Abstract
The ability to regulate the emotional response to threat is critical to healthy emotional function. However, the response to threat varies considerably from person-to-person. This variability may be partially explained by differences in emotional processes, such as locus of control and affective state, which vary across individuals. Although the basic neural circuitry that mediates the response to threat has been described, the impact individual differences in affective state and locus of control have on that response is not well characterized. Understanding how these factors influence the neural response to threat would provide new insight into processes that mediate emotional function. Therefore, the present study used a Pavlovian conditioning procedure to investigate the influence individual differences in locus of control, positive affect, and negative affect have on the brain and behavioral response to predictable and unpredictable threat. Thirty-two healthy volunteers participated in a fear conditioning study in which predictable and unpredictable threat (i.e., unconditioned stimulus) were presented during functional magnetic resonance imaging (fMRI). Locus of control showed a linear relationship with learning-related ventromedial prefrontal cortex (PFC) activity such that the more external an individual’s locus of control, the greater their differential response to predictable versus unpredictable threat. In addition, positive and negative affectivity showed a curvilinear relationship with dorsolateral PFC, dorsomedial PFC, and insula activity, such that those with high or low affectivity showed reduced regional activity compared to those with an intermediate level of affectivity. Further, activity within the PFC, as well we other regions including the amygdala, were linked with the peripheral emotional response as indexed by skin conductance and electromyography. The current findings demonstrate that the neural response to threat within brain regions that mediate the peripheral emotional response are modulated by an individual’s affective state as well as their perceptions of an event’s causality.
Keywords: Affect, Conditioning, Control, fMRI, Fear
1. Introduction
The ability to effectively respond to threats in the environment is critical for healthy emotional function. The response to a threat, however, can vary depending on the circumstances in which the threat occurs. For example, an unpredictable threat elicits a larger emotional response than a predictable threat (Knight et al., 2011). The response to threat also varies considerably from one person to another and appears to be influenced by individual differences in emotion-related processes. For example, the emotional response to threat varies with aspects of anxiety (Grillon et al., 1993; Knight et al., 2011; Wood et al., 2012, 2013). Anxious behavior, however, is influenced by a number of characteristics that vary across individuals. For example, locus of control (i.e., the degree to which an individual believes events are internally versus externally controlled) and affective state (i.e., the degree to which an individual experiences positive and negative emotions in daily life) also vary from person-to-person and appear to influence anxious behavior (Chorpita & Barlow, 1998; Gros et al., 2007; Rotter, 1966; Watson et al., 1988). Therefore, individual differences in locus of control and affective state may also explain variability in the response to threat. However, there is limited prior research on whether inter-subject variability in locus of control, positive affect, and negative affect influence the neural processes that mediate expression of the emotional response. Thus, determining whether individual differences in these attributes influence the response to threat would provide important insight into emotion-related processes.
Pavlovian conditioning is a procedure often used to investigate emotional learning and memory processes. During Pavlovian fear conditioning, an originally innocuous cue (conditioned stimulus; CS) is typically paired with an innately aversive stimulus (unconditioned stimulus; UCS) that produces a reflexive response (unconditioned response; UCR). Repeated pairing of the CS and UCS then comes to elicit a conditioned response (CR) in anticipation of the UCS. Expression of a CR is typically used to index learning, while the UCR is often considered an unlearned response. The UCR, however, also shows learning-related modulation. For instance, prior Pavlovian conditioning research has demonstrated learning-related changes in brain and behavioral responses to predictable compared to unpredictable threat (Baxter et al., 1966; Canli et al., 1992; Dunsmoor et al., 2008). These studies have demonstrated a diminished UCR once the CS-UCS relationship has been learned, a process known as conditioned UCR diminution (Dunsmoor et al., 2008; Kimmel et al., 1967; Knight et al., 2010, 2011; Wood et al., 2012). Thus, as the threat (i.e., the UCS) becomes predictable, the response to the threat (i.e., the UCR) is modulated (Bouton et al., 2001; Domjan, 2005; Wagner & Brandon, 1989). Interestingly, these studies have also found a relationship between UCR expression and negative affect indexed by the State-Trait Anxiety Inventory (STAI; Spielberger, 1983; Knight et al., 2011; Wood et al., 2012) suggesting individual differences in emotional disposition modulate the response to threat. However, questions regarding whether the emotional response to threat also varies with locus of control and positive affect, independent of negative affect, remain unanswered.
Previous research has often taken a categorical approach to the study of anxious behavior. For example, clinical research has traditionally investigated groups with versus without anxiety disorders. This line of research has demonstrated important differences in both brain and behavior between patient and healthy control groups (Monk et al., 2006; Phan et al., 2005; Thayer et al., 1996; Zhao et al., 2007). Even in relatively healthy samples, prior work has often separated participants into groups of “high” and “low” anxiety. These studies have typically found a larger emotional response in “high” compared to “low” anxiety participants (Cook et al., 1992; Grillon et al., 1993; Smith et al., 2005). This categorical division of “high” and “low” anxiety groups however may not fully capture subtleties in the degree to which individual differences in anxiety impact the emotional response.
Therefore, more recent research has focused on differences between individuals, and has demonstrated that brain and behavioral data vary with indices of anxiety in a graded, rather than all or none, manner (Carre et al., 2013; Knight et al., 2011; Sehlmeyer et al., 2011; Wood et al., 2012). For example, functional magnetic resonance imaging (fMRI) research has shown graded changes in the blood-oxygen-level-dependent (BOLD) response that varies with the negative affect indexed by the STAI (Bishop et al., 2004, 2008; Wood et al., 2012; Vytal et al., 2014). Specifically, the BOLD response within prefrontal cortex (PFC), inferior parietal lobule (IPL), and amygdala often show a linear relationship to STAI scores (Bishop et al., 2008; Etkin et al., 2004; Klummp et al., 2011; Wood et al., 2012). Further, functional connectivity studies have found the connectivity strength between areas that include the PFC and amygdala varies with STAI scores (Wheelock et al., 2014; Vytal et al., 2014). This prior work suggests negative affect influences brain processes that mediate the emotional response. Prior research, however, has given limited attention to positive affect which varies independently of negative affect, and may also influence the emotional response (Brown et al., 1998; Gros et al., 2007). Thus, determining the impact of individual differences in positive and negative affectivity on BOLD fMRI and behavioral responses to threat may provide new insight into neural processes that mediate the emotional response.
Most prior work has focused on identifying linear relationships between the brain and behavior. However, non-linear brain-behavior relationships have also been observed. For example, prior emotion research has demonstrated a curvilinear relationship between emotional stimuli, psychophysiological responses, and the BOLD response (Bradley et al., 2001a, 2003; Lang et al., 1998; Wood et al., 2014). It is also well established that there is a curvilinear relationship between emotional arousal and many aspects of cognitive and behavioral performance (Dickman, 2002; Yerkes & Dodson, 1908). In addition, individuals with an “internal” locus of control show increased responses to uncontrollable threats and decreased responses to controllable threats, while those with an “external” locus of control show the opposite pattern (Bonnilli et al., 2004; Lundberg & Frankhausser, 1978). Further, brain structure also varies with locus of control. For example, hippocampal volume increases as locus of control increases (Preussner et al., 2005). This suggests the fMRI signal response may also be influenced by an individual’s locus of control. Taken together, these findings suggest that locus of control may also modulate brain and behavioral responses to threat. Thus, brain regions that mediate expression and regulation of emotion may show linear or non-linear relationships with locus of control and affective state.
