Abstract
Chimerism testing following allogeneic hematopoietic stem cell transplantation (allo-HSCT) in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) represents a promising tool for predicting disease relapse, although its precise role in this setting remains unclear. We investigated the predictive value of T-lymphocyte chimerism analysis at 90 to 120 days following allo-HSCT in 378 patients with AML/MDS who underwent busulfan/fludarabine-based myeloablative preparative regimens. Of 265 (70%) patients with available T-lymphocyte chimerism data, 43% of patients in first or second complete remission (CR1/CR2) at the time of transplant had complete (100%) donor T-lymphocytes at day +90 to +120 compared with 60% of patients in the non-CR1/CR2 cohort (P = .005). In CR1/CR2 patients, donor T-lymphocyte chimerism ≤ 85% at day +90 to +120 was associated with a higher frequency of 3-year disease progression (29%; 95% CI, 18%-46% vs 15%; 95% CI, 9%-23%, HR=2.1, P =.04). However, in the more advanced, non-CR1/CR2 cohort, mixed T-lymphocyte chimerism was not associated with relapse (37%; 95% CI, 20%-66% vs. 34%; 95% CI, 25%-47%, HR=1.3, P =.6). These findings demonstrate that early T-lymphocyte chimerism testing at day +90 to +120 is a useful approach for predicting AML/MDS disease recurrence in patients in CR1/CR2 at the time of transplantation.
INTRODUCTION
Although allogeneic hematopoietic transplantation (allo-HSCT) represents a potential curative therapy for patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), the major cause of treatment failure is disease recurrence.1 The ability to predict relapse before detectable morphologic recurrence may allow for preemptive interventions in high-risk patients such as immune modulation, donor lymphocyte infusion (DLI), or initiation of hypomethylating agents to potentially augment graft versus leukemia (GVL) effects.2-5 Post-transplant peripheral blood chimerism analysis, which quantifies the relative levels of donor and recipient hematopoiesis, represents one potential tool to predict disease recurrence in the post-transplant setting, especially in patients without specific cytogenetic, molecular, or immunophenotypic leukemic signatures that allow more direct monitoring of disease.6
There have been a number of studies that have investigated the efficacy of chimerism analysis in predicting disease relapse, especially with the advent of more sensitive PCR-based methods and lineage-specific analyses to detect donor/recipient hematopoiesis.7 However, the use of chimerism analysis to identify AML patients at high risk to relapse after allo-HSCT remains controversial. Several studies have suggested that mixed donor and recipient chimerism or chimerism kinetics (full donor chimerism to mixed chimerism) can predict disease recurrence,8-12 whereas other studies have suggested that such analyses cannot be used reliably.13-15 Moreover, a substantial number of these studies have focused on pediatric patient populations or have been limited by small patient cohorts or disease heterogeneity inclusive of all leukemia subtypes with variable kinetics of relapse. Results may also vary depending on the preparative regimen. For example, the combination of busulfan and fludarabine, which has been widely used as a myeloablative preparative regimen for myeloid malignancies,16-23 is associated with a relatively high rate of mixed T-lymphocyte chimerism.24 We sought to examine the effect of mixed T-lymphocyte chimerism on the risk of relapse post-transplant in patients with AML or MDS. In this single-institutional retrospective analysis, we report the largest study to-date to our knowledge investigating the potential utility of T-lymphocyte chimerism analysis between post-transplant day +90 to +120 in predicting relapse for AML/MDS patients following myeloablative allo-HSCT using the busulfan-fludarabine preparative regimen and tacrolimus/methotrexate graft-versus-host-disease (GVHD) prophylaxis.
PATIENTS AND METHODS
Patient Eligibility
Patients who underwent allo-HSCT for AML or MDS on five separate sequential clinical protocols involving myeloablative conditioning with busulfan and fludarabine at The University of Texas MD Anderson Cancer Center between 2001 and 2011 were included in this retrospective analysis. Informed consent was obtained from each patient per institutional guidelines prior to enrollment in each clinical trial. The institutional review board also reviewed and approved this retrospective analysis. All patients received myeloablative conditioning consisting of fludarabine 40 mg/m2 with busulfan 130 mg/m2 daily for 4 days or with busulfan given with pharmacokinetic dose adjustment targeting a drug concentration area under the curve of 6000 mM × min. Post-transplant GVHD prophylaxis consisted of methotrexate 5 mg/m2 on days 1, 3, 6, and 11 and tacrolimus which was tapered after four to six months. Patients receiving unrelated donor transplants received antithymocyte globulin (Thymoglobulin) 4.5 mg/kg pretransplant in divided doses.
