Abstract
Introduction
No medical therapies are yet available to slow abdominal aortic aneurysm (AAA) growth. This study sought to investigate the effect of different genders of bone marrow derived mesenchymal stem cells (MSC) on AAA growth in a murine AAA model. Given the decreased rate of AAA in women, it is hypothesized that female MSC would attenuate AAA growth more so than male MSC.
Materials and Methods
Aortas of 8- to 10-week-old male C57Bl/6 mice were perfused with purified porcine pancreatic elastase to induce AAA formation. Bone marrow-derived MSC from male and female mice were dosed via tail vein injection (3 million cells per dose, 500 μL of volume per injection) on post-aortic perfusion days 1, 3, and 5. Aortas were harvested after 14 days.
Results
Mean aortic dilation in the elastase group was 121±5.2% (mean±SEM), while male MSC inhibited AAA growth (87.8±6.9%, P=0.008) compared to elastase. Female MSC showed the most marked attenuation of AAA growth (75.2±8.3% P= 0.0004). Pro-inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and macrophage chemotactic protein-1 (MCP-1) were only decreased in tissues treated with female MSC (p=0.017, p=0.001, and p<0.0001, respectively when compared to elastase).
Conclusions
These data exhibit that female MSC more strongly attenuate AAA growth in the murine model. Furthermore, female MSC and male MSC inhibit proinflammatory cytokines at varying levels. The effects of MSC on aortic tissue offer a promising insight into biologic therapies for future medical treatment of AAAs in humans.
Keywords: Aneurysm, Stem Cell, Gender
1. Introduction
Abdominal aortic aneurysms (AAAs) are characterized by a dilation of the abdominal aorta 150% of its normal diameter and are primarily a disease of elderly male smokers, affecting nearly 7% of the older population (1–3). The natural history of this disease is progressive dilation and eventual rupture, which carries extremely high mortality and morbidity. This disease was primarily responsible for roughly 10,500 deaths in the United States in 2009, and is the 15th leading cause of death in the United States (4). Therefore, medical therapies to treat this deadly disease are necessary for the aging western populations. AAAs are characterized by inflammation involving an elevation of pro-inflammatory cytokines, elastin and collagen disruption, and smooth muscle cell apoptosis, but the exact pathogenic mechanism of AAAs is not well defined (5–7).
Mesenchymal stem cells (MSC) are an emerging medical therapy for multiple disease states. Experimental applications of MSC have reached into treatments for spinal cord injury, acute kidney injury, liver failure, and regeneration of cardiomyocytes post-myocardial infarction (8–10). Cardiac regenerative therapies have branched over into human treatments, as there are a number of trials evaluating the utility of MSC myocardial regeneration for ischemic cardiomyopathy (10). Therapies with MSC have been explored previously in experimental animal models of AAA and have shown beneficial effects on aneurysm formation (11–13). Additionally, reports exist which reveal superior efficacy of female MSC when utilized as therapy for other disease states (14, 15), However, there is a paucity of literature describing the effects of varying genders of stem cells on AAAs. Given the existing trends in gender difference in cardiovascular disease and the increasing frequency of stem cell based therapies, we hypothesized that MSC derived from females will have a more potent effect on experimental AAA formation.
2. Materials and Methods
2.1 Mice
Wild Type (WT) C57Bl/6 were obtained from Jackson Laboratories (The Jackson Laboratory, Bar Harbor, Maine) and maintained in house. Red fluorescent protein (RFP) mice (Strain B6.Cg-Tg(CAG-DsRed*MST)1Nagy/J, Stock Number 006051) were also obtained from Jackson Laboratories (Bar Harbor, Maine) and maintained within our vivarium. All mice were fed minimal phytoestrogen diet (2016 Teklad Diet, Harlan Laboratories, Indianapolis, Indiana). Animal care and use were in accordance with the Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the University of Virginia Institutional Animal Care and Use Committee in compliance with the Office of Laboratory Animal Welfare.
2.2 Aneurysm Model
All mice underwent a surgical model of AAA formation with elastase perfusion as described previously (16, 17). Eight to 12-week old male C57Bl/6 mice underwent induction and maintenance of anesthesia with isoflurane. A midline abdominal incision was created, and the infrarenal aorta was isolated. Proximal and distal control was achieved with 4-0 silk sutures. An aortotomy was created, and a perfusion catheter was inserted into the aorta. The aorta was perfused with 0.35M purified porcine pancreatic elastase or heat-inactivated elastase for 5 minutes. After the allotted time the elastase was evacuated, the aortotomy site was closed, and distal perfusion was restored by untying the proximal and distal silk sutures. The abdomen was closed with 6-0 absorbable polyglactin suture, and the skin was closed with a 6-0 polypropylene suture. The mouse was given buprenorphine for postoperative analgesia. After 14 days, the mouse underwent reinduction of anesthesia and reoperation. The midline was reopened and the aorta was exposed. Video micrometry was used to determine the size ratio between the largest segment of perfused aorta and un-perfused native segment of aorta above above the indentation representing the location of the proximal tie from the perfusion procedure. The aorta was removed and flushed with saline. Aortas were either: 1) snap frozen in liquid nitrogen for real-time polymerase chain reaction (PCR) analysis or protein extraction or 2) incubated overnight in 4% paraformaldehyde solution for immunohistochemistry. Additionally, tissue samples from livers and lungs were procured from the animals after removal of the aorta. These samples were incubated overnight in 4% paraformaldehyde solution for immunohistochemistry.
