Abstract
Introduction
It is well established that sarcopenic patients are at higher risk of postoperative complications and short-term healthcare utilization. Less well understood is how these patients fare over the long-term after surviving the immediate postoperative period. We explored costs over the first postoperative year among sarcopenic patients.
Methods
We identified 1,279 patients in the Michigan Surgical Quality Collaborative database who underwent inpatient elective surgery at a single institution from 2006 to 2011. Sarcopenia, defined by gender-stratified tertiles of lean psoas area, was determined from preoperative CT scans using validated analytic morphomics. Data were analyzed to assess sarcopenia’s relationship to costs, readmissions, discharge location, intensive care unit admissions, hospital length of stay, and mortality. Multivariate models adjusted for patient demographics and surgical risk factors.
Results
Sarcopenia was independently associated with increased adjusted costs at 30, 90, and 180 but not 365 days. The difference in adjusted postsurgical costs between sarcopenic and non-sarcopenic patients was $16,455 at 30 days and $14,093 at one year. Sarcopenic patients were more likely to be discharged somewhere other than home (p <0.001). Sarcopenia was not an independent predictor of increased readmission rates in the postsurgical year.
Conclusion
The effects of sarcopenia on healthcare costs are concentrated in the immediate postoperative period. It may be appropriate to allocate additional resources to sarcopenic patients in the perioperative setting to reduce the incidence of negative postoperative outcomes.
Keywords: sarcopenia, morphomics, cost, discharge disposition, readmissions, healthcare utilization
2. Introduction
Sarcopenia, the age-related degeneration of skeletal muscle mass and function, is increasingly recognized as a burgeoning public health problem. It is associated with increased short-term morbidity and mortality across a wide range of procedures.1,2 While rates of sarcopenia increase in tandem with age, sarcopenia is more predictive of postoperative mortality and length of stay than chronologic age alone.3,4 There is also evidence that sarcopenia can occur rapidly following acute illness and that rate of skeletal muscle mass change is itself predictive of poorer outcomes.5,6 Furthermore, it has long been known that the surgical event is itself a stressor that can strongly negatively impact body composition and that this effect can prolong recovery time.7
Protein depletion is a defining trait among sarcopenic patients and is a key factor contributing to their morbidity. These patients have increased incidence of major complications, including pneumonia and decreased wound healing.8–10 The protein depletion increases the metabolic stress and impairs the immune system, limiting its capacity to respond to insults.11–13 Based on these findings, it is intuitive that perioperative interventions have been considered for sarcopenic patients. Intensive nutrition interventions have been investigated in this context and produced mixed results.14,15 Testosterone and growth hormone replacement aimed at increasing muscle volume and quality have not shown advantage and were associated with adverse effects.16 However, relatively simple resistance training and walking exercises have shown efficacy.16–18 Importantly, exercise addresses the decreased respiratory capacity that is common among sarcopenic patients.19,20 Recent perioperative optimization programs emphasizing these activities demonstrate promising results.21–23 It is important that these programs be considered in the context of cost. More specifically, understanding the cost drivers of care for these at-risk patients will inform targeted and effective clinical interventions.24,25
Within this context, the purpose of this study was to explore the relationship between sarcopenia and postoperative in-hospital costs through and beyond the immediate postoperative hospital stay. To provide further insight into these costs, we also assessed several outcomes known to drive higher costs: length of stay, intensive care unit (ICU) admissions, discharge location, and readmissions. We hypothesized that sarcopenic patients accumulate higher in-hospital costs than their non-sarcopenic counterparts through a variety of related mechanisms. We expected this difference to be visible and significant by the end of the index hospital stay, and for this difference to persist throughout the first year after surgery.