The present study used a Pavlovian conditioning procedure to investigate the effect of individual differences in locus of control, positive affect, and negative affect on the emotional (i.e., brain and behavioral) response to predictable and unpredictable threat. Previous work has demonstrated differences in brain and behavioral responses to predictable and unpredictable threat (Dunsmoor et al., 2008; Knight et al., 2010, 2011; Wood et al., 2012). Individual differences in locus of control and affective state may influence learning-related changes in the brain and behavioral response to threat. However, these differences may also impact the response to threat independent of learning. Therefore, the present study focused on both learning-related changes in the response to threat and the response to threat in general. Given the role of the amygdala, hippocampus, PFC, IPL, and insula in emotional processes, we hypothesized the fMRI signal response would vary linearly or curvilinearly with locus of control, positive affect, and negative affect. Further, we hypothesized that learning-related changes in the neural response would vary (linearly and/or curvilinearly) with individual differences in locus of control, positive affect, and negative affect.
2. Methods
2.1 Participants
Thirty-six healthy participants were recruited from the Birmingham-Metropolitan area. All participants provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board. Four participants were excluded from all analyses for issues affecting general data quality (e.g., failure to follow instructions, non-responsiveness, and excessive movement). Thus, 32 healthy participants were included in the final analyses (12 female, 20 males; 14 Caucasian, 18 African-American; age: M = 18.84, SEM = 0.16, range = 17 – 22 years).
2.2 Stimuli
CS (10s duration) presentations consisted of two pure tones (700 Hz and 1300 Hz). One CS (CS+) co-terminated with the UCS (100-dB white noise, 0.5 s duration), while the other CS (CS-) was presented without the UCS. In addition, the UCS was also presented alone (UCS alone) on some trials. A total of 72 conditioning trials (18 s inter-trial interval) were presented across two fMRI scans (36 trials per scan; 12 CS+, 12 CS-, 12 UCS alone trials). Stimuli were presented in a pseudorandom order such that no more than two trials of any stimulus (CS+, CS-, and UCS alone) were presented consecutively. This study focused on brain and behavioral responses to the UCS (i.e., the threat) when it followed the CS+ (i.e., the CS+UCS) and when the UCS was presented alone (i.e., the UCS alone). Therefore, all analyses are related to trials in which a UCS was presented (i.e., CS+UCS and UCS alone). The response to the CS+ and CS-are important for understanding anticipatory processes that are not the focus of the present report, and will be presented separately.
2.3 Positive and Negative Affect Schedule
Participants completed the Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). The PANAS is a self-report measure consisting of 20 questions related to positive and negative affect (10 questions assessing positive affect, 10 questions assessing negative affect) scored on a one (not very likely) to five (very likely) Likert scale. Positive affect refers to the degree to which the participant’s experiences are pleasurable (e.g., interested, happy, etc.) while negative affect refers to the degree to which the participant’s experiences are unpleasant (e.g., upset, distressing, etc.). PANAS scores were computed as the sum of each affect schedule (positive affect or negative affect) for participants’ affective state in general.
2.4 Locus of Control
Participants’ locus of control was assessed via Rotter’s Locus of Control Questionnaire (Rotter, 1966). The questionnaire consists of 29 items with dichotomous answers that assess the degree to which one’s attributions of an event’s causality is internal (e.g., the individual caused the event) or external (e.g., the environment caused the event).
2.5 Skin Conductance Response and Electromyography
SCR and electromyography (EMG) data were collected using an MRI compatible Biopac MP150 data acquisition system (Biopac Systems; Goleta, CA). SCR data were sampled (10 KHz) from the thenar and hypothenar prominence of the nondominant hand. Analyses were performed offline using Biopac AcqKnowledge 4.1 software as described previously (Knight & Wood, 2011). In short, a 1 Hz low pass filter was applied and data were resampled at 250 Hz. Unconditioned SCRs (calculated as the onset to peak during the 10s after UCS presentation) were calculated as the difference in the participants’ skin conductance level at response onset from the peak skin conductance during the response. SCRs below 0.05 µS were scored as zero. SCR data were square-root transformed prior to statistical analyses.
EMG data were sampled (10 KHz) from the lower orbital portion of the left orbicularis oculi. One electrode was placed below the pupil, and the second electrode was placed lateral to the first (Blumenthal et al., 2005). Analyses were performed offline using Biopac AcqKnowledge 4.1 software based on previous guidelines (Blumenthal et al., 2005; Cook and Miller, 1992). The raw EMG signal was Fast Fourier Transformed to remove frequency domains containing noise (60 Hz Band-Stop filter, 28–400 Hz Kaiser-Bessel Band-Pass filter, combined band-stop filter at fMRI fundamental frequency of 17 Hz). The unconditioned EMG response was calculated as the difference between the mean EMG response during the 20 ms after UCS onset and the maximum response during the 21–150 ms after UCS onset (Blumenthal et al., 2005). We used a linear mixed-effect models to assess the relationship between the self-report scores (i.e., locus of control, positive affect, and negative affect to assess linear relationships, and each 2nd-order term to assess curvilinear relationships), threat predictability, and the psychophysiological response (i.e., SCR and EMG). A linear mixed-effects model was created to assess the influence of the self-report scores (i.e., locus of control, positive affect, and negative affect to assess linear relationships, and each 2nd-order term to assess curvilinear relationships) and threat predictability on SCR. A second model was created to assess the influence of the self-report scores (i.e., locus of control, positive affect, and negative affect to assess linear relationships, and each 2nd-order term to assess curvilinear relationships) and threat predictability on EMG.
2.6 UCS Expectancy
UCS expectancy was measured continuously (40 Hz sampling rate) throughout conditioning as described in prior work (Knight & Wood, 2011). A rating scale ranging from 0 to 100 was displayed on an IFIS-SA LCD monitor (Invivo Crop.; Gainesville FL) using Presentation software (NeuroBehavioral Systems, Inc.; Albany, CA). Participants’ rated their expectation of UCS presentation using a rating bar on a 0 (certain the UCS would not be presented) to 100 (certain the UCS would be presented) scale using an MRI compatible joystick (Current Designs; Philadelphia, PA). UCS expectancy was calculated as the average expectancy rating during the 1s before UCS onset. A linear mixed-effects model was created to assess the influence of the self-report scores (i.e., locus of control, positive affect, and negative affect to assess linear relationships, and each 2nd-order term to assess curvilinear relationships) and threat predictability on UCS expectancy.
2.7 Functional Magnetic Resonance Imaging
MRI scans were obtained on a 3T Siemens Allegra scanner. High resolution anatomical scans (MPRAGE) were collected in the sagittal plane (TR=2300 ms, TE=3.9 ms, flip angle=12°, FOV=25.6 cm, matrix=256×256, slice thickness=1 mm, 0.5 mm gap). The blood-oxygen-level-dependent (BOLD) fMRI signal was measured with a gradient-echo echoplanar pulse sequence in an oblique axial orientation (TR=2000 ms, TE=30 ms, flip angle=70°, FOV=24 cm, matrix=64×64, voxel size=3.75 × 3.75 × 4 mm, no gap). Data analyses were performed with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Echoplanar image (EPI) time-series data were corrected for slice-timing offset, motion corrected, spatially smoothed with a 4 mm full-width-at-half-maximum Gaussian filter, and concatenated. On average, less than 1 mm of motion was observed during the scanning session (M = 0.63, SEM = 0.04). TRs were assessed for high motion by detrending the time series of each voxel up to three Legendre polynomials. The resulting trend was used to calculate the median absolute deviation, which was used to compute voxel-wise outliers. Outliers were defined as voxels with values that deviated more than five times the median absolute deviation. Whole-brain volumes in which more than three percent of voxels were outliers were then temporally censored.
Functional maps were generated at the individual participant level using a general linear regression. Activity was modeled with a gamma variate hemodynamic response function with reference waveforms for all stimuli (i.e., CS+, CS-, CS+UCS, UCS alone) and regressors to account for participant head motion and joystick movement. Regressors of interest for this study modeled the BOLD response to the UCS (i.e., CS+UCS and UCS alone). Percent signal change was used as an index of the amplitude of the BOLD response to UCS presentation. Data were then normalized to the Talairach and Tournoux stereotaxic coordinate system (Talairach and Tournoux, 1988), resampled (i.e., 1 mm3 resolution), and group level analyses were completed.