2.3 Mesenchymal Stem Cell Isolation and Delivery
Mouse mesenchymal stem cell isolation and culture was based on a modified method of the original protocol as described by Guo et al (18) and described in detail by Zhu (19). Namely, mice aged 2–3 weeks underwent euthanasia via isofluorane inhalation and cervical dislocation. The femurs and tibiae were isolated and flushed twice with saline to release the hematopoietic cells. The compact bones were then dissected into 1–3 mm fragments and digested with collagenase II in alpha-MEM with 10% FBS (1 mg/mL by volume, Sigma-Aldrich, St. Luis, MO). Two hours following the digestion, the collagenase II was washed away and the bone fragments were then cultured in MSC culture medium, alpha-MEM with 10% FBS. Media was changed three days following seeding and by 5 days following seeding cultures reached 70–80% confluency. These cultures are available for use from passages 3–8.
Bone marrow-derived MSC were delivered via tail vein injection with 3 million cells per dose at a volume of 500 μL in phosphate-buffered saline. Conditioned media (500 μL) or MSC treatments were given on postoperative days 1, 3, and 5. Finasteride-primed MSC were given in the same manner.
2.4 Treatment Arms
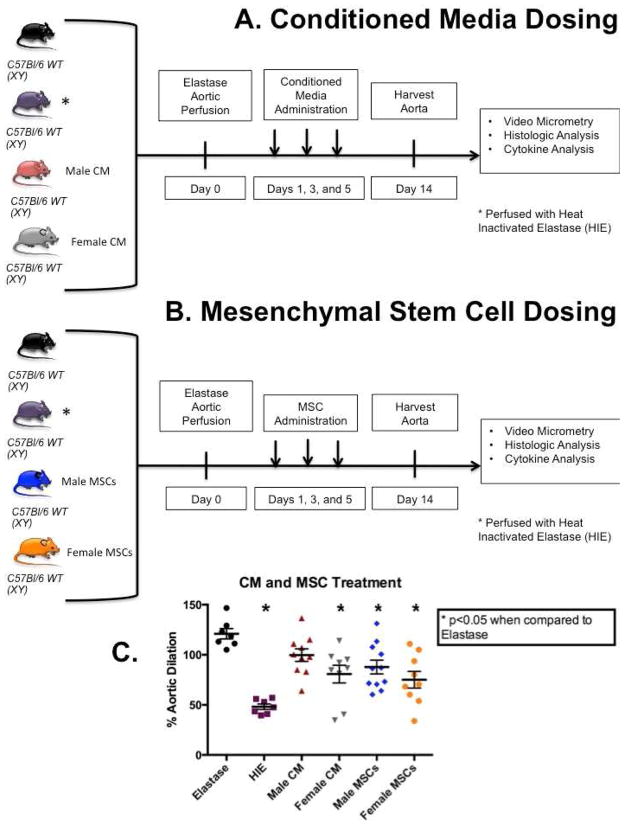
Aortas of 8–12 week old male WT mice were perfused with porcine pancreatic elastase as described above (n=7). Heat inactivated elastase (HIE) was used as a negative control (n=7). These two groups were used as controls for each experimental arm. Two groups of male WT mice were given either conditioned media from male MSC culture (n=10) or conditioned media from female MSC culture (n=9) on postoperative days 1, 3, and 5 after aortic perfusion with elastase [Figure 1A], and a separate treatment arm was given either male MSC (n=11) or female MSC (n=9) in similar fashion [Figure 1B]. Additional arms were performed which WT mice underwent elastase perfusion and received cells derived from male and female RFP mice. These mice were harvested on days 3, 7, and 14 (n=5 per group per gender) in order to utilize confocal microscopy to evaluate the location of MSC in different tissues during multiple progressive time points [Figures 2 and 3]. In separate experiments, male or female MSC were primed with finasteride, a 5-alpha reductase inhibitor (50 μg/mL, Sigma Aldrich, St. Louis, MO) for 48 hours before injection of male or female MSC into the elastase perfused WT male mice. These treated MSC were given days 1, 3, and 5 post-operatively and mice were harvested 14 days following aneurysm formation [Figure 4].
Figure 1.
