Abstract
Inositol is a six-carbon sugar alcohol and is one of nine biologically significant isomers of hexahydroxycyclohexane. Myo-inositol is the primary biologically active form and is present in higher concentrations in the fetus and newborn than in adults. It is currently being examined for the prevention of retinopathy of prematurity in newborn preterm infants. A robust method for quantifying myo-inositol (MI), D-chiro-inositol (DCI) and 1,5-anhydro-D-sorbitol (ADS) in very small-volume (25 μL) urine, blood serum and/or plasma samples was developed. Using a multiple-column, multiple mobile phase liquid chromatographic system with electrochemical detection, the method was validated with respect to (a) selectivity, (b) accuracy/recovery, (c) precision/reproducibility, (d) sensitivity, (e) stability and (f) ruggedness. The standard curve was linear and ranged from 0.5 to 30 mg/L for each of the three analytes. Above-mentioned performance measures were within acceptable limits described in the Food and Drug Administration’s Guidance for Industry: Bioanalytical Method Validation. The method was validated using blood serum and plasma collected using four common anticoagulants, and also by quantifying the accuracy and sensitivity of MI measured in simulated urine samples recovered from preterm infant diaper systems. The method performs satisfactorily measuring the three most common inositol isomers on 25 μL clinical samples of serum, plasma milk, and/or urine. Similar performance is seen testing larger volume samples of infant formulas and infant formula ingredients. MI, ADS and DCI may be accurately tested in urine samples collected from five different preterm infant diapers if the urine volume is greater than 2–5 mL.
Keywords: inositol, clinical samples, sorbitol, LC, diapers
+A:Introduction
Myo-inositol (MI) is the primary biologically active form of inositol. It is a six-carbon sugar alcohol (Fig. 1) and is one of nine biologically significant isomers of hexahydroxycyclohexane. Campling and Nixon (1954) demonstrated that MI is present at higher concentrations in fetal blood and fluids compared with maternal blood in several species including human, and that after birth MI levels drop to adult concentrations within days to weeks. MI is an important component in the synthesis of surfactant (phosphatidyl inositol) and is one of the active intracellular messengers (phosphoinositides) that influence cell signaling through a variety of possible mechanisms (Drummond et al., 1987; Croze and Soulage, 2013). Inositol polyphosphate kinases play a central role in nuclear regulation and development (Seeds et al., 2007) and apoptosis (Majerus et al., 2008). Inositol pyrophosphates are also important in mammalian cell metabolism and signaling (Bennett et al., 2006; Chakaborty et al., 2011; Wilson et al., 2013). D-chiro-inositol (DCI) is also active and recognized as an important messenger in insulin signal transduction (Osterlund et al., 1993). The structurally related compound 1,5-anhydro-D-sorbitol (ADS) is present in adult human serum and may be a marker for glycemic control in patients with type-2 diabetes (Pitkanen, 1990).
Figure 1.
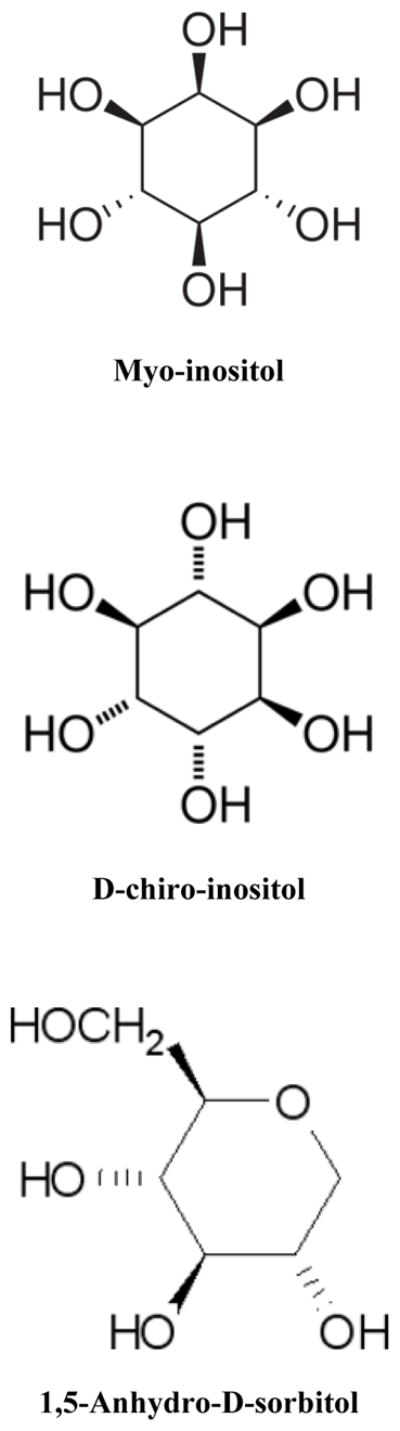
Chemical structures of (a) myo-inositol, (b) D-chiro-inositol and (c) 1,5- anhydro-D-sorbitol are depicted.