Functional maps representing the UCR in percent BOLD signal change elicited by the CS+UCS and UCS alone trials were included in group level analyses with locus of control and PANAS scores. A single linear mixed effects model assessed the relationship between the unconditioned fMRI signal response to threat and the self-report scores (i.e., locus of control, negative affect, positive affect, and each variables 2nd-order term). An additional linear mixed effects model assessed the relationship between the unconditioned fMRI signal response and the behavioral (i.e., SCR and EMG) response. All group level analyses were performed using AFNI’s 3dLME program.
Functional MRI analyses were restricted using an anatomical mask based on the Talairach and Tournoux coordinate system (Talairach and Tournoux, 1988) to a priori regions of interest (ROI) based on prior work from our laboratory (Dunsmoor et al., 2008; Knight et al., 2010; Wheelock et al., 2014; Wood et al., 2012, 2013). These regions included the PFC, IPL, posterior cingulate, insula, hippocampus, and amygdala. Monte Carlo simulations were performed with AFNI’s 3dClustSim program (Cox, 1996) using a voxel wise threshold of p < 0.01 and a cluster volume of 736 mm3 (equivalent to 13 voxels at the originally acquired 3.75 mm x 3.75 mm x 4 mm resolution) to determine the corrected significance threshold of p < 0.05.
3. Results
3. 1 Self-Report Questionnaires
PANAS and Rotter’s Locus of Control questionnaires were included in linear mixed effects analyses on brain and behavioral data to assess the influence of locus of control, positive affect, and negative affect on these measures. All correlations between locus of control, positive affect, and negative affect were nonsignificant (all p > 0.05; Table S1). Prior to analyses, all predictor variables were mean-centered around zero. Polynomial terms were then created from the mean-centered data. One participant was excluded as an outlier from the analyses because of the influence of their data on the model solutions (i.e., leverage values greater than 0.5; Chatterjee & Hati, 1986; Rousseeuw & Leroy, 2005). Positive affect (M = 34.22, SD = 8.66, range = 12 – 49), negative affect (M = 15.91, SD = 4.97, range = 10 – 25), and locus of control (M = 11.09, SD = 3.77, range = 3 – 17) scores for the remaining participants were similar to those reported in prior work (Henry and Crawford, 2004; Rotter, 1966).
3.2 UCS Expectancy
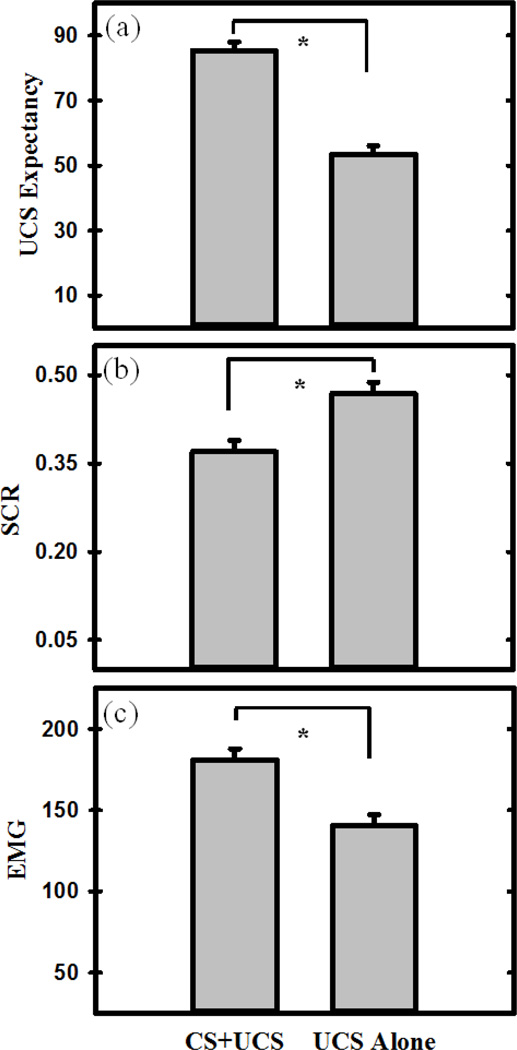
The linear mixed effects model revealed differences in UCS expectancy between CS+UCS and UCS alone trials (Figure 1a). The contrast revealed that UCS expectancy was greater on CS+UCS (predictable) trials than UCS alone (unpredictable) trials (F (1,24) = 155.37, p < 0.001). The model did not reveal any significant relationships between the self-report scores (i.e., locus of control, positive affect, and negative affect) and UCS expectancy ratings (all F < 1.00).
Figure 1.
Learning-related differences in the behavioral response to threat. a) UCS expectancy was greater to predictable (CS+UCS) than unpredictable (UCS alone) trials. b) Unconditioned SCR amplitude was diminished on predictable compared to unpredictable trials (i.e., conditioned UCR diminution). c) EMG response was potentiated on predictable compared to unpredictable trials. The graphs depict the mean UCS Expectancy, SCR, and EMG response to CS+UCS (predictable) and UCS alone (unpredictable) trials. Error bars reflect the within-subjects standard error of the mean (SEM) (Loftus and Masson, 1994). Asterisk indicates a significant difference.
3.3 Skin Conductance Response
SCR data for one participant was incomplete due to technical failure. This participant was therefore excluded from the SCR analyses. The linear mixed effects model revealed a significant difference between unconditioned SCR to CS+UCS (predictable) and UCS alone (unpredictable) trials (Figure 1b) (F (1,23) = 6.94, p < 0.01). Specifically, UCR magnitude was reduced to the CS+UCS compared to the UCS alone. The model did not reveal any significant relationships between the self-report scores (i.e., locus of control, positive affect, and negative affect) and SCR (all F < 1.00).
3.4 Electromyography
The linear mixed-effects model revealed a differential EMG response to CS+UCS (predictable) versus UCS alone (unpredictable) trials (Figure 1c). A significant difference was observed between EMG activity to the CS+UCS versus UCS alone (F (1,24) = 10.76, p < 0.001). Specifically, EMG activity was potentiated by the CS+UCS compared to the UCS alone, demonstrating predictability influenced the unconditioned EMG response to threat. The model did not reveal any significant relationships between the self-report scores (i.e., locus of control, positive affect, and negative affect) and EMG (all F < 1.00).
3.5 Functional MRI
3. 5.1 Individual Differences in the Average Unconditioned fMRI Signal Response
3.5.1.1 Linear Relationships with Average fMRI Signal Response
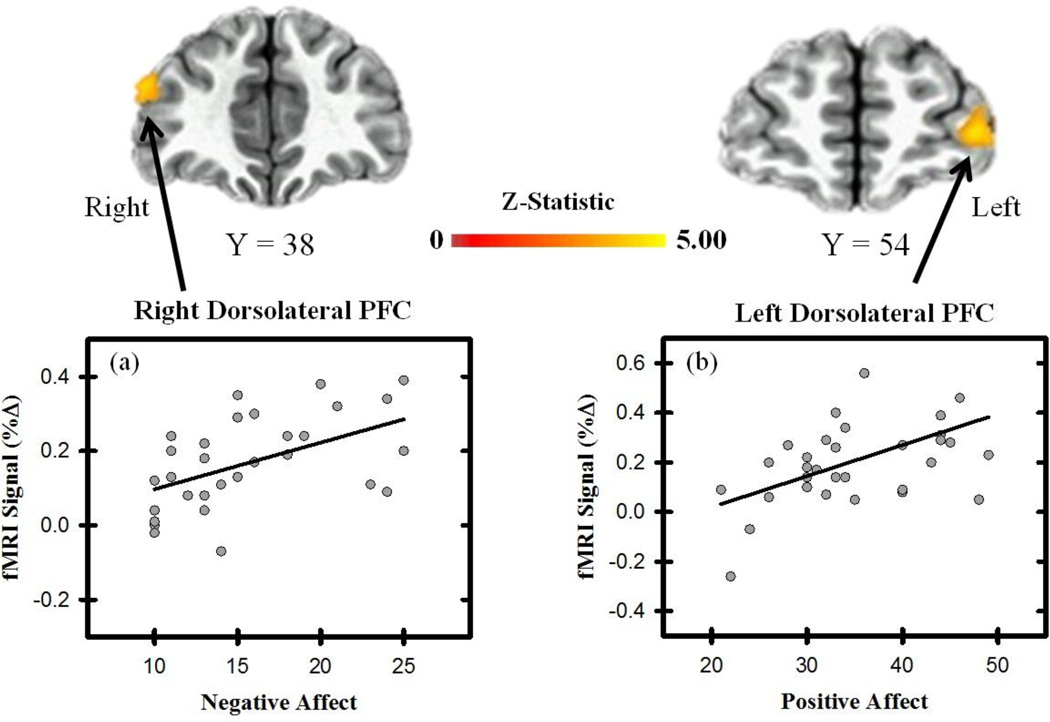
The linear mixed effects model revealed the unconditioned fMRI signal response within several brain regions varied with positive and negative affect (Table 1 & Figure 2). Specifically, a positive linear relationship was observed between positive affect and the average UCR within the superior frontal gyrus (SFG) and dlPFC (Figure 2). A significant positive relationship was also observed between negative affect and the unconditioned fMRI signal response within the dlPFC and dmPFC. No significant relationship was observed between locus of control and the average response to threat (all p > 0.05 corrected). These findings indicate that as positive and negative affect increase, the neural response to threat increases within the PFC.