3. Methods
3.1 Patient Population and Outcomes
We used data from the Michigan Surgical Quality Collaborative (MSQC) clinical registry to identify patients undergoing general surgery at a single institution between 2006 and 2011.26,27 MSQC is a prospectively maintained surgical quality improvement database funded by Blue Cross and Blue Shield of Michigan. This database uses standard data definitions and collection protocols of the American College of Surgeons National Surgical Quality Improvement Programs. All patients identified underwent elective inpatient operations that required at least a 23-hour inpatient observation period. Clinical variables were downloaded from the MSQC database and were supplemented with readmission and discharge disposition data by a review of electronic medical records at the study institution. Clinical data points collected included patient demographic information, preoperative comorbidities, and postoperative morbidity and mortality. Surgical procedures were classified by primary organ of interest and diagnosis into the following categories: appendix, biliary, colorectal/large bowel, endocrine, gastric, hepatic, hernia, pancreas, small bowel, vascular, and other. An internal cost-accounting database at the study institution was used to determine inpatient and outpatient financial data, which were collected from the day of operation to 365 days postoperatively. Financial data were limited to those fees incurred within the institution’s system and were adjusted to account for inflation. Cumulative in-hospital costs were evaluated at 30, 90, 180, and 365 days postoperatively. There were 8,605 unique patients in our database between 2006 and 2011. Of these patients 6,648 had preoperative CT scans, and 3,469 of those were within 90 days of their operative date. 2,179 of these patients were excluded because they were either outpatient or emergent procedures. During review of the CT scans an additional 11 patients were excluded for poor image quality in the lumbar region, yielding a final cohort of 1,279 patients. Cost data were available for all of these patients.
3.2 Analytic Morphomics
Analytic morphomic measurements were performed in a semi-automated manner on collected CT scans using proprietary algorithms programmed in MATLABv13.0 (MathWorks, Natick, MA) according to established methods.28 Briefly, the spinal column vertebral levels were mapped and then cross-sectional area of the left and right psoas muscles at the inferior aspect of the fourth lumbar vertebrae were summed to give the total psoas area. This was then adjusted for fatty infiltration of the muscle using density, measured in Hounsfield units, to yield lean psoas area (LPA).29
3.3 Statistical Analysis
Descriptive statistics were computed for clinical variables, continuous variables were summarized by mean and standard deviation, and categorical variables were summarized by frequency of observation. Tests of significance were performed using Student’s t-tests or Wilcoxon rank sum tests for continuous variables and Fisher’s exact tests and chi-squared tests for categorical variables, where appropriate. LPA measurements were made categorical, and patients were grouped into gender-standardized tertiles of LPA: sarcopenic (small), average (medium), and non-sarcopenic (large). Multivariate analysis was used to assess the risk-adjusted impact of sarcopenia on in-hospital costs and outcomes by controlling for clinical and operative characteristics. Operative time and work relative value units (wRVU) were used to control for operative complexity and case mix disparity among tertiles. First, univariate logistic and linear regression was performed to identify variables appropriate for input into multivariate models. Selected variables were entered into the multivariate models in a stepwise-backward fashion. To account for the non-normal distribution of cost outcomes, these variables were log-transformed before regression analysis. In one case when log transformation was inadequate to achieve normality, a Box-Cox transformation was used instead. Patients who were deceased before a given cost evaluation time point were not included in that cost analysis. All statistical analyses were performed using Stata v13.0 (StataCorp, College Station, TX). A significance level of α=0.05 was used for all significance tests. This study was approved by the University of Michigan Institutional Review Board.
4. Results
4.1 Patient Demographics and Clinical Characteristics
There were 1,279 patients who met the study criteria. Descriptive statistics are included in Table 1. LPA was normally distributed for both males (2,169.0 ± 680.6 mm2) and females (1,400.3 ± 425.5 mm2), and the difference in mean LPA between genders was statistically significant (p <0.001). Sarcopenic patients were older, with a lower body mass index, and were more likely to have dyspnea, diabetes, hypertension, peripheral vascular disease, and non-independent functional status compared to non-sarcopenic patients. Case mix differed significantly among patients in the lowest, middle, and highest tertiles of LPA (p <0.001). Sarcopenic patients underwent a greater number of biliary, large bowel, small bowel, and vascular procedures, while non-sarcopenic patients underwent a greater number of appendectomy, hernia repair, hepatic, and other procedures.