Chimerism Testing
Peripheral blood chimerism analysis was performed using eight highly polymorphic microsatellite markers (purchased from Integrated DNA Technologies, Inc., Coralville, IA) in a multiplex polymerase chain reaction (PCR) assay. Each marker was labeled with a fluorescent tag that allows size separation by using capillary electrophoresis in an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). Data are presented as peaks, and the area under the curve (AUC) represents the percentage of host versus donor hematopoiesis. In addition to looking at overall chimerism status, lineage-specific analysis was also performed by separating out myeloid and T-lymphocyte cell populations using the RoboSep™ Magnetic Cell System and the EasySep whole blood CD3 positive or myeloid selection (anti-CD14 + CD66b) kits (Stem Cell Technologies, Vancouver, BC, Canada).
End Points and Statistical Analysis
The primary objective of the study was to assess the rate of disease progression according to peripheral blood T-lymphocyte chimerism evaluated between day +90 to +120 post-transplant. Leukemic disease progression was defined by morphology as the appearance of >5% blasts on bone marrow aspirate smears. Only patients who were alive and free of disease progression at day +120 after transplantation were eligible for this assessment. The cumulative incidence of disease progression after day +120 (landmark analysis) was estimated considering death before disease progression as a competing risk. Predictors of disease progression were assessed using Cox's proportional regression analysis. Predictors included were: chimerism status, age, gender, cytogenetic risk group, modified European Leukemia Net (ELN) risk stratification,25 donor type, cell type, and fixed or adjusted busulfan dose. Due to the limited number of patients who had both NPM1 and FLT3-ITD mutation status reported in this study, ELN classification was modified to categorize cytogenetically normal patients only on the basis of FLT3-ITD mutation status. Patient and transplant characteristics were compared using Fisher's exact and Chi square test for categorical variables and Mann Whitney's test for continuous variables. Statistical significance was defined at the 0.05 level. Statistical analysis was performed using STATA 11.0 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
RESULTS
Patient Characteristics
A total of 378 consecutive patients who were alive and without evidence of disease progression on day +120 following allo-HSCT met the eligibility criteria and were included in the study. Characteristics of these patients are delineated in Table 1, and characteristics of patients who were excluded from the study due to disease progression prior to day +120 are provided in Supplemental Table S1. In the study-eligible population, three hundred and five (81%) patients had AML and 73 (19%) patients had MDS. Median age was 48 years, and 224 (59%) patients were transplanted in first or second complete remission (CR1/CR2). 158 patients were not in remission or beyond CR2 (non-CR1/CR2). Peripheral blood stem cells were used in 242 (64%) patients whereas bone marrow was used in 36% patients. Over half (52%) of all patients received a matched-related donor transplant. Among the 378 eligible patients in the study, peripheral blood T-lymphocyte and myeloid donor chimerism data between day +90 to +120 was available for 265 (70%) and 286 (76%) patients, respectively.
Table 1.
Patient Characteristics.
