Abstract
Glycosylation of the Fc domain is an important driver of antibody effector function. While assessment of antibody glycoform compositions observed across total plasma IgG has identified differences associated with a variety of clinical conditions, in many cases it is the glycosylation state of only antibodies against a specific antigen or set of antigens that may be of interest, for example, in defining the potential effector function of antibodies produced during disease or after vaccination. Historically, glycoprofiling such antigen-specific antibodies in clinical samples has been challenging due to their low prevalence, the high sample requirement for most methods of glycan determination, and the lack of high-throughput purification methods. New methods of glycoprofiling with lower sample requirements and higher throughput have motivated the development of microscale and automatable methods for purification of antigen-specific antibodies from polyclonal sources such as clinical serum samples. In this work, we present a robot-compatible 96-well plate-based method for purification of antigen-specific antibodies, suitable for such population level glycosylation screening. We demonstrate the utility of this method across multiple antibody sources, using both purified plasma IgG and plasma, and across multiple different antigen types, with enrichment factors greater than 1000-fold observed. Using an on-column IdeS protease treatment, we further describe staged release of Fc and Fab domains, allowing for glycoprofiling of each domain.
Keywords: Antigen, Antibody, Purification, Glycosylation, Effector function
1. Introduction
Research on antibody (Ab) responses has generally focused on assessment of Ab titer. However, Ab quantity provides an incomplete assessment of in vivo activity, which depends upon both Fv and Fc activities that do not necessarily scale linearly with antibody quantity. Such qualitative functional aspects include Fv activities such as neutralization or agglutination, as well as Fc-dependent effector functions. These effector functions are in part post-translationally encoded via variant glycosylation on the conserved (Fc) portion of the antibody (Raju, 2008), which can be recognized by innate immune cells that drive several types of productive responses including antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular viral inhibition (ADCVI) and complement-dependent cytotoxicity (CDC). Long recognized as important to the efficacy of recombinant monoclonal therapeutics in the setting of cancer, these Fc-dependent functions have been identified as potential contributors to the mechanisms of action of even immunomodulatory Abs (Furness et al., 2014), as well as important drivers of protection in a number of infectious disease settings, such as Ebola (Wilson et al., 2000; Zeitlin et al., 2011), smallpox (Benhnia et al., 2013), anthrax intoxication (Bournazos et al., 2014), and in some settings in which broad, antibody-mediated protection has proven challenging to achieve, such as influenza (DiLillo et al., 2014) and HIV (Halper-Stromberg et al., 2014; Pincetic et al., 2014). Among monoclonal therapeutics, a growing number of antibodies have been designed specifically to elicit optimized cell-based killing as a primary mechanism of action, often through engineering of the Fc N-glycan composition (Gasdaska et al., 2012; Jefferis, 2012; Junttila et al., 2010).
The importance of the conserved Fc N-glycan site (N297) to these functions has been shown in numerous settings (Arnold et al., 2007; Jefferis, 1993, 2009), as cleavage of this glycan ablates binding of IgGs to numerous Fc Receptors (FcR). However, fine level modulation of IgG activity based on specific glycoforms is also well-established; the most prominent example being the potentiated ability of afucosylated Abs to strongly bind the FcγR3a and drive ADCC (Ferrara et al., 2011). Variation in the glycosylation patterns of naturally induced antibodies has also been implicated in a variety of autoimmune and infectious diseases (Albert et al., 2008; Mehta et al., 2008; Moore et al., 2005; R. Parekh et al., 1989; R. B. Parekh et al., 1985). Accordingly, recent efforts have begun to focus on understanding the role of natural variation in the glycosylation of antigen-specific antibodies in the setting of infectious disease protection and autoimmune pathology (Ackerman et al., 2013; Espy et al., 2011; Mehta et al., 2008; Scherer et al., 2010; Winkler et al., 2013), as well as beginning to determine the potential role that adjuvants, T cell help, or specific immunization regimens may play in driving the production of Abs with specific glycan profiles via vaccination (Guo et al., 2005; Hess et al., 2013; Oefner et al., 2012; Selman et al., 2012; J. Wang et al., 2011). These studies point toward the potential utility of evaluating variation in IgG glycosylation of Ab specificities of interest at the population level to provide critical information about antibody activity or immune status in vivo; for instance, IgG glycomics may aid in evaluating and comparing candidate vaccines as well as identifying putative mechanistic correlates of protection, or in screening subjects with auto-antibody responses to identify those at risk for more severe disease.
Despite its potential utility, evaluation of IgG glycoforms has not been widely utilized as a clinical biomarker of antibody activity or of immune status. Traditional glycan analysis methods such as high performance liquid chromatography (HPLC) and peptide mass spectrometry (MS) require relatively high sample input, significant operator expertise, and generally operate at low throughput (Huhn et al., 2009). These limitations have made analyzing antigen-specific antibodies, which are often present at very low concentrations, challenging to implement. Furthermore, while MS analysis can greatly reduce sample demands, the isolation of antigen-specific antibodies has generally utilized considerable quantities of antigen (Ackerman et al., 2013). However, recent advances in sensitive and simple glycan analysis methods (Bakovic et al., 2013; Mahan et al., 2014) which allow evaluation with greatly reduced sample quantities and higher throughput, have made high-throughput antigen-specific IgG N-glycan profiling a realistic goal, motivating the development of improved means to affinity purify these rare antibodies more efficiently.
A confounding factor in glycan analysis of antibodies is the potential for N-glycan sites to be present in the Fab region, as N-linked glycosylation motifs are observed in up to 10–30% of Fab fragments among naturally produced polyclonal mixtures (Ritamo et al., 2014). These Fab glycans typically exhibit a strikingly different glycosylation pattern than the Fc, particularly with respect to the level of sialylation (Anumula, 2012; Mimura et al., 2007), making their presence potentially problematic, even at low levels. Intriguingly, Fab glycosylation has been found to impact antigen binding in multiple settings (Rombouts et al., 2015b; Song et al., 2013) and may be a general marker of Abs with relatively high levels of somatic hypermutation, however, it is thought to have a limited role in modulating traditional FcR-driven effector functions. Thus, in order to best determine the role of glycosylation on both antigen recognition as well as the effector potential of an antibody, it is ideal to separate the Fab and Fc domains prior to glycan analysis. Isolation of the Fc domain N-glycan is a challenge relatively unique to the analysis of natural IgG, as candidate therapeutic monoclonal antibodies with N-linked Fab glycosylation motifs are typically not selected for clinical development. While some downstream glycan analysis methods such as glycopeptide-based mass spectrometry can resolve Fab and Fc glycosylation, traditional glycan mass spectrometry, HPLC, and CE-based methods rely on enzymatically or chemically released glycan. Accordingly, these methods require additional upstream processing to isolate Fc glycans. This isolation is generally performed by enzymatic cleavage of the antibody with papain or similar enzymes followed by affinity chromatography-based separation of Fc from Fab prior to glycan release, adding additional manipulation steps likely to reduce overall yield.