Scheme of treatment arms. A) Conditioned media treatment arm (male CM n=10, female CM n=9) B) MSC treatment arm (male MSC n=11, female MSC n=9). C) Aortic diameter measured with video micrometry of mice treated with male and female conditioned media and male and female MSC. The same elastase and heat-inactivated elastase (HIE) controls were used for both treatment arms. Female MSC-derived conditioned media, but not male MSC-derived CM had a significant attenuation of AAA formation compared to elastase (p=0.0013). Male MSC had significant attenuation of AAA formation compared to elastase (p=0.008), while female MSC exhibited more significant attenuation of AAA compared to elastase (p=0.0004).
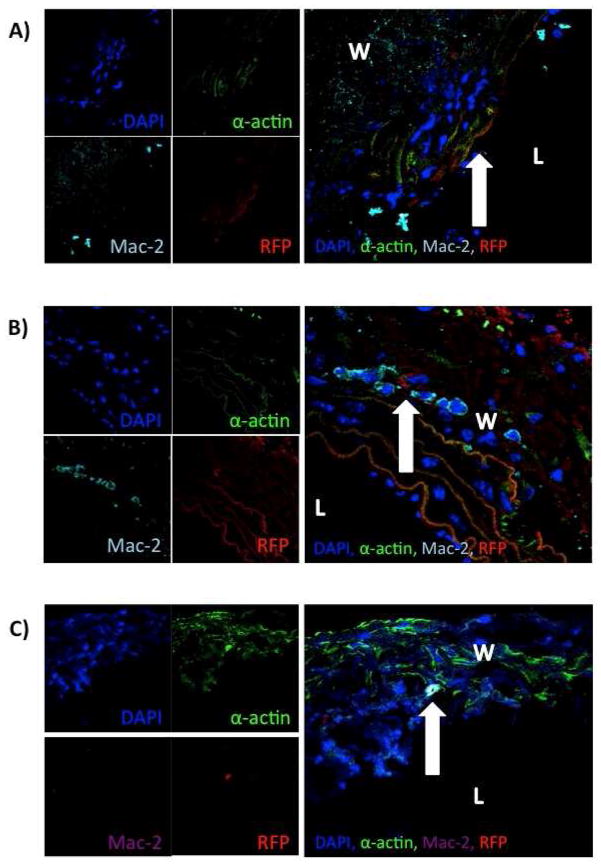
Figure 2.
Confocal microscopy reveals integration of male MSC into aortic wall. A) Convergence of channels of confocal microscopy revealing integration of male MSC into the aortic wall at (arrow) day 3. B) Convergence of channels of confocal microscopy revealing integration of male MSC into the aortic wall (arrow) on day 7. C) Convergence of channels of confocal microscopy revealing integration of male MSC into the aortic wall (arrow) on day 14 (L=lumen, W=wall).
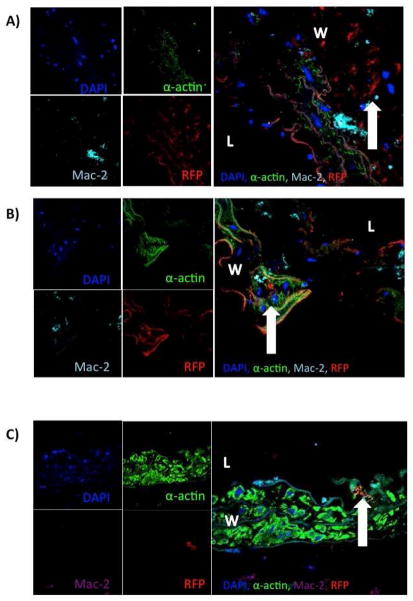
Figure 3.
Confocal microscopy reveals integration of female MSC into aortic wall at multiple time points. A) Convergence of channels of confocal microscopy revealing integration of female MSC into the aortic wall at (arrow) day 3. B) Convergence of channels of confocal microscopy revealing integration of female MSC into the aortic wall (arrow) on day 7. C) Convergence of channels of confocal microscopy revealing integration of female MSC into the aortic wall (arrow) on day 14 (L=lumen).
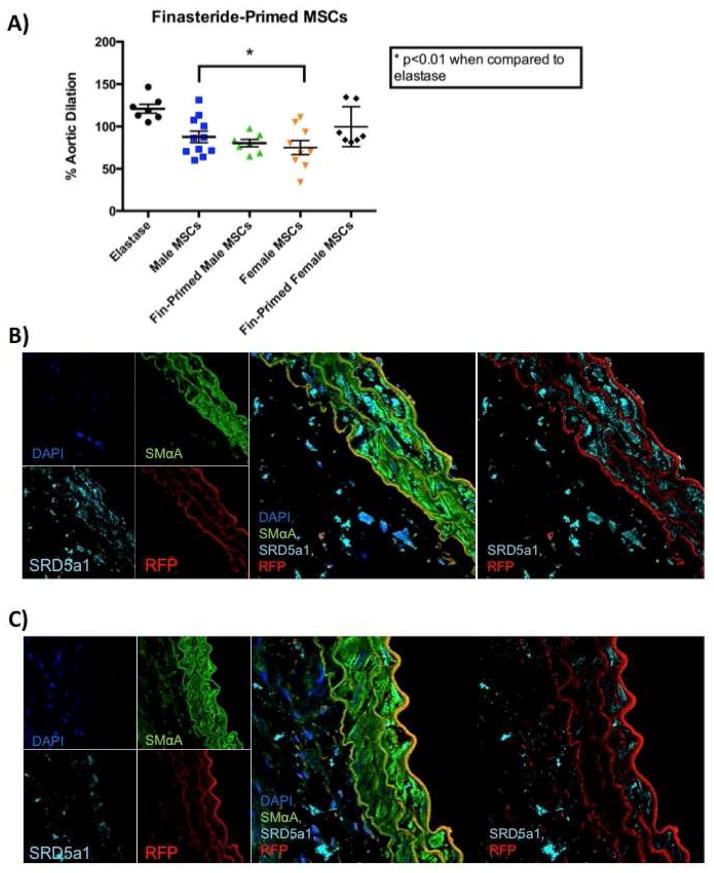
Figure 4.