Human infant cord blood MI levels are much higher than adults and during the last trimester of pregnancy gradually decrease to only moderately elevated levels at term delivery (Lewin et al., 1978; Carver et al., 1997). Preterm delivery results in a fall in blood MI while these unstable infants are unable to feed. Studies by Hallman et al. (1986, 1992) of MI supplementation in preterm infants showed that premature infants receiving MI had lower inspiratory oxygen requirements, lower mean airway pressure and reduced incidence of bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP). In a follow-up study, Friedman et al. (2000) reported on the relationship between early low serum MI levels and an increased probability of premature infants developing stage 3 or 4 ROP. Infants fed a high MI-containing formula and those with higher serum MI concentrations at birth had a statistically significant lower incidence of ROP. A serum MI concentration >215 μM/L was associated with decreased respiratory distress syndrome/BPD/ROP severity and reduced odds for developing severe (i.e. stage 3 or 4) ROP.
Trials have been initiated to assess the safety and efficacy of MI to prevent ROP. The initial studies estimate MI single-dose and multidose pharmacokinetics in preterm infants. This involves collecting a number of low-volume blood and urine samples. Several analytical methods using diverse techniques are available for quantifying MI (Wang et al., 1990; Tagliaferri et al., 2000; Sun et al., 2002; Perello et al., 2004; Kindt et al., 2004; Kim et al., 2012). However, none of these methods could be operated using the low volume samples (<100 μL) available from preterm infants.
We describe a novel technique that was developed and validated using very low-volume (25 μL) clinical samples based on a robust, automated high-performance liquid chromatography with electrochemical detection method to quantify MI, DCI and ADS.
+A:Experimental
+B:Method description
The analytes, MI, DCI and ADS, were extracted from various biological sample matrices using 3% sulfosalicylic acid (SA), which removes protein and renders the samples noninfectious. The extracted samples were injected onto two calcium metal ligand columns (7.8 × 50 mm, Rezex RCM Monosaccharide Guard Column, P/N 03B-0130-K0, Phenomenex, Torrance, CA) connected in series and heated to 65°C, where ADS was separated from MI and DCI using a water mobile phase (Fig. 2). Before elution of MI, ADS and DCI from the second metal ligand guard column, a valve switch tooks place so that the metal ligand columns and a 4 × 50 mm Dionex CarboPac PA1(P/N 43096 ) anion exchange guard column were now in series (Fig. 3). As MI, ADS and DCI elute from the metal ligand columns, they were loaded onto the PA1 guard column. After MI, ADS and DCI were loaded onto the PA1 guard column, the valve returned to its original position and the metal ligand and PA1 guard columns were no longer in series (Fig. 2.) MI, ADS and DCI were separated from other carbohydrates on the PA1 guard column using a 30 mM sodium hydroxide mobile phase that allowed MI, ADS and DCI to quickly pass through the column while strongly retaining most other carbohydrates. As soon as MI, ADS and DCI eluted from the PA1 guard column and onto the 4 × 50 mm Dionex CarboPac anion exchange MA1 guard (P/N 44067) and the 4 × 250 mm Dionex CarboPac anion exchange MA1 (P/N 44066) analytical columns connected in series, another valve switch occurred (Fig. 4). The analytes were further separated on the MA1 guard and analytical columns with a 30 mM sodium hydroxide mobile phase while strongly retained carbohydrates were removed from the PA1 guard column with a 1 M sodium hydroxide mobile phase. After sufficient cleaning of the PA1 guard column with 1 M sodium hydroxide, the valves were returned to their original positions to equilibrate the system before injection of the next sample. (Fig. 2). The PA1 columns contained a polystyrene/divinylbenzene pellicular anion exchange resin with a capacity of 100 μEq while the MA1 columns contained a polystyrene/divinylbenzene pellicular macroporous anion exchange resin with a capacity of 4500 μEq. The eluent from the MA1 columns passed through an electrochemical detector where the analytes were detected by pulsed amperometry and results were calculated by comparison of peak heights for standards of known concentrations with peak heights for samples of unknown concentration. The system also contained ‘dummy’ PA1 and MA1 guard columns that were used to maintain a constant pressure over the MA1 analytical column and prevent the MA1 analytical column packing from collapsing. Using this fully automated procedure increased data consistency, decreased error potential and saved analyst time.