Table 1.
| Average UCR | |||||||
| Linear Effect | Structure | Hemisphere | Z-Statistic |
Talairach Coordinates (X,Y,Z) |
Cluster (mm3) |
||
| Locus of Control | |||||||
| Ventrolateral PFC | Left | 2.95 | −28 | 49 | −2 | 788 | |
| Negative Affect | |||||||
| Dorsomedial PFC | Right | 3.25 | 14 | −2 | 42 | 1179 | |
| Dorsolateral PFC | Right | 3.20 | 47 | 38 | 22 | 955 | |
| Insula | Right | 2.90 | 40 | 5 | 13 | 794 | |
| Positive Affect | |||||||
| SFG | Left | 3.12 | −15 | 18 | 52 | 2645 | |
| Right | 2.99 | 12 | 18 | 48 | 815 | ||
| Dorsolateral PFC | Left | 3.10 | −33 | 53 | 7 | 1045 | |
| Right | 3.16 | 50 | 33 | 26 | 788 | ||
|
Curvilinear Effect Negative Affect |
|||||||
| Insula | Right | −3.01 | 39 | −10 | 14 | 2484 | |
| Dorsomedial PFC | Left | −3.10 | −1 | −22 | 57 | 1632 | |
| Left | −2.84 | −9 | 6 | 39 | 803 | ||
| Right | −2.92 | 13 | −2 | 42 | 783 | ||
| Right | −2.97 | 12 | −2 | 41 | 748 | ||
| SFG | Right | −3.04 | 16 | 63 | 10 | 1188 | |
| Dorsolateral PFC | Left | −3.00 | −35 | 39 | 29 | 945 | |
| Positive Affect | |||||||
| Dorsolateral PFC | Left | −3.03 | −48 | 18 | 17 | 1053 | |
| SFG | Left | −3.14 | −18 | 44 | 37 | 1521 | |
| Right | −3.00 | 16 | 39 | 45 | 1350 | ||
| Left | −3.04 | −18 | 22 | 53 | 945 | ||
| Differential UCR | |||||||
|
Linear Effect Locus of Control |
|||||||
| Ventromedial PFC | Left | 3.26 | −2 | 34 | −3 | 1242 | |
| Right | 3.00 | 6 | 31 | −4 | 759 | ||
| Negative Affect | |||||||
| Posterior Cingulate | Left | −3.12 | −12 | −51 | 19 | 1388 | |
| Dorsolateral PFC | Left | −2.95 | −45 | 38 | 19 | 1241 | |
| SFG | Left | −3.36 | −23 | 10 | 45 | 952 | |
| Curvilinear Effect | |||||||
| Locus of Control | |||||||
| Dorsolateral PFC | Right | −2.95 | 36 | 54 | 17 | 2002 | |
| Ventrolateral PFC | Right | −3.30 | 34 | 32 | −12 | 1009 | |
| Left | −3.15 | −27 | 56 | −4 | 972 | ||
| Negative Affect | |||||||
| Dorsolateral PFC | Left | 3.06 | −50 | 35 | 15 | 1404 | |
| SFG | Left | 3.23 | −24 | 11 | 46 | 1080 | |
| Average UCR | |||||||
|
Linear Effect Unconditioned SCR |
|||||||
| Dorsomedial PFC | Right | 3.13 | 14 | 38 | 15 | 18036 | |
| Right | 3.29 | 5 | 26 | 51 | 1782 | ||
| Posterior Cingulate | Right | 3.24 | 11 | 50 | 9 | 2430 | |
| Ventrolateral PFC | Right | 3.16 | 47 | 20 | −7 | 1728 | |
| Left | 3.14 | −32 | 17 | −13 | 1620 | ||
| Posterior Cingulate | Left | 2.96 | −14 | −59 | 15 | 1539 | |
| Right | 2.86 | 5 | −53 | 30 | 1161 | ||
| SFG | Left | 2.94 | −23 | 35 | 45 | 1107 | |
| Dorsomedial PFC | Left | 3.13 | −2 | −11 | 60 | 756 | |
| Amygdala* | Left | 2.80 | −23 | −2 | −13 | 162 | |
| Right | 3.00 | 29 | 5 | −16 | 270 | ||
| Unconditioned EMG | |||||||
| Dorsomedial PFC | Right | 3.27 | 8 | 38 | 48 | 14769 | |
| Dorsolateral PFC | Right | 3.11 | 53 | 11 | 36 | 6723 | |
| Left | 2.97 | −50 | 23 | 33 | 3942 | ||
| Insula | Left | 2.97 | −32 | 23 | 3 | 1728 | |
| Posterior Cingulate | Left | 3.12 | −14 | −56 | 15 | 1728 | |
| Right | 3.19 | 11 | −53 | 9 | 1566 | ||
| Ventrolateral PFC | Right | 3.02 | 32 | 11 | −13 | 1053 | |
| IPL | Right | 3.05 | 47 | −47 | 24 | 810 | |
|
Curvilinear Effect Unconditioned SCR |
|||||||
| Dorsomedial PFC | Right | −3.11 | 5 | 38 | 48 | 1134 | |
| IPL | Right | −2.87 | 56 | −29 | 24 | 891 | |
| Ventromedial PFC | Right | −3.07 | 2 | 29 | −1 | 864 | |
| Ventrolateral PFC | Left | −3.29 | −32 | 17 | −13 | 810 | |
| Amygdala* | Left | −3.93 | −17 | −5 | −16 | 243 | |
| Unconditioned EMG | |||||||
| Dorsomedial PFC | Left | −3.11 | −5 | 29 | 54 | 13230 | |
| Dorsolateral PFC | Right | −3.02 | 40 | 8 | 42 | 2484 | |
| Right | −3.08 | 53 | 26 | 24 | 1080 | ||
| Ventrolateral PFC | Left | 4.50 | −53 | 26 | −7 | 1350 | |
| Left | −2.87 | −38 | 20 | 9 | 1026 | ||
| Posterior Cingulate | Left | −3.00 | −11 | −56 | 15 | 1269 | |
| Right | −3.28 | 11 | −53 | 9 | 1134 | ||
Coordinates are for the center of mass of the cluster. Z-Statistic corresponds to the average Z-Statistic within the cluster. SCR = Skin conductance response, EMG = Electromyography, PFC = Prefrontal Cortex, SFG = Superior Frontal Gyrus, IPL = Inferior Parietal Lobule.