Finasteride-primed male MSC more significantly inhibit AAA formation. A) Aortic dilation data of MSC and finasteride-primed MSC. B) Confocal microscopy revealing convergence of channels with tissues treated with finasteride-primed male MSC and stained for DNA (blue), smooth muscle cells (green), 5-alpha-reductase (teal), and RFP cells (red). C) Confocal microscopy revealing convergence of channels with tissues treated with female MSC and stained for DNA (blue), smooth muscle cells (green), 5-alpha-reductase (teal), and RFP cells (red).
2.5 Cytokine Array
For the purpose of determining the effects of stem cell application and its effect on the pro-inflammatory cytokine mileu of the aortic wall, mouse cytokine array (R&D Systems, Minneapolis, MN, Mouse Cytokine Antibody Array, Panel A) were performed using isolated protein from mouse aortas harvested at day 14 after elastase perfusion. Protein samples from each group were pooled for analysis, and all samples were run in duplicate (16, 17, 20, 21).
2.6 Histology
Infrarenal aortas were harvested at euthanasia and irrigated with normal saline. Fixation was achieved with overnight incubation in 4% paraformaldehyde followed by paraffin embedding and sectioning at 5μm. Verhoeff-Van Gieson stain was used to evaluate aortic elastin content. Microwave antigen retrieval was performed and antibodies were bound and detected using VectaStain Elite Kit (Vector Laboratories, Burlingame, CA). Antibodies used for immunohistochemistry were anti-mouse CD3 for CD3+ T-cells (1:500; Santa Cruz Biotechnology Inc, Santa Cruz, CA) and anti-rat Mac2 for macrophages (1:10 000; Cedarlane Laboratories, Burlington, Canada. Visualization color development was completed using diaminobenzidine (Dako Corporation, Carpinteria, CA) for Mac2. Images were obtained using a Nikon eclipse Ti imaging system. Blinded cell counts were performed and pixel density analysis was performed in order to quantify elastin and cell content within aortic aneurysm tissues.
Additional murine aortic, lung, and liver cross sections were evaluated with confocal fluorescence immunohistochemistry at multiple time points after aneurysm formation with staining for nuclei (DAPI), macrophages (Mac2 antibody, 1:1000) smooth muscle cells (Smooth muscle alpha actin, 1:1000), pneumocytes (Pro-SPC antibody. 1:200), biliary cells (cytokeratin 19 antibody, 1:200), cells containing 5-α-reductase (SRD5a1 antibody, 1:250), and the RFP tag from the RFP labeled MSC to identify MSC located within the aortic wall and adventitia during aneurysm formation. Cells were visualized using an LSM 710 scanning confocal microscope and results are representative of male WT mice receiving male RFP cells and female RFP cells (n=5 per group).
2.7 Statistical Methods
Statistical analysis was performed using GraphPad Prism Version 6.0e software (Graphpad Software, La Jolla, CA). Maximal aortic dilation (%) was calculated as [(maximal aortic diameter)/(internal control diameter) – 1]. The internal control was an unperfused segment of infrarenal aorta cranial to the proximal ligation site. This section was not perfused with elastase; however, it was susceptible to hemodynamic changes from volume shifts during the operative and postoperative phases, as well as expected animal growth over time. Values are reported as mean±SE of the mean (SEM). Aortic dilation between groups was compared using Analysis of Variance (ANOVA), and post hoc analysis was performed using Tukey correction to determine significance of individual comparisons with α=0.05.
3. Results
3.1 Female MSC Attenuate Experimental AAA Formation When Compared to Male MSC
Aortas from male WT mice perfused with elastase revealed significant dilation when compared to heat inactivated elastase (HIE) (p<0.0001). Elastase-perfused WT mice that received conditioned media from female MSC culture had significant attenuation on AAA formation when compared to untreated elastase perfused mice (p<0.0013) [Figure 1C], while male MSC conditioned media failed to inhibit AAA formation. In contrast to conditioned media experiments, male MSC significantly attenuated AAA formation compared to elastase (p=0.0079), though female MSC had a more significant effect on AAA attenuation (p=0.0004) [Figure 1C]. Representative images of immunohistochemistry reveal no difference in elastin preservation or inflammatory cell infiltration was seen between the mice without and with MSC treatment after elastase perfusion [Supplemental Figure 1A].