Table 1.
Patient demographics and clinical characteristics by lean psoas area.
| Patient characteristics | Small (sarcopenic) lean psoas area, mean ± SD or % |
Medium (average) lean psoas area, mean ± SD or % |
Large (non-sarcopenic) lean psoas area, mean ± SD or % |
p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 66.1 ± 14.0 | 60.3 ± 14.0 | 49.8 ± 13.9 | <0.001 |
| Male gender | 51.3 | 51.2 | 51.2 | 1.0 |
| Body mass index (kg/m2) | 26.0 ± 6.3 | 27.6 ± 6.2 | 31.3 ± 8.7 | <0.001 |
| Clinical characteristics | ||||
| Serum albumin (g/dL) | 3.6 ± 0.7 | 4.0 ± 0.6 | 4.2 ± 0.6 | <0.001 |
| Disseminated cancer | 6.3 | 7.3 | 6.6 | 0.847 |
| Diabetes mellitus | 32.6 | 22.8 | 16.0 | <0.001 |
| Dyspnea | 16.6 | 8.7 | 7.5 | <0.001 |
| Hypertension | 59.0 | 48.4 | 40.1 | <0.001 |
| Independent functional status | 86.2 | 95.1 | 97.7 | <0.001 |
| Peripheral vascular disease | 9.1 | 5.4 | 3.5 | 0.002 |
| Smoker | 18.7 | 16.0 | 19.3 | 0.405 |
4.2 Complications, Length of Stay, and Mortality
The study population had a postoperative complication rate of 24.2%. The unadjusted rate of complications was significantly higher in sarcopenic patients compared to non-sarcopenic patients (33.3% vs. 17.6%, p <0.001). Sarcopenic patients were more likely to be admitted to the ICU postoperatively (18.5%) than non-sarcopenic patients (9.6%, p <0.001). When controlled for patient and procedural covariates, sarcopenia was a significant independent predictor of postoperative ICU admission (OR=2.24, CI 1.38–3.64, p <0.001). Sarcopenic patients also had a significantly longer unadjusted median length of stay (8 days) than non-sarcopenic patients (6 days, p <0.001), and this effect remained significant on multivariate analysis (p <0.001). The unadjusted postoperative mortality rate was significantly higher in sarcopenic than non-sarcopenic patients (16.2% vs 3.8%, p <0.001). On multivariate analysis, sarcopenia remained a strong independent predictor of one-year mortality (OR=3.26, CI 1.73–6.15, p <0.001). Multivariate analysis tables are included as supplemental tables.