Analyses investigating curvilinear relationships within the amygdala and hippocampus were completed using multiple polynomial regressions restricted to an anatomical mask of the amygdala and bilateral hippocampus respectively. Functional maps were small volume corrected using Monte Carlo simulations for the amygdala (cluster volume of 90 mm3 or 1.6 voxels of 3.75 mm x 3.75 mm x 4mm resolution) and the hippocampus (cluster volume of 102 mm3 or 1.8 voxels of 3.75 mm x 3.75 mm x 4mm resolution).
Figure 2.
Unconditioned fMRI signal and affective state. Brain activity to the UCS (average of the CS+UCS and UCS alone) varied linearly with negative affect within the right dlPFC (a), and with positive affect in the left dlPFC (b). Graphs depict the relationship between fMRI signal (% change) and PANAS (positive and negative affect) scores.
3.5.1.2 Curvilinear Relationships with Average fMRI Signal Response
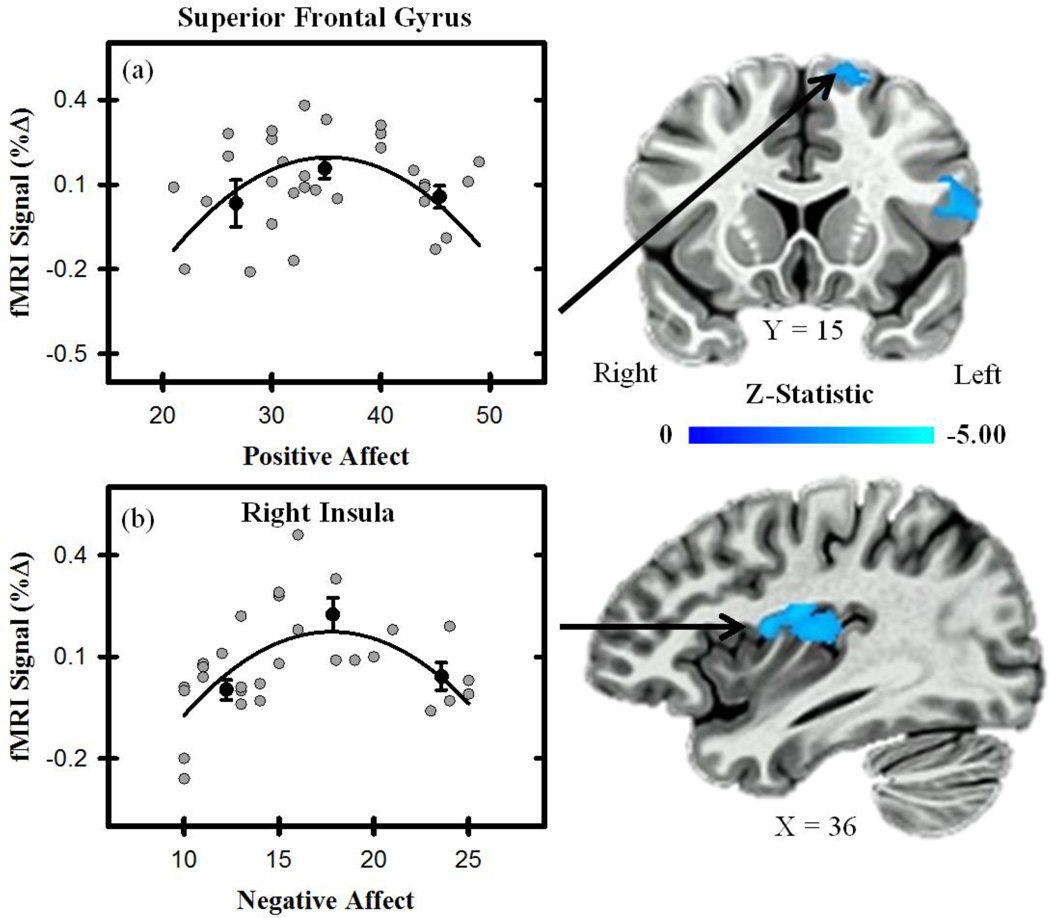
The linear mixed effects model also revealed that positive and negative affect varied curvilinearly with brain activity (Table 1 & Figure 3). Specifically, a curvilinear relationship was observed between positive affect and the unconditioned fMRI signal response within the dlPFC and SFG. A curvilinear relationship was also observed between negative affect and the unconditioned fMRI signal response within the SFG, dmPFC, and insula. No significant curvilinear relationship was observed between locus of control and the average response to threat (all p > 0.05 corrected). These results indicate positive and negative affect have a non-linear relationship with the unconditioned fMRI signal response in several brain regions.
Figure 3.
Unconditioned fMRI signal and affective state. Activity (average to CS+UCS and UCS alone) within the superior frontal gyrus varied curvilinearly with positive affect (a), and activity within the insula varied curvilinearly with negative affect (b). Graphs depict the relationship between fMRI signal (% change) and PANAS (positive and negative affect) scores. Gray dots represent scatter plot of individual participant scores. Black dots represent the % signal change binned by PANAS (positive and negative affect) scores for the top, middle, and bottom third of participants. Error bars reflect the standard error of the mean. Black line represents curvilinear line of best fit.
3.5.2 Individual Differences in the Differential Unconditioned fMRI Signal Response
3.5.2.1 Linear Relationships with the Differential fMRI Signal Response
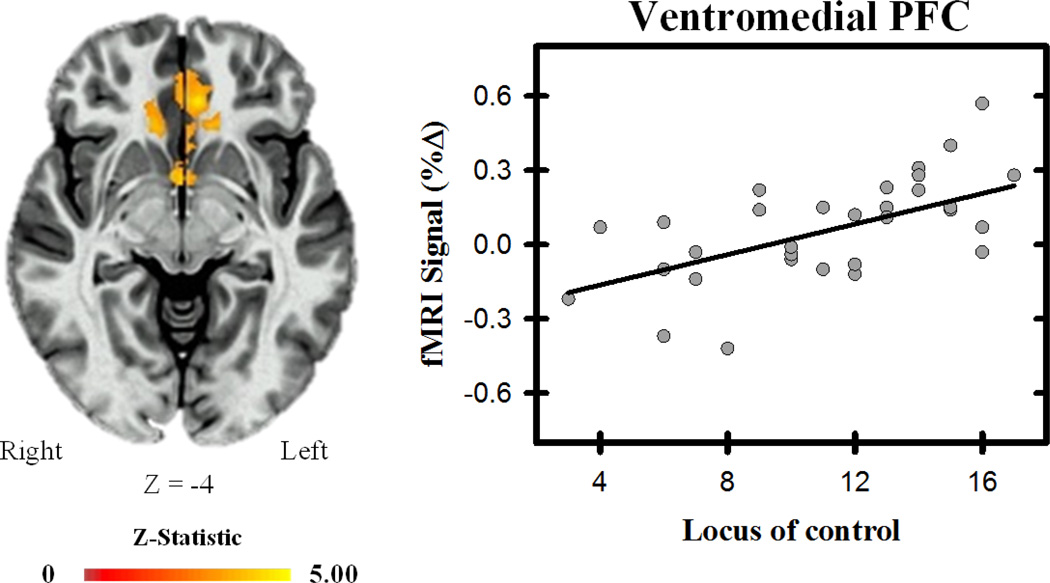
The linear mixed effects model revealed linear effects between locus of control and the differential response to the UCS (i.e., conditioned UCR diminution) within bilateral vmPFC (Table 1 & Figure 4). Specifically, as locus of control became more external, differential activity in the vmPFC increased. The linear mixed effects model revealed that the fMRI signal response to the UCS alone did not vary with locus of control, while a negative relationship was observed between locus of control and the fMRI signal response to the CS+UCS, suggesting the effect is mediated by predictable threat. No linear relationships were observed between positive or negative affect and the differential unconditioned fMRI signal response (all p > 0.05 corrected). Taken together, these data suggest that as individuals move along the spectrum of internal to external locus of control, the response to predictable threat decreases within the vmPFC.