3.2 Mesenchymal Stem Cells Integrate into the Aortic Wall
Confocal microscopy performed on aorta harvested at 3, 7, and 14 day time points revealed integration of MSC from both male and female RFP+ MSC administered by tail vein injection on postoperative days 1, 3, and 5 after elastase perfusion [Figures 2 and 3]. Both male and female RFP MSC are evident within the aortic wall within each time point. Additionally, MSC of both genders expressing RFP integrated into lung tissue and survived at postoperative days 3 and 7 [Supplemental Figures 3 and 4]. Mesenchymal stem cells expressing RFP were not present in lung or liver tissue at day 14 [Supplemental Figures 5 and 6].
3.3 Treatment of Male Mesenchymal Stem Cells with Finasteride Further Inhibits Experimental AAA Formation
Recent reports in the literature have indicated treating MSC with pharmacologic agents while in culture further enhances therapeutic efficacy (22, 23). As androgens have been shown to play a major role in the pathogenesis of AAAs (24, 25), male MSC were treated with finasteride while in cell culture. Finasteride prevents the conversion of testosterone into the more active dihydrotestosterone (DHT) by inhibiting 5-α-reductase. These cells were given to male WT mice after elastase perfusion on postoperative days 1, 3, and 5 Aortas treated with finasteride-primed male MSC exhibited improved attenuation of aneurysms when compared to elastase (p=0.007) and when compared to unprimed male MSC (p=NS) [Figure 4A]. Interestingly, finasteride-primed female MSC did not significantly attenuate aneurysm formation. Histologic analysis suggests macrophages present in aneurysm tissues contain 5-α-reductase [Supplemental Figure 2]. However, a variation of the concentration of 5-α-reductase exists between tissues treated with finasteride primed male and female cells [Figure 4B and 4C].
3.4 Female Mesenchymal Stem Cells Significantly Attenuate Pro-Inflammatory Cytokine Expression in Aortic Aneurysm Tissue
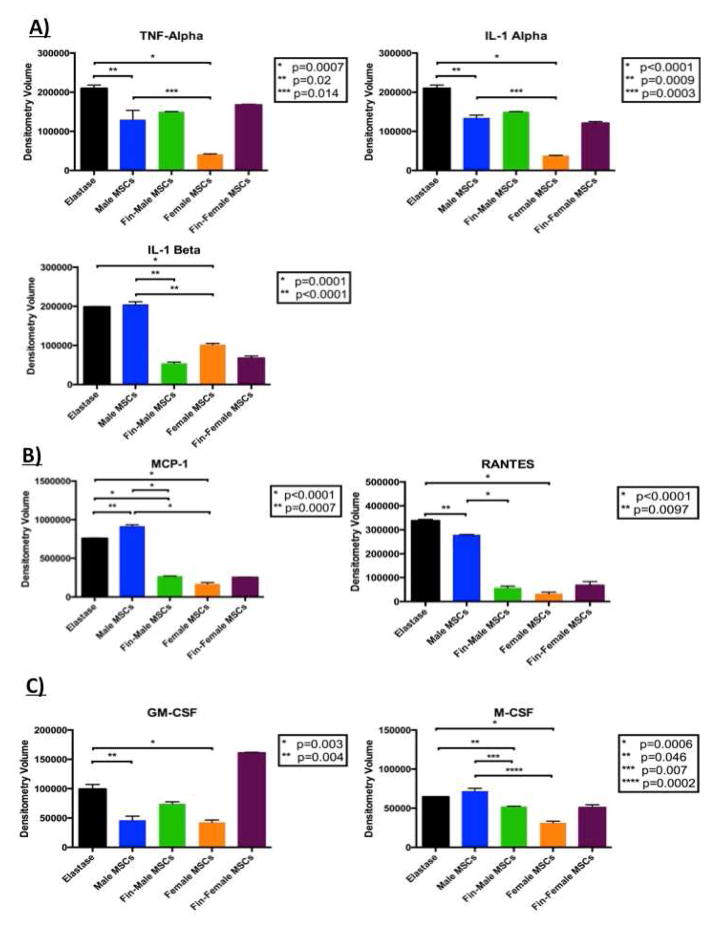
When compared to levels of pro-inflammatory cytokines found in elastase-perfused aortas, mice treated with female MSC exhibited decreased levels of TNF-α, interleukin (IL)-1α, and IL-1β when compared to tissues after treatment with male MSC [Figure 5A]. Aortas treated with female MSC had significantly decreased expression of monocyte chemotactic protein (MCP)-1 and regulated on activation, normal T cell expressed and secreted (RANTES), and male cells exhibited decreased expression of RANTES. [Figure 5B]. Growth factors granulocyte/monocyte - colony stimulating factor (GM-CSF) and macrophage colony stimulating factor (M-CSF) were also significantly decreased in tissues treated with female cells. Male MSC also significantly decreased expression of GM-CSF [Figure 5C].