Figure 2.
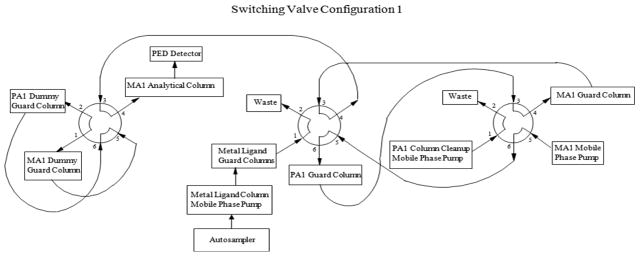
Switching valve configuration 1.
Figure 3.
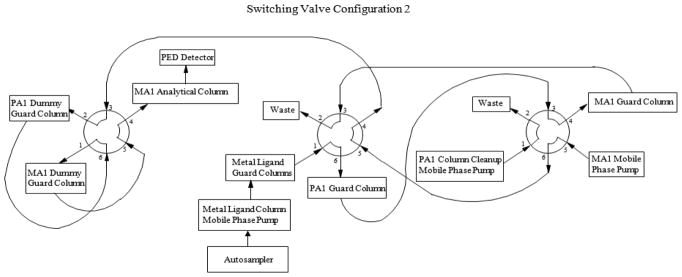
Switching valve configuration 2.
Figure 4.
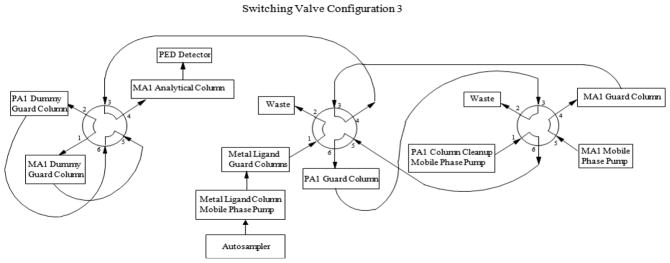
Switching valve configuration 3.
+B:Chemicals and reagents
ADS standard was purchased from Sigma Aldrich (St Louis, MO, USA); DCI and MI were obtained from Abbott Nutrition (Columbus, OH, USA). Deionized water (>15 mΩ) was purified using a Barnstead Nanopure water system (Thermo Scientific, Barnstead, MA, USA). Sodium hydroxide 50% w/w, low-carbonate form was obtained from JT Baker (Phillipsburg, NJ, USA) and SA, ACS grade, was obtained from Fisher Scientific (Pittsburgh, PA, USA). All samples were filtered with a 0.45 μm, 13 mm filter syringe (Whatman, Piscataway, NJ, USA).
+B:Clinical samples
Blood samples were collected from adult donors using six blood collection tubes (all Becton Dickenson); for serum, anti-coagulant free (Red top) and Serum Separator Tubes™; for plasma, sodium citrate, sodium heparin, lithium heparin and sodium EDTA tubes. One-quarter normal saline (USP) was used to simulate urine in diaper recovery experiments.
+B:Preterm infant diapers
Recovery of MI contained in simulated urine delivered to preterm infant diapers was determined for the following diaper systems: WeePee 2 Fluff (Philips Children’s Medical Ventures) with two small cotton balls; Tushies Gel-Free (Tender Care International); Cuddle Buns 21A and 21C (Small Beginnings); PremPampers Swaddlers P-XS (Pampers); and Huggies Preemies Gentle Care P (Huggies Brand). A plastic barrier was placed between the cotton balls and the absorbent diaper when the cotton balls were used.