4.3 Discharge Location & Readmissions
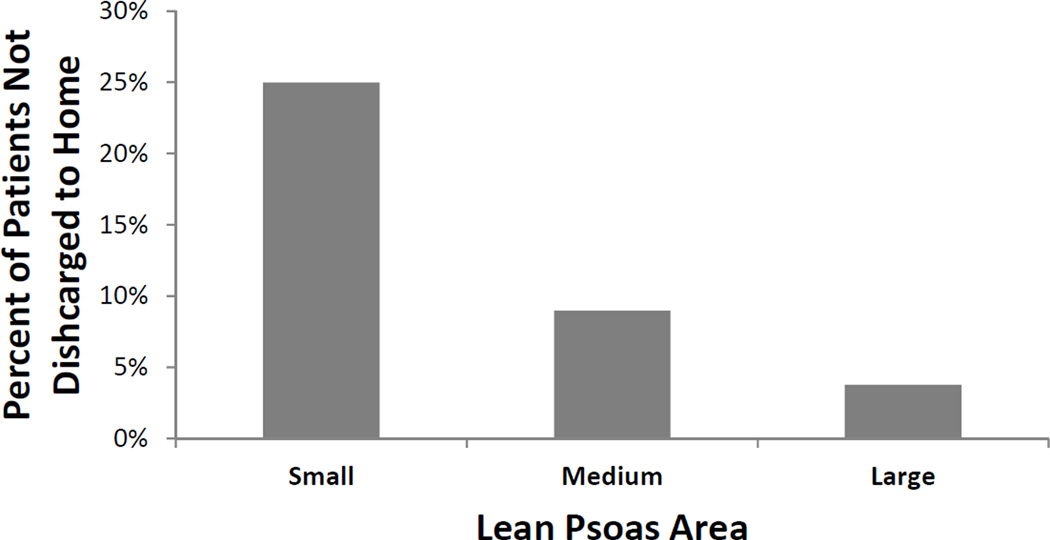
Discharge location varied widely relative to LPA. Unadjusted rates of discharge to somewhere other than home ranged from 3.8% in non-sarcopenic patients to 25.3% in sarcopenic patients (p <0.001). After adjustment for covariates, sarcopenic patients were still significantly more likely to be discharged to a non-home location compared to other patients (Fig 1, OR=4.42, CI=2.28–8.55, p <0.001). Readmission rates during the postoperative year did not vary significantly between sarcopenic and non-sarcopenic patients (40.7% vs. 35.5%, p=0.111), nor was sarcopenia a significant predictor of readmission in multivariate models (p=0.701).
Figure 1.
Adjusted rates of discharge to somewhere other than home across tertiles of lean psoas area. Small (sarcopenic) lean psoas area patients had a significantly greater percentage of non-home discharges compared to large (non-sarcopenic) lean psoas area patients.
4.4 In-Hospital Costs
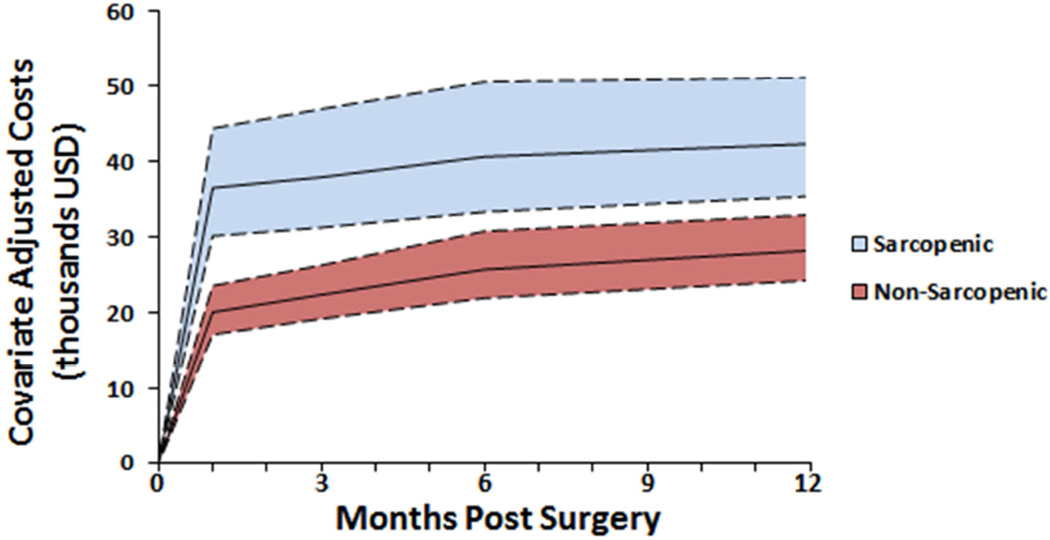
Mean unadjusted in-hospital costs at one year were significantly higher in sarcopenic ($67,525) compared to non-sarcopenic patients ($39,720, p <0.001). The same trends were observed at 30, 90, and 180 day time points (p <0.001 for all). After risk adjustment, sarcopenic status was independently associated with increased costs at 30, 90, and 180 days but not at 365 days (p <0.001, p <0.001, p=0.012, p=0.053 respectively). Continuous LPA was a significant independent predictor of increased costs at all time points. After adjustment for covariates, the difference between sarcopenic and non-sarcopenic patient costs was $16,455 at 30 days and decreased to $14,093 at one year (Fig 2).