Figure 4.
Ventromedial PFC and locus of control. The differential unconditioned fMRI signal response within the vmPFC varied with participants’ locus of control, such that as locus of control became more external, the differential response (i.e., UCS alone minus CS+UCS) increased. Graph depicts the relationship between fMRI signal (% change) and locus of control scores.
3.5.2.2 Curvilinear Relationships with Differential fMRI Signal Response
Significant curvilinear effects between the self-report scores and the differential unconditioned fMRI signal response were also observed (Table 1). Specifically, there was a curvilinear relationship between locus of control and differential BOLD activity within the dlPFC and ventrolateral PFC (vlPFC). The linear mixed effects model revealed a curvilinear relationship between locus of control and the unconditioned fMRI signal response to CS+UCS trials but not the UCS alone, within these regions. A significant curvilinear relationship between negative affect and conditioned UCR diminution in the dlPFC and SFG was also observed. Specifically, a curvilinear relationship between negative affect and the unconditioned fMRI signal response to CS+UCS trials, but not the UCS alone. No significant relationship was observed between positive affect and the differential response to threat. Taken together, these results suggest that locus of control and negative affect interact with stimulus predictability to influence the unconditioned fMRI signal response to threat within the PFC.
3.5.3 Brain and Psychophysiological Response to Threat
3.5.3.1 Linear Relationships between Brain and Psychophysiological Response
A separate linear mixed effects model compared unconditioned SCR and EMG to the fMRI signal response. We observed brain regions that showed linear, curvilinear, or both effects between the unconditioned SCR, EMG, and fMRI signal response (Table 1). A significant positive linear effect was observed between the unconditioned SCR and the unconditioned fMRI signal response within the dlPFC, dmPFC, IPL, insula, posterior cingulate, and amygdala. A significant positive linear relationship was also observed between the unconditioned EMG response and the unconditioned fMRI signal response within the dmPFC, dlPFC, vlPFC, IPL and insula. This data is consistent with results from other studies suggesting that these brain regions mediate the emotional response to threat (Cheng et al., 2003, 2006, 2007; Knight et al., 2005; Wood et al., 2012, 2014).
3.5.3.2 Curvilinear Relationships between Brain and Psychophysiological Response
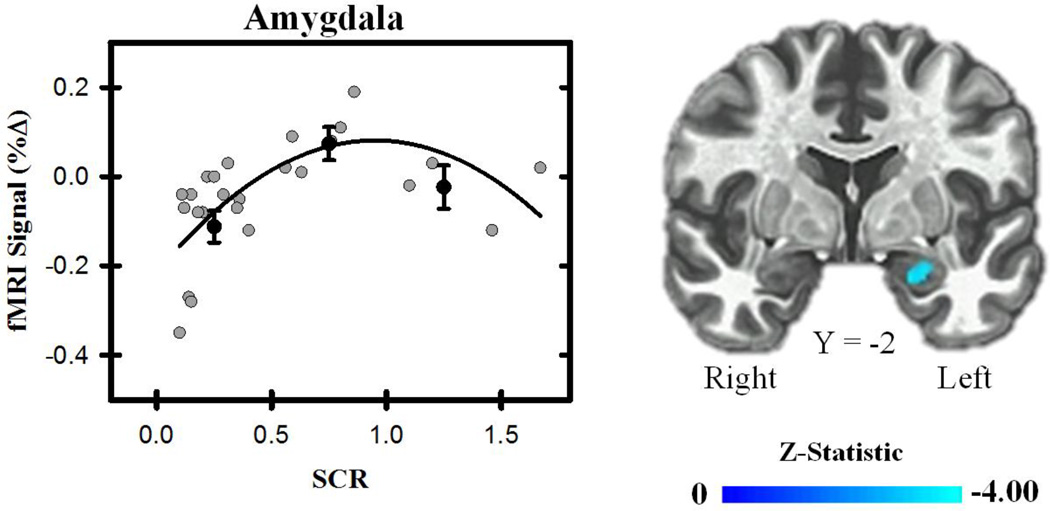
A significant curvilinear effect was also observed between unconditioned SCR (Figure 5) and the fMRI signal response. Specifically, unconditioned SCR varied curvilinearly with the fMRI signal response in the dmPFC, vlPFC, vmPFC, and IPL. We also observed a curvilinear relationship between the unconditioned EMG and fMRI signal response within dmPFC, dlPFC, vlPFC, and posterior cingulate. Due to the relatively small volume of both the amygdala and hippocampus, we performed follow-up analyses in which each brain structure was anatomically masked and corrected for multiple comparisons in relation to their relatively smaller volume. A significant curvilinear effect was observed between the unconditioned fMRI signal response in the amygdala and the unconditioned SCR. No relationship was observed between the unconditioned fMRI signal response in the hippocampus and the unconditioned SCR. No curvilinear relationships between the unconditioned fMRI signal response in the amygdala or hippocampus and unconditioned EMG were observed (all p < 0.05). Taken together, these results suggest the unconditioned behavioral response shares a curvilinear relationship with the unconditioned fMRI signal response to threat in several brain regions.
Figure 5.
Functional MRI signal and behavior. Threat-related brain activity (average of the CS+UCS and UCS alone) varied curvilinearly with participants’ unconditioned SCR within the amygdala. Graph depicts the relationship between fMRI signal (% change) and SCR. Gray dots represent scatter plot of individual participant’s SCR (excluding non-responders). Black dots represent the % signal change binned by unconditioned SCR for the top, middle, and bottom third of participants. Error bars reflect the standard error of the mean. Black line represents curvilinear line of best fit.
3.5.3.3 Relationships between Differential Brain and Psychophysiological Response
The linear mixed effects model assessed the effect of the unconditioned SCR and EMG on the differential (i.e., UCS alone minus CS+UCS) fMRI signal response. No significant linear or curvilinear relationships were observed (p > 0.05, corrected).
4. Discussion
The ability to effectively respond to threats in our environment is important for healthy emotional function. However, the response to threat varies considerably from person-to-person. This variability may be explained, in part, by individual differences in factors that influence emotional processes, such as locus of control and affective state. However, the neural substrates that mediate the effect of locus of control and affective state on the response to threat are not well characterized. Therefore, the current study investigated the relationship that individual differences in locus of control and affective state have with the neural (i.e., BOLD fMRI) and behavioral (i.e., SCR, EMG, UCS expectancy) response to threat. The present findings demonstrate learning-related changes of the fMRI signal response within the vmPFC that vary with an individual’s perceptions of personal control. The results also suggest an individual’s affectivity modulates activity within other prefrontal brain regions responsible for regulating the emotional response to threat (i.e., dlPFC and dmPFC). Taken together, these findings suggest that prefrontal brain regions mediate the influence cognitive-affective predispositions have on threat-relevant learning and the subsequent emotional response.
A novel finding from the current study is that stimulus predictability and locus of control interacted to influence brain activity within the vmPFC. The vmPFC is a central component of a PFC – amygdala network that regulates the emotional response (Delgado et al., 2008; Denny et al., 2014; Hartley & Phelps, 2010; LeDoux, 2000; Ochsner et al., 2012). Functionally, the vmPFC is inversely coupled with the amygdala, such that vmPFC activity is associated with inhibition of the amygdala. In turn, amygdala activity controls the peripheral emotional response (Cheng et al., 2003; Knight et al., 2005; Urry et al., 2006). Thus, the vmPFC appears to modulate learning-related changes in the emotional response. Specifically, prior research has demonstrated activity within the vmPFC that varies with learning-related processes that facilitate emotion regulation (Dunsmoor et al., 2008; Knight et al., 2010; Phelps et al., 2004; Quirk et al., 2000; Sierra-Mercado et al., 2006; Wood et al., 2012, 2013). Consistent with this prior work, the present findings show learning-related changes in vmPFC activity that are associated with learning-related changes in unconditioned SCR. Interestingly, we also observed that as individuals move along the spectrum of internal to external locus of control, the response to predictable threat decreased within the vmPFC. Locus of control represents an individual’s perceptions about an event’s causality, and an external locus of control has been linked to failures of emotion regulation (Burger, 1984; Johnson & Sarason, 1977; Rotter, 1966). Thus, the decreased vmPFC activity associated with an external locus of control may reflect a diminished ability to regulate the emotional response to predictable threat. The present study suggests that learning-related changes in the emotional response are mediated by the vmPFC, and these changes are influenced by an individual’s perceptions of control.