+B:LC-EC conditions
A liquid chromatographic system was used including a gradient pump (Dionex GP50, Thermo Fisher, Pittsburgh, PA, USA) and two isocratic pumps (Bio-Rad 1350T, Hercules, CA, USA), column heater, autosampler (Dionex AS50, or Thermo AS3500, Thermo Fisher) and electrochemical detector (Dionex ED50, Thermo Fisher). Table 1 lists the chromatographic conditions. The valve configurations producing the gradient system were controlled using a ChronTrol relay (ChronTrol Corporation, San Diego, CA) and the AS50 or AS3500 autosampler. The system layout is depicted in Figs 2–4. The timeline and valve configurations were as follows:
Table 1.
CHROMATOGRAPHIC CONDITIONS
|
+B:Preparation of standard solutions and quality control samples
+C:Stock standard solutions
Aliquots of 0.1 ± 0.005 g of ADS standard, 0.1 ± 0.005 g of DCI standard and 0.1 ± 0.005 g of MI standard were each weighed and transferred to separate 100 mL volumetric flasks and dissolved in 3% SA to volume.
+C:Mixed intermediate standard solution
Aliquots of 5.0 mL each of the above-mentioned stock standard solutions of ADS standard, DCI standard and MI were each added to a volumetric flask and diluted to 100 mL with 3% SA.
+C:Working standards
Aliquots of 6.0, 4.0, 2.0 and 1.0 mL of the mixed intermediate standard solution were each diluted to 10 mL with 3% SA then labeled as working standards I–IV. A 1.0 mL aliquot of each of the working standards II– IV was diluted to 10 mL with 3% SA then labeled as working standards V–VII.
+B:Procedure
+C:Sample preparation
A 25 μL volume sample and 175 μL 3% SA were thoroughly mixed by vortex for at least 15 s, then filtered through a 0.45 μm syringe filter and placed into an autosampler vial insert for analysis.
+C:HPLC analysis
A 20 μL volume of the filtered sample mixture was injected for analysis. The concentrations of ADS, DCI and MI in samples were determined by comparison of peak heights from samples of known volumes with peak heights of standards of known concentrations.
+B:Method validation
The inositol method was validated with respect to (a) selectivity, (b) accuracy/recovery, (c) precision/reproducibility, (d) sensitivity, (e) stability and (f) ruggedness as reported below.
+A:Results and discussion
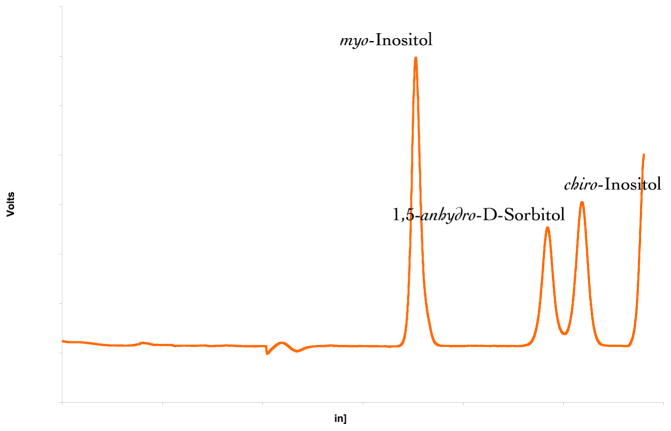
The chromatogram for the analytes is depicted in Fig. 5. MI elutes at approximately 17 min and ADS and DCI elute at approximately 24 and 26 min, respectively. A plot of the respective standard concentrations for MI, ADS and DCI vs peak heights was linear (typical r2 ≥ 0.9999, 0.999 and 0.9999, respectively; minimum acceptable r2 ≥ 0.998) throughout the standard concentration range.
Figure 5.
Chromatogram of myo-inositol, D-chiro-inositol and 1,5-anhydro-D-sorbitol.