Figure 5.
Females more potently alter cytokine profiles and finasteride-primed MSC more closely resemble cytokine profiles of female MSC. Cytokine array results for A) pro-inflammatory cytokine, B) chemokines, and C) growth factor profiles present in aortic tissues after treatment with male MSC, finasteride-primed male MSC, or female MSC.
3.5 Treatment of Male Mesenchymal Stem Cells with Finasteride Decreases the Pro-inflammatory Cytokine Milieu
Cytokine arrays were performed on aortic tissue treated with finasteride-primed male MSC and compared with cytokine levels from non-primed MSC. Treatment with finasteride-primed male MSC decreased pro-inflammatory cytokine IL-1β levels when compared to levels of non-primed male MSC. Similarly, tissues treated with finasteride-primed male MSC had decreased expression MCP-1, RANTES, and M-CSF when compared to levels of non-primed MSC [Figure 5].
4. Discussion
The present study documents the beneficial role of MSC in the treatment of experimental AAAs in a murine model, and, furthermore, revealed a difference in the efficacy of varying genders of MSC on AAA formation. Female mesenchymal stem cells have a more potent effect on the attenuation of experimental aneurysm formation and decrease the overall inflammatory profile of aneurysmal aortic tissue. We also demonstrated that both genders of MSC have integrated into the aneurysmal aortic wall at multiple time points. Furthermore, priming male MSC with finasteride, an anti-androgen, potentiates the anti-inflammatory properties of male MSC. This is the first report demonstrating the greater effectiveness of female MSC in the treatment of experimental AAA and demonstrates that priming male stem cells makes them relatively more effective inhibitors of AAA formation.
Mesenchymal stem cells have been shown to have beneficial effects in experimental aneurysm formation and inflammation commonly associated with AAAs. Hashizume and colleagues exhibited attenuation of angiotensin II-induced AAAs in Apolipoprotein E knockout utilizing bone marrow derived MSC (11). This group also co-cultured bone marrow-derived mesenchymal stem cells with macrophages and smooth muscle cells and revealed decreased expression of matrix metalloproteinases (MMPs) and TNF-α in macrophages and preserved elastin content in SMCs when compared to cells cultured without MSC. Our lab used human placenta-derived female MSC in a murine elastase perfusion model of experimental AAA formation and exhibited decreased aneurysm formation and decreased levels of pro-inflammatory cytokines, particularly IL-17 (12). These experiments illustrate the promising anti-inflammatory properties of MSC. Gender differences of MSC with regards to anti-inflammatory and regenerative properties have been previously illustrated in an ischemic myocardium model, though the efficacy of treatment with different genders of has not been applied in AAA models. Treatment of ischemic myocardium with female MSC reveal improved myocardial function and decreased levels of tissue TNF-α and increased levels of vascular endothelial growth factor (VEGF) compared to tissue treated with male MSC or vehicle (14). Similar results were noted when female and male MSC were exposed to lipopolysaccharide and hypoxia (15), which suggests TNF-α expression plays a significant role in the more prominent anti-inflammatory properties of female MSC. However, a definitive mechanism has not been well defined in applications for AAAs.
Mesenchymal stem cells possess an innate ability to hone to areas of tissue injury and inflammation. Localization and integration into injured tissues has been identified in acute kidney injury (26), liver failure (8), and post myocardial infarction (10), though integrative properties of MSC have not been well described in the pathology associated with AAA. The mechanism explaining the ability of MSC to identify areas of injury after systemic administration has not been well defined, though experiments involving MSC application after dermal injury in a murine model suggest complex paracrine signaling involving the activation of macrophages and endothelial progenitor cells (27). Identification of MSC in the aortic wall at multiple time points and up to14 days is encouraging and suggests continued paracrine signaling well after the inflammatory insult has occurred and cells have been administered. Our experiments utilizing confocal microscopy have indicated a predilection for MSC to integrate into lung tissues in addition to inflammatory aortic tissue. Turnbull and colleagues have utilized a large animal model to successfully identify MSC in aortic walls of experimental porcine AAA up to 7 days after AAA formation (13). Additionally, harvested aortas revealed significantly increased capillary density and levels of VEGF (13), further corroborating the findings of the increased expression of VEGF by Crisostomo et al. (14, 15). Despite the evidence of MSC integration into aneurysmal tissues in animal models, the overall pathogenesis and disease progression is different in humans, and it is unclear whether MSC integration will be as evident in human aneurysms.