In addition to the positive linear relationship between locus of control and brain activity, we also observed positive linear relationships between affective state and brain activity within dlPFC and dmPFC. Specifically, we observed a positive linear relationship between negative affect and brain activity within right dlPFC and dmPFC, and a positive linear relationship between positive affect and brain activity within bilateral dlPFC and left SFG. Dorsolateral PFC and dmPFC appear to support emotion regulation via top-down processes (Ochsner et al., 2005; Silvers et al., 2013). These brain regions are also involved in processes that support selective attention and negative reactions to threatening stimuli, and are modulated by negative affectivity as measured by the STAI (Bishop et al., 2009; Kalisch & Gerlicher, 2014; Shackman et al., 2011; Wood et al., 2012). Thus, this prior work suggests affectivity may influence the selective attention processes mediated by the PFC. Interestingly, individuals with elevated anxiety tend to show hypervigilance to threats within their environment (MacLeod, 1986; Mogg et al., 2004; Mogg & Bradley, 1998). The linear relationships we observed between affectivity and PFC activity in the present study suggest the dlPFC and dmPFC may mediate the hypervigilance to threat that typically accompanies elevated negative affectivity.
Interestingly, we observed laterality differences in the relationships negative and positive affect have with dlPFC activity. Specifically, while positive affectivity varied with both left and right dlPFC activity, negative affectivity varied only with right dlPFC activity. Previous emotion research has demonstrated laterality differences within the PFC related to affective information processing, wherein the right hemisphere was predominately associated with negative emotion (i.e., avoidance processes), while the left was predominately associated with positive emotion (i.e., approach processes) (Schwartz et al., 1978; Allen et al., 2001; Davidson & Irwin, 1999; Davidson, 2004). Thus, the present findings are consistent with prior work that suggests left and right hemispheres of the PFC differentially contribute to affective processing.
Curvilinear effects were also observed between affective state (i.e., PANAS) and the neural response within the dmPFC, dlPFC, and insula. Specifically, individuals with relatively high and low affectivity showed a diminished neural response to threat compared to those with an intermediate level of affectivity. These results suggest that individual differences in affectivity may have a non-linear impact on the brain activity that supports emotion regulation processes. Nonlinearity between brain function and behavior has been previously reported (Buchel et al., 1998; Elliot et al., 2003). For example, prior work has demonstrated visual cortex activity varies curvilinearly with the emotional valence of stimuli while medial PFC activity varies curvilinearly with reward value (Bradley et al., 2003; Elliot et al., 2003). Non-linear relationships have also been observed in psychophysiological indices of the emotional response (Bradley et al., 2001a, 2001b; Wood et al., 2014), wherein high arousal pleasant and unpleasant images elicit greater SCR than neutral images. This increased SCR is likely due to changes in amygdala activity that mediates expression of the peripheral emotional response (Cheng et al., 2003, 2006; Knight et al., 2005), and these findings are consistent with the view that the PFC’s ability to regulate amygdala activity is modulated by an individual’s affectivity.
The current study demonstrated both linear and curvilinear relationships between brain and behavioral responses to threat. Our findings are largely consistent with prior research that has demonstrated the amygdala, insula, PFC, IPL and PCC control and regulate important components of the emotional response (Baskin-Sommers et al., 2012; Hurleman et al., 2015; Kalisch & Gerlicher, 2014; Knight et al., 2005; LeDoux, 2000; Ochsner et al., 2005; Robinson et al., 2014; Wood et al., 2012). However, the present study demonstrates that there are a variety of linear and curvilinear relationships between cognitive-affective function, brain activity, and behavioral responses. Thus, the specific dynamics by which these various relationships result in the final peripheral emotional response requires further investigation. More specifically, future research should aim to determine the relative contributions of the various linear and curvilinear brain-behavior relationships to the final observed psychophysiological response. For instance, it is well established that the amygdala is important for learning-related modulation of the peripheral emotional response via descending projections to the hypothalamus (Critchley, 2002; Cheng et al., 2003; Helmstetter et al., 1992; Helmstetter & Bellgowan, 1994; Knight et al., 2005). However, the hypothalamus also receives projections from other brain regions, such as the PFC (Buijs & Van Eden, 2000; Floyd et al., 2001). Specifically, the vmPFC has direct projections to hypothalamus, as does the amygdala, to trigger the peripheral emotional response (Buijs & Eden, 2000). Therefore, PFC and amygdala projections to the hypothalamus appear to act in tandem to control somewhat independent aspects of the peripheral emotional response. Thus, the peripheral emotional response is likely a combination of multiple brain regions that support distinct cognitive-affective processes working together. This view is consistent with previous work that has found functional coupling between PFC, amygdala, and hypothalamic activity (Grecius et al., 2003; Kilpatrick et al., 2006; Wheelock et al., 2014). However, the relative contributions of these and other brain regions, as well as how these contributions vary across cognitive-affective tasks, to the final peripheral emotional response requires further study.
Conclusions
The present study demonstrates that cognitive-affective predispositions (i.e., locus of control and affective state) modulate threat-related activity within brain regions that mediate the emotional response to threat. Specifically, perceptions of control influenced learning-related activity within the vmPFC. Further, positive and negative affectivity varied with threat-related activation within dlPFC and dmPFC, such that a diminished neural response was observed in those with relatively low and high affectivity. Interestingly each of these prefrontal brain regions plays an important role in the regulation of the emotional response. The present study demonstrates the importance of considering the effect cognitive-affective predispositions have on the brain-behavioral response. Understanding these relationships may be particularly important for understanding emotion-related disorders in which altered cognitive-affective processes may have a profound impact on the formation of conditioned threat-related associations.
Supplementary Material
Highlights.
Individual differences in the learning-related threat response were investigated.
The peripheral emotional response varied with threat predictability.
Learning-related ventromedial PFC activity varied with locus of control.
Threat-related neural activity varied with affectivity in a curvilinear fashion.
Acknowledgements
The authors would like to thank Edwin W. Cook III for statistical assistance as well as Josh Shumen for aid in collecting the data. This research was funded by the National Institute of Mental Health of the National Institutes of Health under award number R01-MH098348 (S. M. & D. C. K.), and the University of Alabama at Birmingham, Office for Equity and Diversity’s CMFSDP Fellowship (N.G.H.).