+B:Selectivity
A single analyte-associated peak was demonstrated from each of six replicate water solutions and four human serum test samples for each of the analytes added to samples at both the lower and upper limits of quantification. The average (SD) retention times (water solution) were: MI, 17.47 (0.15) and 17.46 (0.22) min; ADS, 23.29 (0.27) and 23.28 (0.39) min; and DCI, 25.59 (0.29) and 25.63 (0.38) min for the respective lower and upper limits. Chromatographic behaviors of scyllo-, epi-, allo- and muco-inositols did not interfere with determination of MI, ADS or DCI. In separate experiments using unspiked serum samples, fractions from the separations were collected and pooled, and the contents of the peak fractions were assessed by proton-NMR spectra (data not shown). These tests confirmed the identities of MI and ADS in peaks 1 and 2. The unspiked serum DCI peak fractions contained insufficient amounts of material for NMR analysis.
+B:Accuracy/recovery
Accuracy and recovery were determined at the lower, mid-range and upper limits of quantification. Four human serum samples were each spiked with about 4.0, 80 or 160 mg/L of the individual analytes then tested and average percentage recoveries calculated. The average (SD) lower, mid-range and upper limit percent recoveries were: for MI, 93.4 (4.6), 97.3 (5.5) and 94.5 (4.0); for ADS , 98.1 (18.7), 99.4 (8.7) and 95.8 (4.4); and for DCI , 108.3 (5.3), 100.2 (6.2) and 98.2 (5.3).
+B:Precision/reproducibility
A single human serum sample was spiked with each of three combinations of low, mid-range, and high concentrations of each of the analytes (Table 2). The spiked samples were aliquoted, frozen and distributed to Abbott Nutrition and Texas Tech University Health Sciences Center for testing. On four separate days each laboratory thawed one sample aliquot and tested the sample in five replicates for each analyte. Data were analyzed to determine within-day, day-to-day and site-to-site precision and reproducibility (Table 3). Data show close replication of sample concentration results between the test sites. Total RSD was similar for each analyte at both test sites and overall precision was between ±6.55 and ±16.2%.
Table 2.
Sample composition for precision/reproducibility testing
| Analyte | Serum sample relative concentration | ||
|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | |
| Myo-Inositol | High | Mid-range | Low |
| 1,5-Anhydro-D- sorbitol | Low | High | Mid-range |
| D-Chiro-Inositol | Mid-range | Low | High |
Table 3.
Precision/reproducibility
| Mean concentration (mg/L) | Within day RSD (%) | Day-to-day RSD (%) | Total RSD (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Spike | AN | TT | Average | AN/TT × 100 | AN | TT | RMS average | AN | TT | RMS average | AN | TT | RMS RSD |
| Myo- Inositol | Low | 10.1 | 10.5 | 10.3 | 96.2 | 2.64 | 1.93 | 2.31 | 7.64 | 13.4 | 10.9 | 8.09 | 13.5 | 11.2 |
| Med | 71.5 | 72.2 | 71.8 | 99.0 | 2.72 | 1.72 | 2.28 | 1.57 | 12.4 | 8.86 | 3.14 | 12.6 | 9.15 | |
| High | 132 | 135 | 133 | 97.8 | 3.31 | 2.31 | 2.85 | 1.97 | 10.2 | 7.35 | 3.85 | 10.5 | 7.88 | |
| D-Chiro Inositol | Low | 11.2 | 11.1 | 11.2 | 101 | 3.65 | 4.42 | 4.05 | 9.74 | 19.8 | 15.6 | 10.4 | 20.3 | 16.2 |
| Med | 71.7 | 74.5 | 73.1 | 96.2 | 2.54 | 2.34 | 2.44 | 2.96 | 9.53 | 7.06 | 3.89 | 9.81 | 7.46 | |
| High | 131 | 136 | 133 | 96.3 | 2.66 | 1.32 | 2.10 | 1.15 | 8.70 | 6.21 | 2.90 | 8.80 | 6.55 | |
| 1,5 ADS | Low | 15.7 | 16.9 | 16.3 | 92.9 | 2.98 | 3.51 | 3.26 | 8.63 | 14.6 | 12.0 | 9.13 | 15.0 | 12.4 |
| Med | 71.4 | 72.6 | 72.0 | 99.2 | 2.82 | 2.15 | 2.51 | 2.99 | 9.20 | 6.84 | 4.11 | 9.45 | 7.29 | |
| High | 130 | 133 | 131 | 97.7 | 2.87 | 2.10 | 2.51 | 1.31 | 11.7 | 8.35 | 3.15 | 11.9 | 8.71 | |
Abbreviations: 1,5 ADS, 1,5-anhydro-D-sorbitol; AN, Abbott Nutrition; TT, Texas Tec.; RSD, relative standard deviation (%); total RSD, square root (within day SD2 + day-to-day SD2).