Fortunately, the significance of the Fc domain in vivo has led to the evolution of microbial proteins whose functions are to restrict antibody effector function. The best known example of such a molecule is Protein A, which is utilized by Staphylococcus aureus to mis-orient IgG; however, microbes possessing a number of diverse alternative means of antibody-evasion exist (Collin and Killian, 2014). Enzymes such as IdeS and SpeB restrict the Fc domain by cleaving Abs in their hinge region, and a number of glycosidases with activity against IgG and IgA glycans have been identified. While one of these glycosidases, EndoS is relatively specific to the IgG Fc domain (M. Collin and Olsen, 2001) and is consequently of interest in therapy of antibody-mediated autoimmune diseases (Collin et al., 2008) Endo S cleaves the IgG glycan after the N-linked acetylchitobiose core, which is variably fucosylated. Thus, its use as an alternative to the pan N-glycosidase PNGaseF is limited by the resulting loss of resolution of core-fucosylation, which is known to dramatically modulate IgG Fc binding to FcγR3a and FcγR3b. Nonetheless, collectively these microbial defense mechanisms represent useful biotechnological tools for IgG glycan analysis. Here, IdeS (von Pawel-Rammingen et al., 2002), a hinge protease, was chosen as a means to separate antibody Fc for glycan profiling.
In this work, we present a 96 well plate-based method for microscale purification of antigen-specific antibodies in high throughput, suitable for profiling of large-scale, population-based studies, such as vaccine trials or clinical cohorts. In addition, the method can be used to separately elute the Fc domain alone without additional steps, via an on-resin digestion with the IdeS enzyme that cleaves the hinge portion of the antibody. With this method we demonstrate isolation of various antigen-specific antibodies from human and non-human primate (NHP) samples in sufficient yield to permit highly quantitative chromatography-based glycan analysis. This method has proved useful across a variety of types of antigens, including peptides, and for purification of even epitope-specific antibodies. We have been able to quantify routine antigen-specific Ab enrichment of several hundred fold over serum concentrations in clinically relevant settings, as well as the ability to obtain useful glycan data from relatively small sample volumes (200 μL of plasma).
2. Materials and methods
2.1. Sample processing
IgG from human or NHP plasma was either separated from other common serum proteins via Melon Gel purification according to the manufacturer's instructions (Thermo 45214) or simply diluted 10-fold in PBS and then filtered through a 0.22 μm syringe filter (Millipore SLGP033RB). Filtered or purified samples were then concentrated to approximately 10 mg/mL total antibody concentration via centrifugal concentration (Amicon UFC801024). Pooled polyclonal human IgG from healthy donors, IVIG, Sigma (#I2511-10 mg), and HIV-infected subjects, HIVIG (NIH AIDS Reagent Program #3957), were used as controls.
2.2. Preparation of affinity resin cartridges
HIV gp41, gp120, p24, and influenza HA antigens (Immune Technologies IT-001-005p, IT-001-0027p, IT-001-017p and IT-003-0011p), and SIVmac239 gp120 (IT-001-022p) were diluted to 0.1 mg/mL in 20 mM Tris pH 8.2 to which a 5-fold molar excess of 10 mM Sulfo-NHS-Biotin (Themo 21335) dissolved in dH2O was added. Biotinylation was allowed to proceed for 1 h at RT with end-over-end mixing. To remove excess biotin, the biotinylated antigen was then buffer exchanged 3 times into Phosphate Buffered Saline (PBS) using Amicon spin concentrators with 10 min spins at 3000 ×g (Amicon UFC801024); final volumes were brought up with PBS to establish a biotinylated antigen concentration of 0.5 mg/mL. A synthetic N-terminally biotinylated cyclic SIVsmE543 V2 peptide (Barouch et al., 2012) (JPT Peptide Technologies GmbH); GenBank U72748) was likewise prepared in PBS. Agilent Bravo Streptavidin Cartridges (Agilent G5496-60010) were loaded into the provided 96-well cartridge racks and 170 μL of PBS was added to each tip. Loaded tip racks were placed in receiver plates and spun at 1000 ×g for 2 min. A 100 μL volume of biotinylated antigen in PBS (50 μg antigen) was then loaded on each tip and spun through at 50 ×g for 10 min. The PBS wash and antigen loading steps were then repeated to ensure maximum antigen binding. Finally, tips were washed with 100 μL of PBS + 0.05% Tween20 (1000 ×g for 2 min) and then twice with 100 μL PBS (1000 ×g for 2 min) and stored in PBS at 4 °C for up to a week.
2.3. Antigen-specific antibody purification
A 200 μL volume of filtered or concentrated antibody sample (~2 mg total Ab) was added to each cartridge prior to centrifugation at 50 ×g for 10 min. For higher yield, flowthrough was retained, cartridges were washed with 150 μL of PBS (1000 ×g for 2 min) and the flowthrough then reloaded and centrifuged for a second time at 50 ×g for 10 min. After sample loading, the column was washed with 100 μL of PBS+ 0.05% Tween20 (300 ×g for 3 min) and then twice with 100 μl of PBS (1000 ×g for 2 min). After washing, bound protein was eluted; for whole IgG purification, elution was performed with 50 μL of 100 mM sodium citrate pH 2.9, centrifuged at 50 ×g for 10 min. Before citrate elution, the tip racks were placed into fresh receiver plates (Griener Bio-One 781906), with 20 μL of 0.5 M sodium phosphate dibasic added to each well to neutralize the pH. After low pH elution the antigen-conjugated cartridges were regenerated by 2× washes with 150 μL of PBS (1000 ×g for 2 min) and stored in 50 μL PBS at 4 °C, for pH stable antigens, or as desired. For Fc-specific purification, elution was carried out with IdeS (Genovis A0-FR1-050) diluted 50-fold in dH2O, giving a final enzyme concentration of 2000 units/mL. A 50 μL volume of this solution was added to each cartridge and incubated at 37 °C for 1 h. After this digestion reaction, released Fc domain was eluted via centrifugation (50 ×g for 10 min). Antigen-bound Fab was subsequently eluted via the citrate elution protocol, and tips were washed, regenerated and stored as described previously. In all centrifugation steps, great care was taken to avoid or eliminate air bubbles observed in the sample reservoirs, as the centrifugation conditions used are insufficient to force liquid through such an obstruction.