Figure 2.
Adjusted costs in sarcopenic and non-sarcopenic patients through the postoperative year (mean with 95% CI). Sarcopenic status was independently associated with increased costs at 1, 3, and 6 months but not at 1 year.
5. Discussion
This study shows that sarcopenia is primarily a predictor of short-term in-hospital costs following surgery, not longer-term costs of care. The largest difference in costs is noted at 30 days after surgery; thereafter, the difference in costs between sarcopenic and non-sarcopenic patients is modest. While the difference in costs at later time points is technically statistically significant it seems that this significance is actually a reflection of the differences accumulated within the first 30 days. The drivers of cost during the index hospital stay include significantly longer hospital stays and increased ICU admission rates. Surprisingly, sarcopenic patients had similar readmission rates as non-sarcopenic patients. In all, efforts to improve the care of sarcopenic patients and reduce costs should focus on the acute surgical admission.
The difference in discharge disposition (home vs. non-home) found in this study represents a significant source of additional costs that are not captured in our in-hospital cost measurements. Medicare reimbursement for skilled nursing facility care can be as high as $3,100 per week.30 This is particularly notable because the odds of non-home discharge were over four-fold higher for sarcopenic patients in this study as compared to non-sarcopenic patients. As an illustration, if we assumed a two week stay in a skilled nursing facility at $3,100 per week for non-home discharges within our study population, there would be an additional $130,000 per 100 patients cost accumulated by sarcopenic patients compared to non-sarcopenic patients. This represents a significant limitation of this analysis.
Taken together these data provide strong evidence that sarcopenia is a robust predictor of negative postoperative outcomes and their consequent in-hospital costs. Furthermore, the longitudinal nature of these data indicate that focusing on costs in the immediate postoperative period as well as reducing the number of discharges to rehabilitation and skilled nursing facilities may yield important decreases in costs over the short and long term. As a potentially remediable variable, sarcopenia appears to be an attractive target to improve surgical care. The cause and effect relationship of sarcopenia, comorbid disease burden, and postoperative complications is a complex one that will require more prospective work to thoroughly understand. However, sarcopenia appears to be an effective way to identify high-risk surgical patients who are candidates for clinical intervention. Targeted interventions to train patients and optimize their nutrition status have shown promising results.31–33 Data such as those detailed in this manuscript help craft a business case for interventions in these high-risk patients. Initial data from the authors’ institution has shown that implementation of a broad perioperative optimization program in these patients appears to decrease costs and length of stay while improving the patient experience.34
There are several important limitations of this study. Only patients with preoperative CT scans were included, which may have selected for patients or procedures with greater morbidity and mortality. Data were not available on preoperative nutritional interventions or muscle function, both of which would have strengthened the analysis. This study was limited to a single institution, and since payer reimbursements are unique to every institution our findings may not be generalizable to other institutions. Furthermore, our institution is a tertiary care center, and it is possible that some readmissions and their associated costs were missed when patients were readmitted to local hospitals. Additionally, because we chose to censor deceased patients in our cost analyses it is possible that differences in mortality lead to differences in costs that are not reflected in our results. Finally, this study is limited by the fact that it is a retrospective single institution study.
6. Conclusions
Overall this study builds on a body of evidence implicating sarcopenia as a strong predictor of postoperative morbidity, mortality, and costs. We evaluated the effects of sarcopenia over a full year after surgery, showing that the effects of sarcopenia on in-hospital costs are concentrated in the immediate postoperative period. Incorporating analytic morphomics into preoperative evaluation in the future may decrease costs by improving patient selection for surgery and enabling targeted interventions to decrease negative outcomes.