+B:Sensitivity
Method operating sensitivity is set by the lowest standard concentration (0.5 mg/L). Since the samples were diluted 1:8 prior to testing, the lowest quantifiable sample concentration was 4.0 mg/L. This is an acceptable lower limit for most clinical samples. However, the detector signal-to-noise ratio at this concentration would allow for at least a 100× decrease in the lower detection limit, if needed.
+B:Stability
MI, ADS and DCI standards at concentrations between 1.0 and 30 mg/L stored refrigerated in 3% SA were stable for at least 12 weeks. MI, ADS and DCI at concentrations between 10 and 130 mg/L in untreated human plasma were stable following each of eight freeze–thaw cycles. Finally, the three analytes were stable in SA-treated human plasma stored at room temperature for at least 64 h.
+B:Ruggedness
Samples were analyzed at two different laboratories with different equipment, test solutions, standards and control samples. Despite differing environments, all analytes showed excellent agreement. Mean laboratory results for the three analytes were 97.4% (range, 101–92.9%, Table 3). The method also yielded accurate and equivalent results using blood serum or plasma collected using any of four anti-coagulants (data not shown). The method has also been used successfully to quantify MI, ADS and DCI in samples of infant formulas, infant formula ingredients and human milk.
+B:Recovery of urine MI from diapers
+C:Accuracy/recovery
Twelve individual diapers of each type were weighed. Then 5.0 or 15.0 mL of 9 or 54 mg/L MI dissolved in 1:4 normal saline (0.225% NaCl) was added to the diapers (four sample sets, in triplicate). The diapers were folded over and incubated for 30–60 min at room temperature and reweighed, with the weight difference determining the amount of sample added to the diaper. The absorbent material was then removed from the diaper and placed in a 30 mL syringe that was placed in a Harvard syringe pump to compress the material and express the fluid. The fluid volume recovered was recorded and the sample was stored frozen for later testing. Results are shown in Table 4. Average MI percentage recoveries were similar at 5.0 and 15.0 mL loading (102.7%, 99.22%). Average percentage recoveries for the diaper types were: WeePee, 94.43; Tushies, 110.8; Cuddlebuns, 93.68; PremPampers, 103.0; and Huggies, 103.0.
Table 4.
Inositol recovery from diapers
| Diaper solution | WeePee | Tushies | Cuddle Buns | PremPampers | Huggies No Gel | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MI @ 8 mg/L |
MI @ 54 mg/L |
MI @ 8 mg/L |
MI @ 54 mg/L |
MI @ 8 mg/L |
MI @ 54 mg/L |
MI @ 8 mg/L |
MI @ 54 mg/L |
MI @ 8 mg/L |
MI @ 54 mg/L |
|||||||||||
| mL added | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 | 5 | 15 |
| Recovery (%) | 92.2 | 95.9 | 89.9 | 99.7 | 120 | 107 | 114 | 102 | 96.0 | 92.5 | 89.9 | 96.3 | 111 | 97.9 | 101 | 102 | 120 | 101 | 92.9 | 97.9 |
| RSD (%, n = 3) | 1.80 | 1.56 | 5.38 | 3.20 | 12.7 | 5.57 | 5.06 | 3.07 | 3.49 | 3.73 | 0.77 | 2.04 | 7.27 | 3.22 | 2.69 | 1.45 | 11.36 | 1.63 | 6.61 | 3.13 |
+C:Sensitivity
Sensitivity is determined by the minimum urine volume added that yields a testable sample volume. Nine individual diapers of each type were weighed. Then 5.00, 2.00 or 1.00 mL of 1:4 normal saline was added to each of three diapers per type. The diapers were incubated for 30–60 min and reweighed, with the weight difference determining the amount of sample added to the diaper. The absorbent material was then removed from the diaper and placed in a 30 mL syringe that was placed in a Harvard syringe pump to compress the material and express the sample fluid. The fluid volume recovered was recorded and the lowest initial sample volume that consistently yielded an expressed sample volume of >200 μL was recorded. Results are shown in Table 5 and indicate that the minimum urine volume yielding a testable amount of sample was between 2.0 and 5.0 mL, depending on the diaper type. Inositol concentrations in simulated urine extracted from each diaper type were stable for >30 days when stored frozen.