2.4. Evaluation of affinity purified antibodies
Yield and specificity of antigen-specific antibodies were evaluated using a previously published multiplexed assay (Brown et al., 2012). Briefly, coupled microspheres were diluted in Assay Buffer (PBS + 0.1% BSA + 0.05% Tween20), creating a working mixture of 12.5 microspheres per μl. Using a black, clear bottom 384-well plate (Greiner Bio One, 781906) 40 μL of the working microsphere mixture (500 beads per well total) was added to 10 μL of each IgG sample. IgG samples were either the elution fraction, or the starting material or flow through diluted 10× in Assay Buffer; for titration experiments, all samples were then serially diluted 1:2 with Assay Buffer. Plates were covered and incubated for 2 h at room temperature on an XYZ plane plate shaker (IKA). Following this incubation, plates were then washed five times with 65 μL of Assay Wash (PBS-1×, 0.1% BSA, 0.5% Triton-X100) using a plate washing system (BioTek 405). Antigen-specific antibody was detected with R-phycoerythrin (PE)-conjugated anti-Hu IgG specific Ab (Southern Biotech 9040-09) or PE-conjugated anti-Rhesus IgG (Southern Biotech 6200-09), as appropriate, at 0.65 μg/mL, with 50 μL/well. After a 1 h incubation at room temperature on the shaker, plates were washed five times with 65 μL of sheath fluid, and microspheres were resuspended in 50 μL of sheath fluid.
A Bio-Plex array reader (FlexMap 3D, Bio-Plex Manager 5.0, Bio-Rad) acquired the microspheres, and the Median Fluorescence Intensity (MFI) of bound detection antibody was assessed. Background signal, defined as the average MFI observed for each microsphere set when incubated with the PE detector antibody in the absence of antibody sample, was subtracted from the MFI for each sample. HIVIG (NIH AIDS Reagent Program 3957), IVIG (Sigma I2511) and anti-gp120 IgG1 b12 (NIH AIDS Reagent Program 2640) served as controls.
Enrichment of antigen-specific Ab was calculated as follows. The antibody sample loaded onto the columns was diluted along a concentration series and measured for binding to the antigen of interest as described above. Fluorescence intensity was plotted against Ab concentration to generate a dilution curve. From this curve, the minimum concentration of Ab at which binding signal 3× above background signal was determined. Enrichment factors for the purified vs loaded material were calculated as the ratio of this minimum in the antibody load versus eluate, as follows: [concentration of input Ab at which signal 3-fold above background was observed] / [concentration of purified Ab at which signal 3-fold above background was observed]. To further assess the relative purity of gp120-specific antibodies, titration curves were generated against the targeted antigen gp120, and off-target antigens p17 and p24. Effective concentration midpoints (EC50s) were fit from these titration curves using GraphPad Prism.
2.5. IgG glycan analysis
CE-based glycan analysis, and isolation of total plasma IgG Fc via IdeS treatment and subsequent Protein G-based separation was conducted as previously described (Mahan et al., 2014). For HPLC-based glycan analysis, antibodies were denatured and subjected to enzymatic glycan release using PNGase F per manufacturer's instructions (NEB). Denatured protein was precipitated with 200 μL ice-cold ethanol and centrifuged at 11,000 g for 10 min. The recovered supernatant, which contained the released glycan, was dried using a centrifugal vacuum evaporator (CentriVap® from Labconco). Glycan labeling solution was prepared by dissolving 5 mg of 2-aminobenzamide (2-AB, Sigma-Aldrich) and 6 mg sodium cyanoborohydride in 30 μL of acetic acid and 70 μL of DMSO. A 5 μL volume of this solution was added to each dried glycan sample and incubated at 65 °C for 3 h. Following incubation, samples were brought up to 75% acetonitrile. Excess labeling solution was removed by passage through a microcrystalline cellulose cake packed on a 0.45 μm GHP filter plate (Pall Corporation). Cakes were prepared by centrifugation at 3750 rpm for 5 min of 200 μL of 200 mg/mL microcrystalline cellulose per well, followed by two 200 μL washes with water, and equilibration three times with 200 μL 80% acetonitrile at 3750 rpm for 5 min. The labeling reaction mixture was then loaded on the plate and centrifuged at 2000 rpm for 10 min. After washing with 150 μL 80% acetonitrile 3 times, the N-glycans were eluted in 200 μl water by centrifugation at 2000 rpm for 10 min. The eluted fraction was collected for HPLC analysis.
Labeled N-glycan samples injected were diluted 1:3 with acetonitrile with a volume at 100 μL. GlyKO 2-AB-human IgG N linked library (Prozyme) was used as a standard. 2AB-glycan separation was performed essentially as described previously (Melmer et al., 2011; Ruhaak et al., 2008). HPLC (Agilent technologies 1200 series) was carried out using a TSKgel Amide-80 HPLC-column (size 2 × 150 mm, Tosoh Bioscience). Gradient conditions were used as follows: Solvent A was 60 mM ammonium formate in 75% acetonitrile, pH 7.0, and solvent B was 115 mM ammonium formate in 54% acetonitrile, pH 4.5. Two sets of gradient conditions were used. Initial conditions were 21% B at a flow rate of 0.2 mL/min followed by 53% B over 80 min followed by 53-100% B over the next 12 min. Then the column was re-equilibrated in 21% B for 20 min between samples, resulting in a run time of 110 min per sample. The column temperature was 45 °C, and 2-AB was excited at 250 nm and fluorescence detected at 428 nm. Peak identities were identified and confirmed via use of glycan standards as well as by glycosidase treatment, and were quantified using Agilent ChemStation software.
3. Results
3.1. Method overview
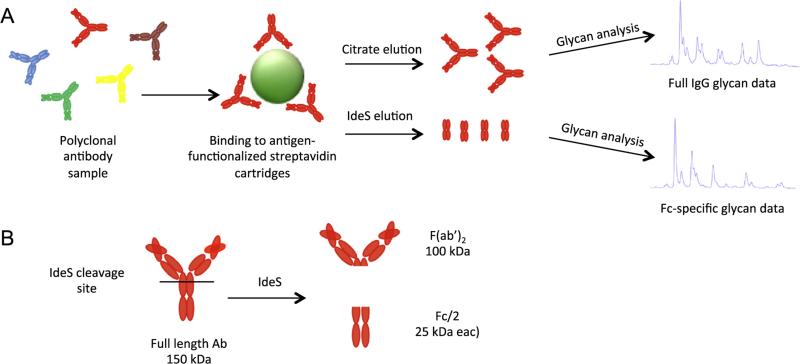
An overview of the sample processing, antigen-specific purification and glycan analysis method as used for high-throughput analysis of antibody samples is shown in Fig. 1. To summarize, the antigen of interest is biotinylated and conjugated to streptavidin-functionalized agarose cartridges. After washing, polyclonal antibody, either purified IgG or unpurified serum is passed through the cartridges via centrifugation, during which antigen-specific antibodies bind while non-binding IgG is removed in the flowthrough. The antigen-specific antibodies can then be eluted either via low-pH citrate elution, which disrupts antigen binding and releases the full antibody, or by IdeS treatment, which results in cleavage of the antibody hinge region and releases the Fc portion while leaving the Fab bound to the cartridge. Bound Fabs can subsequently be eluted via citrate, if desired. This process can be performed in 96-well format in less than one day. In addition, the antigen-conjugated tips can be reused several times if regenerated properly, with minimal loss of antibody binding capacity depending on the antigen used, and undetectable carryover between samples. The primary advantage afforded by the IdeS elution relative to pH-based elution is to allow for analysis of only Fc glycan while eliminating two more traditional downstream processing steps, enzymatic cleavage of the antibody Fc followed by protein A or other separation of Fab from Fc, prior to glycan analysis.