Pharmacologic priming of mesenchymal stem cells is a novel method of enhancing drug delivery and therapeutic effects of MSC (22, 23). As androgens have been shown to have a detrimental effect on the inflammatory state of the aortic wall and formation of AAA (24, 25), priming MSC with finasteride, an anti-androgen drug commonly used in prostate cancer, exhibits a more significant attenuation of experimental aneurysm, as well as a decrease of the inflammatory profile of aortic tissues, when compared to unprimed male MSC. Finasteride is a potent 5-α-reductase inhibitor, but any anti-inflammatory properties this drug may possess, especially with regard to AAA formation, have not been well documented. Our results demonstrate priming male MSC with finasteride exhibits aneurysm attenutation and cytokine alteration similar to that of female MSC thereby underlining the impact of pharamacological intervention of gender-specific MSC.
5. Conclusions
Gender differences exist in cardiovascular disease as female gender imparts a protective effect against cardiovascular disease. This protective effect is evident in the treatment of experimental aortic aneurysms with molecular therapies utilizing different genders of MSC. We demonstrate female MSC more significantly inhibit aneurysm progression and inflammation in the aortic wall. Furthermore, MSC are present in aortic tissues at 14 days. Finally, priming male MSC with finasteride resulted in therapeutic and anti-inflammatory characteristics similar to those of female MSC. Currently, no definitive medical therapies exist for AAAs, and the application of MSC from differing gender sources offer promise into future biologic therapies for AAAs.
Supplementary Material
Supplemental Figure 1. A) Immunohistochemistry of aortas treated with elastase, heat-inactivated elastase (HIE), male MSC, and female MSC (scale bar = 100 μm). B) Time course of aortic dilation from mice perfused with elastase alone (n=3), male MSC (n=5), and female MSC (n=5). Day 3 exhibited no significant difference between groups; however, day 7 exhibits significantly different aortic dilation between elastase and MSC groups.
Supplemental Figure 2. SRD5a staining in male WT mice at day 14. Convergence channels of confocal microscopy staining for DNA (blue), macrophages (green), 5-alpha reductase (purple), and smooth muscle cells (teal) in a WT mouse aorta at day 14 after induction of aneurysm.
Supplemental Figure 3. Murine lung at day 3 after aneurysm formation, after MSC administration on postoperative day 1, contains RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), pneumocytes (light blue), and mesenchymal stem cells (red). A) Murine lung with evidence of female RFP MSC. B) Murine lung with evidence of male RFP MSC.
Supplemental Figure 4. Murine lung at day 7 after aneurysm formation, after MSC administration on postoperative days 1, 3 and 5, contains RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), pneumocytes (light blue, Pro-SPC), and mesenchymal stem cells (red). A) Murine lung with evidence of female RFP MSC. B) Murine lung with evidence of male RFP MSC.
Supplemental Figure 5. Murine lung at day 14 after aneurysm formation, after MSC administration on postoperative days 1, 3, and 5, does not contain RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), pneumocytes (light blue, Pro-SPC), and mesenchymal stem cells (red). A) Murine lung without evidence of female RFP MSC. B) Murine lung without evidence of male RFP MSC.
Supplemental Figure 6. Murine liver at day 14 after aneurysm formation, after MSC administration on postoperative days 1, 3, and 5, does not contain RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), biliary cytokeratin (green), and mesenchymal stem cells (red). A) Murine liver without evidence of female RFP MSC. B) Murine liver without evidence of male RFP MSC.
Acknowledgments
Funding
This project was supported by Award Number 2T32 HL007849 from the National Heart, Lung, and Blood Institute (NHLBI) (J. Davis, PI: Irving L. Kron, MD), Award Number 2R01 HL081629 from the NHLBI (PI Gilbert R. Upchurch, Jr., MD), and Award Number 14SDG18730000 from the American Heart Association National Scientist Development Award (PI Morgan Salmon, PhD). The content is solely the responsibility of the authors and does not necessarily represent the views of the NHLBI or the AHA.
We would like to thank Sheila Hammond, Cindy Dodson, and Tony Herring for their knowledge and technical expertise.
Footnotes
Author Contributions:
Study conception and design: JD, MS, NP, GU
Acquisition of data: JD, MS, GS, GU
Analysis and interpretation of data: JD, MS, NP, GL, AS, GA, GU
Drafting of manuscript: JD, MS
Critical revision: JD, MS, AS, GA, GU
Disclosures
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Morgan Salmon, Email: morgan.angulo310@gmail.com.
Nicolas H. Pope, Email: NP7G@hscmail.mcc.virginia.edu.
Guanyi Lu, Email: GL9T@hscmail.mcc.virginia.edu.
Gang Su, Email: GS4GV@hscmail.mcc.virginia.edu.
Ashish K. Sharma, Email: aks2n@cms.mail.virginia.edu.
Gorav Ailawadi, Email: gorav@virginia.edu.
Gilbert R. Upchurch, Jr., Email: GRU6N@hscmail.mcc.virginia.edu.
