PubMed] [Google Scholar]
- 23.Bover J, Urena P, Brandenburg V, Goldsmith D, Ruiz C, et al. Adynamic Bone Disease: From Bone to Vessels in Chronic Kidney Disease. Seminars in nephrology. 2014;34:626–640. doi: 10.1016/j.semnephrol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Chen YW, Peng Y, Foley RN, St Peter WL. Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:602–611. doi: 10.1053/j.ajkd.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Akaberi S, Clyne N, Sterner G, Rippe B, Reihner E, et al. Temporal trends and risk factors for parathyroidectomy in the Swedish dialysis and transplant population – a nationwide, population-based study 1991 – 2009. BMC nephrology. 2014;15:75. doi: 10.1186/1471-2369-15-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng SP, Lee JJ, Liu TP, Yang TL, Chen HH, et al. Parathyroidectomy improves symptomatology and quality of life in patients with secondary hyperparathyroidism. Surgery. 2014;155:320–328. doi: 10.1016/j.surg.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Annals of surgery. 2009;250:618–630. doi: 10.1097/SLA.0b013e3181b76d2b. [DOI] [PubMed] [Google Scholar]