Fig. 1.
Assay schematic. A) Polyclonal antibodies present in either purified IgG or serum are bound to antigen-coated cartridges and washed to remove all non-specific IgG. Antigen-specific antibodies can then be eluted with either low pH citrate buffer, which releases the full Ig, or an IdeS enzyme solution, which cleaves and releases the Fc portion allowing separation of Fab′2 and Fc domains. Full Ig, cleaved Fc, or cleaved Fab′2 can then be used for glycan analysis. B) Schematic of IdeS cleavage resulting in unbound Fc fragments.
3.2. Application across multiple antigen types
In order to determine the robustness of the purification protocol, antigen-specific Ab purifications were conducted against a series of distinct antigens, including HIV gp120, gp41, and p24 proteins, influenza hemagglutinin (HA), and an SIV V2 loop peptide (Fig. 2A-E). A multiplex titering experiment was performed to quantify the level of antigen-specific antibody present in the loaded IgG sample, and flowthrough and eluted fractions. In each case, binding of the purified Ab was significantly enhanced against the antigen of interest. We conducted a similar experiment to isolate epitope-specific antibodies from a vaccinated rhesus macaque, using a cyclic V2 peptide from SIV gp120 instead of a full-length protein antigen. The binding capacity of the eluted epitope-specific Abs against the cyclic peptide of interest, as compared to the starting material and flowthrough is shown in Fig. 2E. The flowthrough shows a drastic depletion of peptide-binding activity, demonstrating that the method can perform well at scavenging antibody from dilute samples, and could be used to produce samples depleted of antibody subsets of interest.
Fig. 2.
Purification of diverse specificities, reproducibility and quality of purified antibody. (A–E) Antibodies specific for HIV gp120 (A), gp41 (B), p24 (C), an SIV V2 loop peptide (D), and the influenza HA protein (E) were purified from HIVIG (A/B/C), IVIG (E), or an SIV-vaccinated macaque (D). The ability of the antibody load (Input), flowthrough (FT), and the eluted antibody fraction (Elution) to bind to each antigen of interest across a range of sample concentrations was measured via an antigen-specific multiplex assay. The concentration versus binding signal of the gp120-specific monoclonal antibody b12 is shown as a benchmark of sample purity (A). Results are representative of at least 3 replicates. (F–H) HIV gp120-specific Abs were purified from HIVIG in quadruplicate. Yield (F) and ability to bind gp120, p24 and p17 were evaluated for each fraction of each purification replicate (G). Extended binding curves were used to calculate the reciprocal EC50s of each sample relative to the average observed across the loaded antibody sample toward each antigen (H).
To further demonstrate that eluted samples are of high purity, we compared the titration profile of HIV gp120-specific antibody purified as described to that of the monoclonal anti-gp120 antibody b12 in Fig. 2A. Both the purified antigen-specific Abs and the monoclonal Ab b12 used as a benchmark demonstrated overlapping signals in the linear range of the binding curve, suggesting that purified samples are primarily composed of antibodies against the antigen of interest. As a point of comparison, the concentration of total antibody in the starting material needed for similar gp120 binding was roughly 500-fold higher, suggesting that the purified gp120-specific antibodies from this sample were enriched 500-fold.
Process reproducibility and the purity of eluted antibodies were further investigated in 4 replicate experiments, in which gp120-specific antibodies were purified from 2 mg of HIVIG (Fig. 2F–H). Eluted antigen-specific Abs were quantified for each run, and demonstrated reproducible yields (Fig. 2F). The purity of each fraction was evaluated by quantifying binding to several HIV antigens including the targeted antigen gp120, and two off-target antigens, p17 and p24 (Fig. 2G). Reciprocal EC50 binding values were calculated based on extended titration curves for the input, flowthrough and elution fractions from each purification. The change in prevalence of each antibody specificity is plotted relative to that observed in the HIVIG input sample, and demonstrate both enhanced binding toward the target antigen (gp120) as well as reduced ability of the purified fraction to bind other antigens (Fig. 2H). As shown in Fig. 2H, the eluted fraction demonstrated a <10-fold enhancement in binding to gp120, whereas recognition of HIV p24 and p17 antigens was decreased by several orders of magnitude, and was essentially indistinguishable from signals observed for control gp120-specific monoclonal antibodies.
3.3. Quantitation of antigen-specific antibody enrichment
In order to quantify enrichment factors we fit the linear range of the binding curves to determine what minimum concentration of antibody would result in detectable binding to the antigen of interest. Table 1 summarizes the resulting data and enrichment factors of the purified material relative to the starting antibody source from the experiments shown in Fig. 2. This data suggests enrichment of factors of 20–400 fold across many antigens, with an apparent dependence of antibody prevalence in the starting sample. The peptide-specific antibody enrichment could only be roughly calculated as saturated signal was observed for the eluted fraction at all concentrations evaluated in this experiment.
Table 1.
Enrichment of antigen-specific Abs from pooled antibody sources. Antibodies specific for several viral antigens were purified from pooled polyclonal antibody samples. Antigen binding data for the input and elution material was used to calculate the enrichment of antigen-specific antibody for each sample. Enrichment factors are representative of 3 or more purification replicates.
| Antigen | Enrichment |
|---|---|
| HA | 400× |
| p24 | 20× |
| gp41 | 40× |
| gp120 | 20–500× |
| SIV gp120 | 20–500× |
| V2 loop peptide | >1000× |
3.4. Analysis of yield
One of the main obstacles for glycan analysis of antigen or disease-specific antibodies is their potentially low prevalence in cases other than acute or chronic infection. This quantitative barrier can pose a particular challenge to evaluating vaccine-induced antibodies, which tend to be present at much lower concentrations than antibodies produced during infection. This method was therefore designed in large part to isolate the quantity of antibody needed for downstream glycan analysis by HPLC, UPLC, or CE. Sample requirements will always vary based on the prevalence of antigen-specific antibody, but using a nominal 2 μg IgG quantity cutoff for reliable glycan quantitation, sufficient antibody was generally recovered from 200 μL of starting serum or 2 mg of total purified IgG. As a demonstration, gp120-specific Abs were purified from a set of 44 HIV infected patients. A total of 2 mg of IgG was purified from the plasma of each patient, and antigen purification carried out as described. The quantity of purified Ab from each sample was quantified and is presented in Fig. 3A. Using a nominal 2 μg cutoff, sufficient material was collected after a single round of purification to obtain an antigen-specific Ab glycan readout from 95.5% of samples (42 of 44).
Fig. 3.