Sources
- 1.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. Journal of Vascular Surgery. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators Archives of Internal Medicine. 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 3.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group Annals of Internal Medicine. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung HC. Deaths: final data for 2009. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2011;60:1–116. [PubMed] [Google Scholar]
- 5.Carmo M, Colombo L, Bruno A, Corsi FR, Roncoroni L, et al. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery. 2002;23:543–549. doi: 10.1053/ejvs.2002.1620. [DOI] [PubMed] [Google Scholar]
- 6.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, et al. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 7.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, et al. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: a protein array study. Journal of Vascular Surgery. 2007;45:574–580. doi: 10.1016/j.jvs.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. 2121 e2111–2113. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nature Medicine. 2004;10(Suppl):S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 10.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 11.Hashizume R, Yamawaki-Ogata A, Ueda Y, Wagner WR, Narita Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. Journal of Vascular Surgery. 2011;54:1743–1752. doi: 10.1016/j.jvs.2011.06.109. [DOI] [PubMed] [Google Scholar]
- 12.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, et al. Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012;126:S38–45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbull IC, Hadri L, Rapti K, Sadek M, Liang L, et al. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. The Journal of Surgical Research. 2011;170:e179–188. doi: 10.1016/j.jss.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, et al. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142:215–221. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, et al. Sex dimorphisms in activated mesenchymal stem cell function. Shock. 2006;26:571–574. doi: 10.1097/01.shk.0000233195.63859.ef. [DOI] [PubMed] [Google Scholar]
- 16.Johnston WF, Salmon M, Su G, Lu G, Stone ML, et al. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:294–304. doi: 10.1161/ATVBAHA.112.300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmon M, Johnston WF, Woo A, Pope NH, Su G, et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013;128:S163–174. doi: 10.1161/CIRCULATIONAHA.112.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Z, Li H, Li X, Yu X, Wang H, et al. In vitro characteristics and in vivo immunosuppressive activity of compact bone-derived murine mesenchymal progenitor cells. Stem Cells. 2006;24:992–1000. doi: 10.1634/stemcells.2005-0224. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature protocols. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 20.Johnston WF, Salmon M, Pope NH, Meher A, Su G, et al. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 2014;130:S51–59. doi: 10.1161/CIRCULATIONAHA.113.006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston WF, Salmon M, Su G, Lu G, Ailawadi G, et al. Aromatase is required for female abdominal aortic aneurysm protection. Journal of Vascular Surgery. 2014 doi: 10.1016/j.jvs.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. Journal of the American College of Cardiology. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Pessina A, Bonomi A, Cocce V, Invernici G, Navone S, et al. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PloS one. 2011;6:e28321. doi: 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makrygiannis G, Courtois A, Drion P, Defraigne JO, Kuivaniemi H, et al. Sex Differences in Abdominal Aortic Aneurysm: The Role of Sex Hormones. Annals of Vascular Surgery. 2014;28:1946–1958. doi: 10.1016/j.avsg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, et al. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circulation Research. 2012;110:e73–85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Togel F, Hu Z, Weiss K, Isaac J, Lange C, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. American journal of physiology Renal Physiology. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS one. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A) Immunohistochemistry of aortas treated with elastase, heat-inactivated elastase (HIE), male MSC, and female MSC (scale bar = 100 μm). B) Time course of aortic dilation from mice perfused with elastase alone (n=3), male MSC (n=5), and female MSC (n=5). Day 3 exhibited no significant difference between groups; however, day 7 exhibits significantly different aortic dilation between elastase and MSC groups.
Supplemental Figure 2. SRD5a staining in male WT mice at day 14. Convergence channels of confocal microscopy staining for DNA (blue), macrophages (green), 5-alpha reductase (purple), and smooth muscle cells (teal) in a WT mouse aorta at day 14 after induction of aneurysm.
Supplemental Figure 3. Murine lung at day 3 after aneurysm formation, after MSC administration on postoperative day 1, contains RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), pneumocytes (light blue), and mesenchymal stem cells (red). A) Murine lung with evidence of female RFP MSC. B) Murine lung with evidence of male RFP MSC.
Supplemental Figure 4. Murine lung at day 7 after aneurysm formation, after MSC administration on postoperative days 1, 3 and 5, contains RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), pneumocytes (light blue, Pro-SPC), and mesenchymal stem cells (red). A) Murine lung with evidence of female RFP MSC. B) Murine lung with evidence of male RFP MSC.
Supplemental Figure 5. Murine lung at day 14 after aneurysm formation, after MSC administration on postoperative days 1, 3, and 5, does not contain RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), pneumocytes (light blue, Pro-SPC), and mesenchymal stem cells (red). A) Murine lung without evidence of female RFP MSC. B) Murine lung without evidence of male RFP MSC.
Supplemental Figure 6. Murine liver at day 14 after aneurysm formation, after MSC administration on postoperative days 1, 3, and 5, does not contain RFP MSC. Convergence of confocal microscopy channels staining for DNA (blue), biliary cytokeratin (green), and mesenchymal stem cells (red). A) Murine liver without evidence of female RFP MSC. B) Murine liver without evidence of male RFP MSC.