Abbreviations footnote
- CS
conditioned stimulus
- UCS
unconditioned stimulus
- CS+
CS that predicts the UCS
- CS-
CS that predicts the omission of the UCS
- CS+UCS
UCS that follows the CS+
- CR
conditioned response
- UCR
unconditioned response
- SCR
skin conductance response
- PFC
prefrontal cortex
- IPL
inferior parietal lobule
- PCC
posterior cingulate cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JJ, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38:685–693. [PubMed] [Google Scholar]
- Baskin-Sommers AR, Curtin JJ, Larson CL, Stout D, Kiehl KA, Newman JP. Characterizing the Anomalous Cognition-Emotion Interactions in Externalizing. Biological Psychology. 2012;91:48–58. doi: 10.1016/j.biopsycho.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R. Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of Experimental Psychology. 1966;71:447–451. doi: 10.1037/h0022977. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Brett M, Lawrence A. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bollini AM, Walker EF, Hamann S, Kestler L. The Influence of perceived control and locus of control on the cortisol and subjective response to stress. Biological Psychology. 2004;67:245–260. doi: 10.1016/j.biopsycho.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Mineka S, Barlow DH. A Modern learning theory perspective on the etiology of Panic Disorder. Psychological Review. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001a;1:276–299. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001b;1:300–319. [PubMed] [Google Scholar]
- Bradley MM, Sabantinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the endocrine system. Progress in Brain Research. 2000;126:117–132. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- Burger JM. Desire for control, locus of control, and proneness to depression. Journal of Personality. 1984;52:71–89. doi: 10.1111/j.1467-6494.1984.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Canli T, Detmer WM, Donegan NH. Potentiation or diminution of discrete motor unconditioned responses (rabbit eyeblink) to an aversive Pavlovian unconditioned stimulus by two associate processes: Conditioned fear and a conditioned diminution of unconditioned stimulus processing. Behavioral Neuroscience. 1992;106:498–508. doi: 10.1037//0735-7044.106.3.498. [DOI] [PubMed] [Google Scholar]
- Carre A, Gierski F, Lemogne C, Tran E, Raucher Chene D, Limosin F. Linear association between social anxiety symptoms and neural activations to angry faces: from subclinical to clinical levels. Social Cognitive and Affective Neuroscience. 2013;9:880–886. doi: 10.1093/scan/nst061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Hadi AS. Influential observations, high leverage points, and ouliters in linear regression. Statistical Science. 1986;1:379–393. [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: Stimulus processing versus response expression. Behavioral Neuroscience. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards JA, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning and Memory. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The Development of anxiety: The Role of control in the early environment. Psychological Bulletin. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Cook EW, Miller GA. Digital filtering: Background and tutorial for psychophysiologists. Psychophysiology. 1992;29:350–367. doi: 10.1111/j.1469-8986.1992.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Cook EW, 3rd, David TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of clinical Psychology. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Electrodermal responses: What happens in the brain. The Neuroscientist. 2002;8:132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Science. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: Perspectives in frontal EEG asymmetry research. Biological Psychology. 2004;67:219–234. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD. Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Social Cognitive and Affective Neuroscience. 2014;9:403–411. doi: 10.1093/scan/nss148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman SJ. Dimensions of arousal: Wakefulness and vigor. Human Factors. 2002;44:429–442. doi: 10.1518/0018720024497673. [DOI] [PubMed] [Google Scholar]
- Domjan M. Pavlovian conditioning: a functional perspective. Annual Reviews in Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. NeuroImage. 2008;40(2):811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot R, Newman JL, Longe OA, William Deakin JF. Differential Response Patterns in the Striatum and Orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. The Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Klemenagen K, Dudman J, Rogan M, Hen R, Kandel E, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to the hypothalamus in the rat. The Journal of Comparative Neurology. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Foot M, Davis M. Fear-potenitated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33(8–9):566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Gros DF, Antony MM, Simms LJ, McCabe RE. Psychometric Properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): Comparison to the State-Trait Anxiety Inventory. 2007 doi: 10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: The Neurocircuitry of emotion Regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiology & Behavior. 1992;51:1271–1276. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to te basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behavioral Neuroscience. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Arndt S, Schlaepfer TE, Reul J, Maier W, Scheele D. Diminished appetitive startle modulation following targeted inhibition of prefrontal cortex. Scientific reports. 2015;5:1–6. doi: 10.1038/srep08954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JH, Sarason IG. Life stress, depression and anxiety: internal-external control as a moderator variable. Journal of Psychomatic Research. 1978;22:205–209. doi: 10.1016/0022-3999(78)90025-9. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Gerlicher AMV. Making a mountain out of a molehill: On the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neuroscience & Biobehavioral Reviews. 2014;42:1–8. doi: 10.1016/j.neubiorev.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kimmel E. Judments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of Experimental Psycholoy. 1967;73:532–543. doi: 10.1037/h0024333. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depress. Anxiety. 2011;28:194–201. doi: 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. NeuroImage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Lewis EP, Wood KH. Conditioned Diminution of the Unconditioned Skin Conductance Response. Behavioral Neuroscience. 2011;125:626–631. doi: 10.1037/a0024324. [DOI] [PubMed] [Google Scholar]
- Knight DC, Wood KH. Investigating the neural mechanisms of aware and unaware fear memory with fMRI. Journal of Visualized Experiments. 2011;56:e3083. doi: 10.3791/3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bardley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Nangia V. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Psychophysiological reactions to noise as modified by personal control over noise intensity. Biological Psychology. 1978;6:51–59. doi: 10.1016/0301-0511(78)90006-6. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A Cognitive-motivational analysis of anxiety. Behavior Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Pine DS. Ventrolateral Prefrontal Cortex Activation and Attentional Bias in Response to Angry Faces in Adolescents with Generalized Anxiety Disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. TRENDS in Cognitive Science. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biological Psychiatory. 2005;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. NeuroImage. 2005;28:815–826. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C. Towards a mechanistic understanding of pathological anxiety: the dorsal medial prefrontal-amygdala ‘aversive amplification’ circuit in unmedicated generalized and social anxiety disorders. The Lancet Psychiatry. 2014;1(4):294–302. doi: 10.1016/S2215-0366(14)70305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter JB. Generalized Expectancies for Internal Versus External Control of Reinforcement. Psychological Monograps: General and Apploed. 80:1–28. [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. The Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ, Lerow AM. Robust regression and outlier detection. Hoboken, NJ: Wiley and Sons; 1987. [Google Scholar]
- Schwartz GE, Davidson RJ, Maer F. Right hemisphere lateralization for emotion in the human brain: interactions with cognition. Science. 1975;190:286–288. doi: 10.1126/science.1179210. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Konrad C. Neural correlates of trait anxiety in fear extinction. Psychol. Med. 2011;41:789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. European Journal of Neuroscience. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN. In: The neuroscience of emotion regulation: Basic mechanisms and their role in development, aging and psychopathology. The Handbook of Cognitive Neuroscience, Vol. I. Ochsner KN, Kosslyn SM, editors. New York, NY: Oxford University Press; 2013. [Google Scholar]
- Smith JC, Bradley MM, Lang PJ. State anxiety and affective physiology: effects of sustained exposure to affective pictures. Biological Psychiatry. 2005;69:247–260. doi: 10.1016/j.biopsycho.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Adults. Redwood City, CA: Mind Garder; 1983. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Thayer JF, Friedman B, Borkovec TD. Autonomic Characteristics of Generalized Anxiety Disorder and Worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (ÆSOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theories. Hillsdale, NJ: Erlbaum; 1989. pp. 149–189. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wheelock MD, Sreenivasan KR, Wood KH, Ver Hoef LW, Desphande G, Knight DC. Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. NeuroImage. 2014;102:904–912. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. NeuroImage. 2012;60:787–799. doi: 10.1016/j.neuroimage.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Wood KH, Kuykendall D, Ver Hoef LW, Knight DC. Neural Substrates Underlying Learning-Related Changes of the Unconditioned Fear Response. The Open Neuroimaging Journal. 2013;7:41–52. doi: 10.2174/1874440001307010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KH, Ver Hoef LW, Knight DC. The Amygdala Mediates the Emotional Modulation of Treat-Elicited Skin Conductance Response. Emotion. 2014;14:693–700. doi: 10.1037/a0036636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Overstreet C, Charney DR, Robinson OJ, Grillon C. Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. Journal of Psychiatry & Neuroscience. 2014;39:321–219. doi: 10.1503/jpn.130145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The Relation of Strength of Stimulus to Rapidity of Habit-Formation. Journal of Comparative Neurology and Psychology. 1908:459–482. [Google Scholar]
- Zhao XH, Wang PJ, Li CB, Hu ZH, Xi Q…, Tang XW. Altered default mode network activity in patient with anxiety disorders: An fMRI study. European Journal of Radiology. 2007;63:373–378. doi: 10.1016/j.ejrad.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.