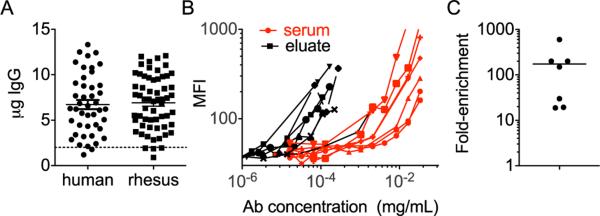
Yield and enrichment factors. A) The yield of HIV gp120-specific antibodies isolated from a set of 44 HIV-positive subjects and SIV gp120-specific antibodies from 60 vaccinated rhesus macaques is presented. B) Antigen (gp120) binding titration curves of loaded antibody samples (serum) and eluted fractions (eluate) from a representative subset of the rhesus purifications (n = 7) from part A are plotted. C) The fold-enrichment of antigen specific antibodies as determined by calculation of the minimal antibody concentration at which binding signals 3× above background were observed for loaded and eluted serum samples described in B.
3.5. Application across various species
In order to further demonstrate the breadth of this method, antigen-specific purifications were carried out on the serum of several model organisms, including rhesus macaque. SIV gp120-specific Abs were purified from a set of 60 serum samples (200 μL) from SIV-vaccinated rhesus macaques (Fourati et al., 2014). The yield of SIV-specific rhesus IgG is presented in Fig. 3A. To summarize, after purification, nominally sufficient Ab was obtained from 56 of the 60 samples (93.3%) for glycan characterization. Antibody binding capacity against SIV gp120 was calculated for the serum used as input, as well as the antigen-purified antibodies for a subset of animals via the multiplex method described previously (Fig. 3B). Enrichment factors were calculated based on the maximum dilution at which binding at least 3-fold over background was observed. Enrichment factors of the SIV gp120-specific Abs ranged from 20 to 500-fold following purification (Fig. 3C). The range in enrichment values appears to be generally due primarily to varying amounts of antigen-specific antibody present in each animal. Thus, using this method, sufficient quantities of antigen-specific antibody were recovered to permit glycan data from human clinical samples and vaccinated rhesus macaques to be obtained with a high rate of success, and similar results have been observed utilizing murine samples (data not shown).
3.6. Glycosylation of Antigen-specific Abs
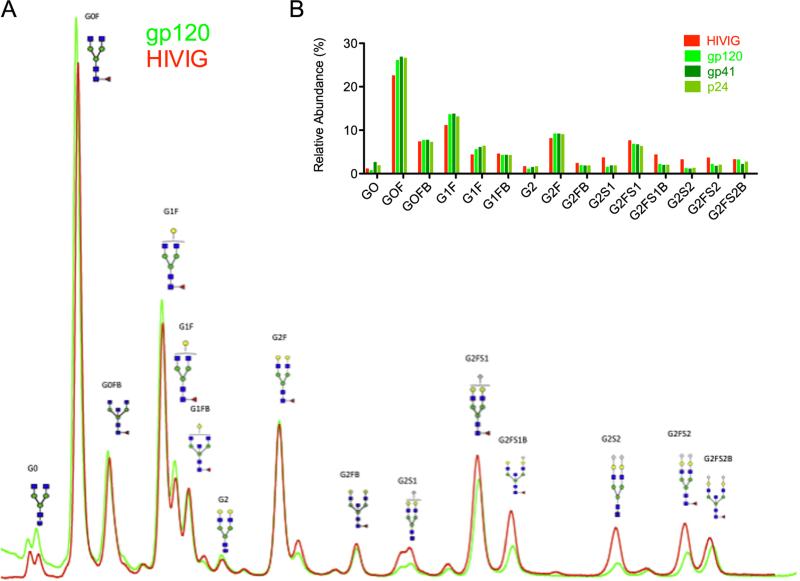
As a test case, antigen-specific antibodies recognizing one of several viral antigens (HIV gp120, gp41 and p24, as well as influenza HA) were isolated from a single starting sample of HIVIG. Since HIVIG is a pooled IgG source, this experiment aggregates Ab from a large number of subjects. An HPLC-based method (Ruhaak et al., 2008) was used to determine prevalence of various glycan species present on the starting material and the various antigen-specific Abs, in which IgG glycans were released enzymatically, dried, and then fluorescently labeled prior to separation via chromatography. To give a sense of the raw data generated in glycan analysis, a representative overlay of the HPLC trace of glycoform prevalences in the starting material (HIVIG) versus those of purified gp120-specific antibody is shown in Fig. 4A. Relative peak areas were calculated from the traces and are summarized in Fig. 4B. Glycosylation profiling shows a consistent shift between the bulk antibody and the antigen-specific Abs (Fig. 4B). Interestingly, across different target antigens, all of the HIV-specific antibodies showed a similar trend in glycosylation, in which greater levels of fucosylation and bisection, lower levels of digalactosylated (G2) and sialylated (S) glycans were observed, as compared to the bulk or total plasma antibody.
Fig. 4.
Glycosylation profiles of multiple antigen-specificities from a single source. A) Representative HPLC glycan data, demonstrating the overlay of chromatography traces for bulk HIVIG glycoforms (red) and gp120-specific antibodies purified from HIVIG (green) with peak identities and representative structural cartoons. B) Relative abundance of glycoforms present in HIVIG, and among gp120, gp41, and p24-specific antibodies. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. IdeS treatment for isolation of antibody Fc
Conducting an IdeS digest-based elution allows for purification of only the Fc portion of the antibodies of interest. The conserved Fc glycan is responsible for modulating binding to many Fc-receptors (Pincetic et al., 2014) and thus is critical to modulating effector function. To identify potential differences in the Fc glycan of antigen specific Ab versus bulk plasma IgG, we combined the cartridge-based antigen-purification method with IdeS treatment (Fig. 1A–B) on samples from four chronically infected HIV+ subjects to isolate the Fc portion of gp120-specific antibodies. In parallel, IdeS treatment followed by Protein G purification (Mahan et al., 2014) was also carried out on the bulk antibody from these subjects to isolate bulk Fc. Glycan analysis was conducted on both groups by CE, generating a matched data set regarding the glycosylation profiles of total bulk plasma IgG Fc and gp120-specific IgG Fc (Fig. 5). Fig. 5A–F shows the differences observed between the bulk and antigen specific antibodies in common classes of glycan structures known to influence antibody function, such as the levels of galactose (n = 0, 1, 2), core fucose, terminal sialic acid, and bisecting GlcNac. The average prevalences of each of the 19 glycoforms resolved by CE are presented in Fig. 5G, as are levels of galactosylation (5H) and sialyation (5I). In this data set, a clear and consistent trend toward lower levels of galactose and sialic acid, as well as a higher level of bisecting GlcNac among gp120-specific antibodies were observed.
Fig. 5.