Table 5.
Diaper minimum urine recoveries
| Diaper | WeePee | Tushies | Cuddle Buns | PremPampers | Huggies No Gel | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fluid added (mL) | 5.0 | 2.0 | 1.0 | 5.0 | 2.0 | 1.0 | 5.0 | 2.0 | 1.0 | 5.0 | 2.0 | 1.0 | 5.0 | 2.0 | 1.0 |
| Fluid recovered (g) Average, n = 3 | 2.20 | 0.53 | 0.00 | 0.83 | 0.06 | 0.00 | 2.17 | 0.73 | 0.07 | 3.43 | 0.57 | 0.03 | 1.93 | 0.2a | 0.00 |
| RSD (n = 3) | 0.66 | 0.06 | — | 0.31 | — | — | 0.59 | 0.12 | — | 0.21 | 0.15 | — | 0.38 | 0.26 | — |
| Lowest volume to recover ≥0.2 g (mL) | 2.0 | 5.0 | 2.0 | 2.0 | 5.0 |
a2/3 <0.2 |
|||||||||
The average fluid recovered was 0.2 g, but two of the three samples yielded less than 0.2 g at 2.0 mL of fluid added.
+A:Conclusions
Results from validation of the method described support the use of electrochemical detection to quantify the content of MI, ADS and DCI in very low volume human clinical samples. Performance measures were within acceptable limits described in the Food and Drug Administration’s Guidance for Industry: Bioanalytical Method Validation (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidanc es/ucm070107.pdf). Furthermore, the method has been successfully employed to measure MI, ADS and DCI content in human serum, urine, breast milk and infant formula samples in a large clinical trial in preterm infants (Phelps et al., 2013). MI, ADS and DCI may be accurately tested in urine samples collected from at least five common preterm infant diapers if the excreted urine volume is greater than 2–5 mL.
Acknowledgments
This work was supported by Abbott Nutrition, and the National Institutes of Health (U10 HD40521, U10 HD46000 and UL1 TR42). The authors are grateful for the technical assistance of Erica Burnell, RN (diaper studies) and Marti S. Bergana, PhD (proton-NMR analyses).
Abbreviations used
- ADS
1,5-anhydro-D-sorbitol
- BPD
bronchopulmonary dysplasia
- DCI
D-chiro-inositol
- MI
myo-inositol
- ROP
retinopathy of prematurity
- SA
sulfosalicylic acid
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Bennett M, Onnebo SM, Azevedo C, Saiardi A. Inositol pyrophosphates: metabolism and signaling. Cellular and Molecular Life Sciences. 2006;63(5):552–564. doi: 10.1007/s00018-005-5446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campling JD, Nixon DA. The inositol content of foetal blood and foetal fluids. Journal of Physiology. 1954;126:71–80. doi: 10.1113/jphysiol.1954.sp005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver JD, Stromquist CI, Benford VJ, Minervini G, Benford SA, Barness LA. Postnatal inositol levels in preterm infants. Journal of Perinatology. 1997;17(5):389–392. [PubMed] [Google Scholar]
- Chakarborty A, Kim S, Snyder SH. Inositol pyrophosphates as mammalian cell signals. Science Signaling. 2011;23(4):188. doi: 10.1126/scisignal.2001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:1811–1827. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Drummond AH, Joels LA, Hughes PJ. The interaction of lithium ions with inositol lipid signaling systems. Biochemical Society Transactions. 1987;15(1):32–35. doi: 10.1042/bst0150032. [DOI] [PubMed] [Google Scholar]
- Friedman CA, McVey J, Borne MJ, James M, May WL, Temple DM, Robbins KK, Miller CJ, Rawson JE. Relationship between serum inositol concentration and development of retinopathy of prematurity: a prospective study. Journal of Pediatric Ophthalmology and Strabismus. 2000;37(2):79–86. doi: 10.3928/0191-3913-20000301-06. [DOI] [PubMed] [Google Scholar]