Supplementary Material
Acknowledgments
Disclosures:
SCW and MJE are cofounders of Prehab Technologies, LLC. This company focuses on perioperative optimization of surgical patients. PSK was supported by a grant from the National Institutes of Health under award number T35HL007690.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Study conception and design: Kirk, Cron, Terjimanian, Wang, Campbell, Englesbe
Acquisition of data: Kirk, Friedman, Cron
Analysis and interpretation of data: Kirk, Friedman, Cron, Terjimanian, Werner, Englesbe
Drafting of manuscript: Kirk, Friedman, Werner
Critical revision: Cron, Terjimanian, Wang, Campbell, Englesbe
References
- 1.Zembroń-Łacny A, Dziubek W, Rogowski Ł, Skorupka E, Dąbrowska G, Polskiego AW. Sarcopenia: monitoring, molecular mechanisms, and physical intervention. Physiological research/Academia Scientiarum Bohemoslovaca. 2014;63(6):683–691. doi: 10.33549/physiolres.932692. [DOI] [PubMed] [Google Scholar]
- 2.Kim JH, Lim S, Choi SH, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014;69(10):1244–1252. doi: 10.1093/gerona/glu050. [DOI] [PubMed] [Google Scholar]
- 3.Englesbe MJ, Terjimanian MN, Lee JS, et al. Morphometric age and surgical risk. Journal of the American College of Surgeons. 2013;216(5):976–985. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waits SA, Kim EK, Terjimanian MN, et al. Morphometric age and mortality after liver transplant. JAMA surgery. 2014;149(4):335–340. doi: 10.1001/jamasurg.2013.4823. [DOI] [PubMed] [Google Scholar]
- 5.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Annals of surgery. 2012;256(2):255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 6.Zarinsefat A, Terjimanian MN, Sheetz KH, et al. Perioperative changes in trunk musculature and postoperative outcomes. Journal of Surgical Research. 2014;191(1):106–112. doi: 10.1016/j.jss.2014.03.056. [DOI] [PubMed] [Google Scholar]
- 7.Mathur S, Plank LD, Hill AG, Rice MA, Hill GL. Changes in body composition, muscle function and energy expenditure after radical cystectomy. BJU international. 2008;101(8):973–977. doi: 10.1111/j.1464-410X.2007.07337.x. [DOI] [PubMed] [Google Scholar]
- 8.Windsor JA, Hill GL. Protein depletion and surgical risk. Australian and New Zealand Journal of Surgery. 1988;58(9):711–715. doi: 10.1111/j.1445-2197.1988.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 9.Windsor JA, Hill GL. Risk factors for postoperative pneumonia. The importance of protein depletion. Annals of surgery. 1988;208(2):209. doi: 10.1097/00000658-198808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haydock DA, Hill GL. Impaired wound healing in surgical patients with varying degrees of malnutrition. Journal of Parenteral and Enteral Nutrition. 1986;10(6):550–554. doi: 10.1177/0148607186010006550. [DOI] [PubMed] [Google Scholar]
- 11.Hill G. Impact of nutritional support on the clinical outcome of the surgical patient. Clinical Nutrition. 1994;13(6):331–340. doi: 10.1016/0261-5614(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 12.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. The American journal of clinical nutrition. 1995;62(1):30–39. doi: 10.1093/ajcn/62.1.30. [DOI] [PubMed] [Google Scholar]
- 13.Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Molecular aspects of medicine. 2005;26(3):181–201. doi: 10.1016/j.mam.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Solerte SB, Gazzaruso C, Bonacasa R, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. The American journal of cardiology. 2008;101(11):S69–S77. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Campbell WW. Synergistic use of higher-protein diets or nutritional supplements with resistance training to counter sarcopenia. Nutrition reviews. 2007;65(9):416–422. doi: 10.1111/j.1753-4887.2007.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 16.Borst SE. Interventions for sarcopenia and muscle weakness in older people. Age and ageing. 2004;33(6):548–555. doi: 10.1093/ageing/afh201. [DOI] [PubMed] [Google Scholar]