Fc glycan analysis of IdeS-eluted antigen specific antibodies. Antibodies specific to HIV gp120 were purified from four chronically infected HIV patients, and Fc domains released using an on-cartridge IdeS elution. These samples, and Fc domain isolated from total plasma IgG (bulk) were profiled via capillary electrophoresis for glycan composition. A–F) The relative prevalences of several major classes of glycoforms are presented for each matched gp120 and bulk Fc sample. G–I) The relative prevalences of all identifiable glycan species (G), differentially galactosylated (H), and sialylated (I) forms averaged across subjects. A paired Student's t-test was conducted to determine the significance of differences observed in glycan prevalences between specificities (*p < 0.05, **p < .005).
4. Discussion
The importance of effector functions as impacted by the glycosylation patterns of monoclonal antibody therapeutics is well established (Jefferis, 2012), and numerous observations have been made as to variation of total plasma IgG glycosylation profiles in infectious disease (Moore et al., 2005), autoimmunity (R. B. Parekh et al., 1985; Selman et al., 2011), and cancer (Saldova et al., 2007). For each of these major disease classes, associations between IgG glycoform prevalences and outcomes have also been observed (Ackerman et al., 2013; Kodar et al., 2012; R. B. Parekh et al., 1988), suggestive of the potential value of IgG glycoprofiles as biomarkers of various immune diseases. However, coupling the known functional relevance of glycovariation to these disease associations points toward potential causative relevance of IgG glycans as contributors to clinical status, and motivates the development and expanded deployment of tools to facilitate IgG glycan evaluation.
To date, relatively few studies have looked at the glycovariation patterns of antigen-specific antibodies rather than among the total plasma IgG glycome at a population level, likely due to the throughput limitations of glycan determination and antibody purification. However, in the several studies that have reported on variation of antigen-specific antibody glycoprofiles, support for mechanistic associations with disease status and significant evidence for regulation of this post-translational modification have been found. Among these, glycoprofile changes among antigen-specific antibodies over time following vaccination have been observed (Selman et al., 2012), and a role for T cell help in modulating IgG glycosylation defined (Hess et al., 2013; Oefner et al., 2012). In addition, altered glycosylation of virus-specific IgG has been associated with altered antiviral antibody function among HIV-infected subjects (Ackerman et al., 2013), anti-citrullinated peptide/protein antibodies (ACPA) isolated from subjects with rheumatoid arthritis have been observed to posses altered glycosylation that can precede disease onset and differ between serum and within synovial fluid (Lundstrom et al., 2014; Rombouts et al., 2015a; Scherer et al., 2010), and glycovariation among anti-proteinase 3 antibodies has been found to correlate with disease activity as determined by clinical vasculitis score (Wuhrer et al., 2015). Successful allergy immunotherapy has been associated with the induction of sialylated allergen-specific antibodies (Oefner et al., 2012). Furthermore, therapeutic anti-RhD allotypic antibodies have likewise been associated with sialylation (Winkler et al., 2013), whereas the severity of hemolytic disease of the fetus and newborn has been associated with pathologic anti-RhD and anti-human platelet antigen antibodies with low levels of fucose (Kapur et al., 2014a; Kapur et al., 2014b; Wuhrer et al., 2009). These studies have begun to establish the role that even relatively small shifts in glycosylation patterns have in predicting a range of useful factors, such as responses to vaccination (J. R. Wang et al., 2015) or susceptibility to antibody-mediated disease (Winkler et al., 2013).
Our goal was to develop a means to purify antigen-specific antibodies that would have cross-species and cross-antigen applicability and that could be deployed to facilitate IgG glycoprofiling of large cohorts. While IgG glycosylation profile shifts are often observed among total plasma antibody, antigen purification enables exclusive resolution of the glycan profiles of antibodies that may be associated with disease pathology or protection. To this end, we describe development of a high-throughput method for isolating antigen-specific antibodies that reliably isolated sufficient material for glycan characterization by either CE or HPLC. Purifications utilizing a variety of protein antigens and even peptides were successful from hundreds of samples from varied sources. High purity, as determined by comparison to a monoclonal antibody, was observed, and high specificity was further demonstrated by the loss of recognition of other antigen types from eluted samples. Enrichment factors of 100-fold were routinely achieved, with rates greater than 1000-fold attained for rare antigen-specificities. As examples of downstream utility, IgG glycan prevalences among different antigen specificities from the same initial sample were characterized. Furthermore, to avoid potentially confounding differences between Fc and Fv glycosylation, incorporation of an efficient on-column proteolytic cleavage step using the microbial enzyme IdeS allowed for isolation of the Fc domain and resolution of the single N-linked glycosylation site key to IgG effector function. Because the Fab′2 domains can be subsequently eluted via a pH shift, evaluation of Fab glycans could likewise be conducted. In particular, the IdeS-based Fc elution method offers a very useful one-step method to isolate only the Fc portion of antigen-specific antibodies of interest without the need for additional sample transfers, digests, and Fc-purification steps following affinity purification prior to glycan analysis. Lastly, while a centrifuge-based protocol was employed for this study, the process described utilizes cartridges designed for automation using a liquid handling robot. Automation would likely greatly increase assay ease as well as potentially minimize the formation of flow-restricting air bubbles at the cartridge surface, as liquid handlers can move liquid bi-directionally at well-defined flow rates.
While this work has focused on the utility of antigen-purification for the specific goal of glycan characterization, purified antigen-specific antibodies may also be useful for other purposes, such as assessment of relative antigen binding or Fc receptor binding affinity values across a polyclonal pool (Boesch et al., 2014). With optimization, or for less prevalent antibody specificities, this method is able to remove antigen-specific antibodies present in the flowthrough to below the limit of detection, achieving a nearly complete recovery of the antibody of interest, and demonstrating potential utility in the evaluation of samples depleted of various specificities. Coupled to the demonstrated ability to isolate even epitope-specific antibodies, questions as to the functional relevance of very precise sub-populations of complex polyclonal mixtures could begin to be answered. As an alternative to the antigen-driven affinity selection described here, isolation of specific IgG subclasses, idiotypes, allotypes, or light chain variants could also be conducted with appropriate ligands.
Because antibodies can recognize antigens bivalently, there exists the potential for this purification procedure to result in co-elution of antigen present in serum, or even of immune complexes, carrying antigen/pathogen and antibodies specific to other components of the complex. While we have not observed evidence of either co-purification of serum antigen or of immune complexes, their presence in a sample could confound analysis, and therefore this possibility should be evaluated on a case-by-case basis. Similarly, for any novel antigen utilized, care should be taken to compare yield from samples known and known not to contain antigen-specific antibodies in order to assess the potential contribution of poor antigen folding, or post-translational modifications to result in the isolation of poly-reactive antibodies that may bind glycan or hydrophobic regions, for example. In our experience, the multiplex bead assay used here has served as a facile means to assess specificity and compare purification fractions without requiring as much material as a traditional ELISA assay.