- Hallman M, Jarvenpaa AL, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Archives of Disease in Childhood. 1986;61(11):1076–1083. doi: 10.1136/adc.61.11.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol supplementation in premature infants with respiratory distress syndrome. New England Journal of Medicine. 1992;326(19):1233–1239. doi: 10.1056/NEJM199205073261901. [DOI] [PubMed] [Google Scholar]
- Kim BH, Park JY, Jang JB, Moon DC. LC-MS/MS method for the quantification of myo- and chiro-inositol as the urinary biomarkers of insulin resistance in human urine. Biomedical Chromatography. 2012;26(4):429–433. doi: 10.1002/bmc.1682. [DOI] [PubMed] [Google Scholar]
- Kindt E, Shum Y, Badura L, Snyder PJ, Brant A, Fountain S, Szekely-Klepser G. Development and validation of an LC/MS/MS procedure for the quantification of endogenous myo-inositol concentrations in rat brain tissue homogenates. Analytical Chemistry. 2004;76(16):4901–4908. doi: 10.1021/ac049746w. [DOI] [PubMed] [Google Scholar]
- Lewin LM, Melmed S, Passwell JH, Yannai Y, Brish M, Orda S, Boichis H, Bank H. Myo-inositol in human neonates: serum concentrations and renal handling. Pediatric Research. 1978;12:3–6. doi: 10.1203/00006450-197801000-00002. [DOI] [PubMed] [Google Scholar]
- Majerus PW, Zou J, Marjanovic J, Kisseleva MV, Wilson MP. The role of inositol signaling in the control of apoptosis. Advances in Enzyme Regulation. 2008;48:10–17. doi: 10.1016/j.advenzreg.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund RE, Jr, McGill JB, Herskowitz I, Kipnis DM, Santiago JV, Sherman WR. D-Chiro-inositol metabolism in diabetes mellitus. Proceedings of the National Academy of Sciences USA. 1993;90(21):9988–9992. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello J, Isern B, Costa-Bauza A, Grases F. Determination of myo-inositol in biological samples by liquid chromatography–mass spectrometry. Journal of Chromatography B Analytical Technologies in the Biomedical Life Sciences. 2004;802(2):367–370. doi: 10.1016/j.jchromb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Phelps DL, Ward RM, Williams RL, et al. Pharmacokinetics and safety of a single dose of myo-inositol in preterm infants of 23–29 wk. Pediatric Research. 2013;74(6):721–729. doi: 10.1038/pr.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen E. 1. 5-Anhydro-D-glucitol – a novel type of sugar in the human organism. Scandinavian Journal of Clinical Laboratory Investigation. 1990;50(suppl 201):55–62. [PubMed] [Google Scholar]
- Seeds AM, Frederick JP, Tsui MMK, York JD. Roles for inositol polyphosphate kinases in the regulation of nuclear processes and developmental biology. Advances in Enzyme Regulation. 2007;47:10–25. doi: 10.1016/j.advenzreg.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TH, Heimark DB, Nguygen T, Nadler JL, Larner J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochemical and Biophysical Research Communications. 2002;293(3):1092–1098. doi: 10.1016/S0006-291X(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Tagliaferri EG, Bonetti G, Blake CJ. Ion chromatographic determination of inositol in infant formulae and clinical products for enteral feeding. Journal of Chromatography A. 2000;879(2):129–135. doi: 10.1016/s0021-9673(00)00323-x. [DOI] [PubMed] [Google Scholar]
- Wang WT, Safar J, Zopf D. Analysis of inositol by high-performance liquid chromatography. Analytical Biochemistry. 1990;188(2):432–435. doi: 10.1016/0003-2697(90)90632-j. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Livermore TM, Saiardi A. Inositol pyrophosphates: between signaling and metabolism. Biochemical Journal. 2013;452(3):369–370. doi: 10.1042/BJ20130118. [DOI] [PubMed] [Google Scholar]