- 17.Rydwik E, Lammes E, Frandin K, Akner G. Effects of a physical and nutritional intervention program for frail elderly people over age 75. A randomized controlled pilot treatment trial. Aging clinical and experimental research. 2008;20(2):159–170. doi: 10.1007/BF03324763. [DOI] [PubMed] [Google Scholar]
- 18.Pahor M. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2006 doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 19.Pahor M, Kritchevsky S. Research hypotheses on muscle wasting, aging, loss of function and disability. The journal of nutrition, health & aging. 1997;2(2):97–100. [PubMed] [Google Scholar]
- 20.Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. Journal of aging research. 2011 doi: 10.4061/2011/569194. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CK, Leng X, Hsu F-C, et al. The impact of sarcopenia on a physical activity intervention: the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P) The journal of nutrition, health & aging. 2014;18(1):59–64. doi: 10.1007/s12603-013-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Carli F, Lee L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surgical endoscopy. 2013;27(4):1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 23.Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 24.Sheetz KH, Waits SA, Terjimanian MN, et al. Cost of major surgery in the sarcopenic patient. Journal of the American College of Surgeons. 2013;217(5):813–818. doi: 10.1016/j.jamcollsurg.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG Group ESA. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156(3):521–527. doi: 10.1016/j.surg.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Campbell DA, Kubus JJ, Henke PK, Hutton M, Englesbe MJ. The Michigan surgical quality collaborative: a legacy of Shukri Khuri. The American Journal of Surgery. 2009;198(5):S49–S55. doi: 10.1016/j.amjsurg.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Campbell DA, Englesbe MJ, Kubus JJ, et al. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Archives of Surgery. 2010;145(10):985–991. doi: 10.1001/archsurg.2010.220. [DOI] [PubMed] [Google Scholar]
- 28.Lee JS-J, He K, Harbaugh CM, et al. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. Journal of vascular surgery. 2011;53(4):912–917. doi: 10.1016/j.jvs.2010.10.111. [DOI] [PubMed] [Google Scholar]
- 29.Parenteau CS, Zhang P, Holcombe S, Kohoyda-Inglis C, Wang SC. Can anatomical morphomic variables help predict abdominal injury rates in frontal vehicle crashes? Traffic injury prevention. 2014;15(6):619–626. doi: 10.1080/15389588.2013.852665. [DOI] [PubMed] [Google Scholar]
- 30.Sharareh B, Le NB, Hoang MT, Schwarzkopf R. Factors Determining Discharge Destination for Patients Undergoing Total Joint Arthroplasty. The Journal of arthroplasty. 2014;29(7):1355–1358. e1351. doi: 10.1016/j.arth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Valkenet K, van de Port IG, Dronkers JJ, de Vries WR, Lindeman E, Backx FJ. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clinical rehabilitation. 2011;25(2):99–111. doi: 10.1177/0269215510380830. [DOI] [PubMed] [Google Scholar]
- 32.Hoogeboom TJ, Dronkers JJ, van den Ende CH, Oosting E, Van Meeteren NL. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clinical rehabilitation. 2010 doi: 10.1177/0269215510371427. [DOI] [PubMed] [Google Scholar]
- 33.Dronkers J, Lamberts H, Reutelingsperger I, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clinical rehabilitation. 2010;24(7):614–622. doi: 10.1177/0269215509358941. [DOI] [PubMed] [Google Scholar]
- 34.Friedman J, Lussiez A, Sullivan J, Wang S, Englesbe M. Implications of Sarcopenia in Major Surgery. Nutrition in Clinical Practice. 2015:0884533615569888. doi: 10.1177/0884533615569888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.