Overall, this facile and high-throughput affinity purification method may serve to facilitate development of a better understanding of how the humoral immune system regulates IgG activity via glycosylation during an adaptive response. Identification of various signals that lead plasmablasts to secrete antibodies with varied glycans will potentially enable rational modulation to drive altered antibody activity in the setting of vaccination or autoimmunity. Based on the accumulating evidence that glycan profile changes that differ with clinical status may be mechanistically associated with disease, robust tools for assessing the glycosylation status of antigen-specific antibodies may be key to identifying means to rationally modify antibody activity via glycan alteration.
Acknowledgments
These studies were conducted with support from the Bill and Melinda Gates Foundation OPP1032817; NIH 1R01AI102691 and 5R01AIl08028903. The authors acknowledge E. Billings of MHRP for the design of the cyclic peptide. The following reagent was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Catalog #3957, HIV-IG from NABI and NHLBI. The opinions herein are those of the authors and should not be construed as official or representing the views of the U.S. Department of Defense or the Department of the Army, or the U.S. National Institutes of Health.
References
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Alter G. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 2013;123(5):2183. doi: 10.1172/JCI65708. http://dx.doi.org/10.1172/JCI65708sxf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc. Natl. Acad. Sci. U. S. A. 2008;105(39):15005. doi: 10.1073/pnas.0808248105. http://dx.doi.org/10.1073/pnas.0808248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumula KR. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods. 2012;382(1–2):167. doi: 10.1016/j.jim.2012.05.022. http://dx.doi.org/10.1016/j.jim.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007;25:21. doi: 10.1146/annurev.immunol.25.022106.141702. http://dx.doi.org/10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Bakovic MP, Selman MH, Hoffmann M, Rudan I, Campbell H, Deelder AM, Wuhrer M. High-throughput IgG Fc N-glycosylation profiling by mass spectrometry of glycopeptides. J. Proteome Res. 2013;12(2):821. doi: 10.1021/pr300887z. http://dx.doi.org/10.1021/pr300887z. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89. doi: 10.1038/nature10766. http://dx.doi.org/10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhnia MR, Maybeno M, Blum D, Aguilar-Sino R, Matho M, Meng X, Crotty S. Unusual features of vaccinia virus extracellular virion form neutralization resistance revealed in human antibody responses to the smallpox vaccine. J. Virol. 2013;87(3):1569. doi: 10.1128/JVI.02152-12. http://dx.doi.org/10.1128/JVI.02152-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch AW, Brown EP, Cheng HD, Ofori MO, Normandin E, Nigrovic PA, Ackerman ME. Highly parallel characterization of IgG Fc binding interactions. MAbs. 2014;6(4):915. doi: 10.4161/mabs.28808. http://dx.doi.org/10.4161/mabs.28808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S, Chow SK, Abboud N, Casadevall A, Ravetch JV. Human IgG Fc domain engineering enhances antitoxin neutralizing antibody activity. J. Clin. Invest. 2014;124(2):725. doi: 10.1172/JCI72676. http://dx.doi.org/10.1172/JCI72676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J. Immunol. Methods. 2012;386(1–2):117. doi: 10.1016/j.jim.2012.09.007. http://dx.doi.org/10.1016/j.jim.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Killian M. Bacterial modulation of Fc effector functions. In: Ackerman ME, Nimmerjahn F, editors. Antibody Fc: Linking Adaptive and Innate Immunity. Elsevier Inc.; 2014. p. 317. [Google Scholar]
- Collin M, Olsen A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20(12):3046. doi: 10.1093/emboj/20.12.3046. http://dx.doi.org/10.1093/emboj/20.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Shannon O, Bjorck L. IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc. Natl. Acad. Sci. U. S. A. 2008;105(11):4265. doi: 10.1073/pnas.0711271105. http://dx.doi.org/10.1073/pnas.0711271105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require Fc gamma R interactions for protection against influenza virus in vivo. Nat. Med. 2014;20(2):143. doi: 10.1038/nm.3443. http://dx.doi.org/10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy C, Morelle W, Kavian N, Grange P, Goulvestre C, Viallon V, Guilpain P. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener's). Arthritis Rheum. 2011;63(7):2105. doi: 10.1002/art.30362. http://dx.doi.org/10.1002/art.30362. [DOI] [PubMed] [Google Scholar]
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Benz J. Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. U. S. A. 2011;108(31):12669. doi: 10.1073/pnas.1108455108. http://dx.doi.org/10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourati S, Vaccari M, Gordon SN, Schifanella L, Cameron M, Keele BF, Franchini G. Modulation of RAS pathways as a biomarker of protection against HIV and as a means to improve vaccine efficacy. AIDS Res. Hum. Retrovir. 2014;30:A99. [Google Scholar]
- Furness AJ, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 2014;35(7):290. doi: 10.1016/j.it.2014.05.002. http://dx.doi.org/10.1016/j.it.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Gasdaska JR, Sherwood S, Regan JT, Dickey LF. An afucosylated anti-CD20 monoclonal antibody with greater antibody-dependent cellular cytotoxicity and B-cell depletion and lower complement-dependent cytotoxicity than rituximab. Mol. Immunol. 2012;50(3):134. doi: 10.1016/j.molimm.2012.01.001. http://dx.doi.org/10.1016/j.molimm.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Guo N, Liu Y, Masuda Y, Kawagoe M, Ueno Y, Kameda T, Sugiyama T. Repeated immunization induces the increase in fucose content on antigen-specific IgG N-linked oligosaccharides. Clin. Biochem. 2005;38(2):149. doi: 10.1016/j.clinbiochem.2004.10.002. http://dx.doi.org/10.1016/j.clinbiochem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Nussenzweig MC. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989. doi: 10.1016/j.cell.2014.07.043. http://dx.doi.org/10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Ehlers M. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J. Clin. Invest. 2013;123(9):3788. doi: 10.1172/JCI65938. http://dx.doi.org/10.1172/JCI65938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9(4):882. doi: 10.1002/pmic.200800715. http://dx.doi.org/10.1002/pmic.200800715. [DOI] [PubMed] [Google Scholar]
- Jefferis R. The glycosylation of antibody molecules: functional significance. Glycoconj. J. 1993;10(5):358. [PubMed] [Google Scholar]
- Jefferis R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol. Sci. 2009;30(7):356. doi: 10.1016/j.tips.2009.04.007. http://dx.doi.org/10.1016/j.tips.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys. 2012;526(2):159. doi: 10.1016/j.abb.2012.03.021. http://dx.doi.org/10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Sliwkowski MX. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70(11):4481. doi: 10.1158/0008-5472.CAN-09-3704. http://dx.doi.org/10.1158/0008-5472.can-09-3704. [DOI] [PubMed] [Google Scholar]
- Kapur R, Della Valle L, Sonneveld M, Hipgrave Ederveen A, Visser R, Ligthart P, Vidarsson G. Low anti-RhD IgG-Fc-fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn. Br. J. Haematol. 2014a;166(6):936. doi: 10.1111/bjh.12965. http://dx.doi.org/10.1111/bjh.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur R, Kustiawan I, Vestrheim A, Koeleman CA, Visser R, Einarsdottir HK, Vidarsson G. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014b;123(4):471. doi: 10.1182/blood-2013-09-527978. http://dx.doi.org/10.1182/blood-2013-09-527978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj. J. 2012;29(1):57. doi: 10.1007/s10719-011-9364-z. http://dx.doi.org/10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- Lundstrom SL, Fernandes-Cerqueira C, Ytterberg AJ, Ossipova E, Hensvold AH, Jakobsson PJ, Zubarev RA. IgG antibodies to cyclic citrullinated peptides exhibit profiles specific in terms of IgG subclasses, Fc-glycans and a fab-Peptide sequence. PLoS ONE. 2014;9(11):e113924. doi: 10.1371/journal.pone.0113924. http://dx.doi.org/10.1371/journal.pone.0113924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, Alter G. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J. Immunol. Methods. 2014 doi: 10.1016/j.jim.2014.12.004. http://dx.doi.org/10.1016/j.jim.2014.12.004. [DOI] [PMC free article] [PubMed]
- Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, Block TM. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. J. Virol. 2008;82(3):1259. doi: 10.1128/JVI.01600-07. http://dx.doi.org/10.1128/JVI.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmer M, Stangler T, Premstaller A, Lindner W. Comparison of hydrophilic-interaction, reversed-phase and porous graphitic carbon chromatography for glycan analysis. J. Chromatogr. A. 2011;1218(1):118. doi: 10.1016/j.chroma.2010.10.122. http://dx.doi.org/10.1016/j.chroma.2010.10.122. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Ashton PR, Takahashi N, Harvey DJ, Jefferis R. Contrasting glycosylation profiles between Fab and Fc of a human IgG protein studied by electrospray ionization mass spectrometry. J. Immunol. Methods. 2007;326(1–2):116. doi: 10.1016/j.jim.2007.07.014. http://dx.doi.org/10.1016/j.jim.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Mestecky J. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 2005;19(4):381. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, Ehlers M. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J. Allergy Clin. Immunol. 2012;129(6):1647. doi: 10.1016/j.jaci.2012.02.037. http://dx.doi.org/10.1016/j.jaci.2012.02.037(e1613) [DOI] [PubMed] [Google Scholar]
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988;1(8592):966. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- Parekh R, Isenberg D, Rook G, Roitt I, Dwek R, Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J. Autoimmun. 1989;2(2):101. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Ravetch JV. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 2014;15(8):707. doi: 10.1038/ni.2939. http://dx.doi.org/10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008;20(4):471. doi: 10.1016/j.coi.2008.06.007. http://dx.doi.org/10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Ritamo I, Cloutier M, Valmu L, Neron S, Rabina J. Comparison of the glycosylation of in vitro generated polyclonal human IgG and therapeutic immunoglobulins. Mol. Immunol. 2014;57(2):255. doi: 10.1016/j.molimm.2013.10.005. http://dx.doi.org/10.1016/j.molimm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Scherer HU. Anti-citrullinated protein antibodies acquire a proinflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2015a;74(1):234. doi: 10.1136/annrheumdis-2013-203565. http://dx.doi.org/10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- Rombouts Y, Willemze A, van Beers JJ, Shi J, Kerkman PF, van Toorn L, Toes RE. Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann. Rheum. Dis. 2015b doi: 10.1136/annrheumdis-2014-206598. http://dx.doi.org/10.1136/annrheumdis-2014-206598. [DOI] [PubMed]
- Ruhaak LR, Huhn C, Waterreus WJ, de Boer AR, Neususs C, Hokke CH, Wuhrer M. Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal. Chem. 2008;80(15):6119. doi: 10.1021/ac800630x. http://dx.doi.org/10.1021/ac800630x. [DOI] [PubMed] [Google Scholar]
- Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Rudd PM. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17(12):1344. doi: 10.1093/glycob/cwm100. http://dx.doi.org/10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, Toes RE. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620. doi: 10.1002/art.27414. http://dx.doi.org/10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M, Deelder AM. IgG fc N-glycosylation changes in Lambert–Eaton myasthenic syndrome and myasthenia gravis. J. Proteome Res. 2011;10(1):143. doi: 10.1021/pr1004373. http://dx.doi.org/10.1021/pr1004373. [DOI] [PubMed] [Google Scholar]
- Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, Wuhrer M. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell Proteomics. 2012;11(4) doi: 10.1074/mcp.M111.014563. http://dx.doi.org/10.1074/mcp.M111.014563(M111 014563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Oren DA, Franco D, Seaman MS, Ho DD. Strategic addition of an N-linked glycan to a monoclonal antibody improves its HIV-1-neutralizing activity. Nat. Biotechnol. 2013;31(11):1047. doi: 10.1038/nbt.2677. http://dx.doi.org/10.1038/nbt.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Pawel-Rammingen U, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21(7):1607. doi: 10.1093/emboj/21.7.1607. http://dx.doi.org/10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Balog CIA, Stavenhagen K, Koeleman CAM, Scherer HU, Selman MHJ, Wuhrer M. Fc-glycosylation of IgG1 is modulated by B-cell stimul. Molecular & cellular proteomics. 2011;10(5) doi: 10.1074/mcp.M110.004655. http://dx.doi.org/10.1074/mcp.M110.004655(M110.004655-M004110.004655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JR, Guan WD, Yau LF, Gao WN, Zhan YQ, Liu L, Jiang ZH. Glycomic signatures on serum IgGs for prediction of postvaccination response. Sci. Rep. 2015;5:7648. doi: 10.1038/srep07648. http://dx.doi.org/10.1038/srep07648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JA, Hevey M, Bakken R, Guest S, Bray M, Schmaljohn AL, Hart MK. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287(5458):1664. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- Winkler A, Berger M, Ehlers M. Anti-rhesus D prophylaxis in pregnant women is based on sialylated IgG antibodies. F1000Res. 2013;2:169. doi: 10.12688/f1000research.2-169.v1. http://dx.doi.org/10.12688/f1000research.2-169.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Porcelijn L, Kapur R, Koeleman CA, Deelder A, de Haas M, Vidarsson G. Regulated glycosylation patterns of IgG during alloimmune responses against human platelet antigens. J. Proteome Res. 2009;8(2):450. doi: 10.1021/pr800651j. http://dx.doi.org/10.1021/pr800651j. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Stavenhagen K, Koeleman CA, Selman MH, Harper L, Jacobs B, Morgan MD. Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients feature show low levels of bisection, galactosylation and sialylation. J. Proteome Res. 2015 doi: 10.1021/pr500780a. http://dx.doi.org/10.1021/pr500780a. [DOI] [PubMed]
- Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, Olinger GG. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. U. S. A. 2011;108(51):20690. doi: 10.1073/pnas.1108360108. http://dx.doi.org/10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]