| Overall N (%) | T-Lymphocyte chimerism available N (%) | T-Lymphocyte chimerism available CR1/CR2 N (%) | T-Lymphocyte chimerism available Non-CR1/CR2 N (%) | |
|---|---|---|---|---|
| Total patients | 378 | 265 | 164 | 101 |
| Sex | ||||
| Female | 185 (49) | 125 (47) | 75 (46) | 50 (50) |
| Male | 193 (51) | 140 (53) | 89 (54) | 51 (50) |
| Age | ||||
| ≤50 | 231 (61) | 156 (59) | 99 (60) | 57 (56) |
| >50 | 147 (39) | 109 (41) | 65 (40) | 44 (44) |
| Diagnosis | ||||
| AML | 305 (81) | 186 (70) | 152 (93) | 61 (60) |
| MDS | 73 (19) | 79 (30) | 12 (7) | 40 (40) |
| Disease Status at Transplant | ||||
| CR1 | 158 (42) | 119 (45) | ||
| CR2 | 66 (17) | 45 (17) | ||
| Not CR | 154 (41) | 101 (38) | ||
| Cytogenetics | ||||
| Good Risk | 50 (13) | 36 (14) | 17 (10) | 19 (19) |
| Intermediate Risk | 196 (52) | 136 (51) | 93 (57) | 43 (43) |
| Poor Risk | 121 (32) | 86 (32) | 50 (30) | 36 (36) |
| Unknown | 11 (3) | 7 (3) | 4 (2) | 3 (3) |
| FLT3 ITD Mutation | ||||
| Yes | 45 (12) | 36 (14) | 31 (19) | 5 (5) |
| No | 224 (59) | 161 (61) | 95 (58) | 66 (65) |
| N/A | 109 (29) | 68 (26) | 38 (23) | 30 (30) |
| Modified ELN Classification | ||||
| Favorable | 114 (30) | 82 (31) | 50 (30) | 32 (32) |
| Intermediate 1* | 32 (8) | 27 (10) | 24 (15) | 5 (5) |
| Intermediate 2 | 75 (20) | 53 (20) | 38 (23) | 15 (15) |
| Adverse | 85 (22) | 60 (23) | 29 (18) | 31 (31) |
| Cg normal, FLT3 ITD N/A | 56 (15) | 30 (11) | 16 (10) | 14 (14) |
| Cg N/A | 16 (4) | 11 (4) | 7 (4) | 4 (4) |
| Stem Cell Source | ||||
| Bone Marrow | 135 (36) | 92 (35) | 54 (33) | 38 (38) |
| Peripheral Blood | 242 (64) | 173 (65) | 110 (67) | 63 (63) |
| Cord Blood | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
| Donor type | ||||
| Match-related donor | 195 (52) | 137 (52) | 92 (56) | 45 (44) |
| Other | 183 (48) | 128 (48) | 72 (44) | 56 (55) |
| GVHD prophylaxis | ||||
| Tacrolimus/Methotrexate | 335 (89) | 237 (89) | 149 (91) | 88 (87) |
| Tacrolimus/Methotrexate/Pentostatin | 42 (11) | 28 (11) | 15 (9) | 13 (13) |
| Other | 1 (0) | 0 (0) | 0 (0) | 0 (0) |
CR1 indicates 1st complete remission; CR2, 2nd complete remission; ELN, European Leukemia Net; Cg, cytogenetics; N/A, not available; GVHD, graft-versus-host-disease.
Intermediate-I included only FLT3-ITD mutant patients due to limited number of patients with both NPM1 and FLT3-ITD mutation reported
Mixed T-lymphocyte Chimerism and Disease Status at Transplant
There was a significant association between disease status at transplant and day +90 to +120 donor T-lymphocyte chimerism. Of 164 patients in the CR1/CR2 cohort, 43% of patients had complete donor T-lymphocyte chimerism compared with 60% of patients in the non-CR1/CR2 cohort (P = 0.005). Most (90%) patients in the CR1/CR2 and non-CR1/CR2 cohorts achieved complete (100%) or near complete (99%) donor myeloid chimerism by day +90 to +120 (Table 2). Because of the high rates of full donor myeloid chimerism by day +90 to +120 in all patients signifying the low value of myeloid chimerism at this time point, subsequent analyses focused solely on the impact of T-lymphocyte chimerism and post-transplant disease progression. Moreover, given the differences in donor T-lymphocyte chimerism based on disease status at transplant, predictors of disease progression were analyzed separately for CR1/CR2 and non-CR1/CR2 patients at the time of transplant.
Table 2.
Myeloid and T-lymphocyte chimerism at day +90 to +120 according to disease status at transplant.
| CR1/CR2 | Non-CR1/CR2 | P | |
|---|---|---|---|
| % Donor T-lymphocyte chimerism | N=164 (%) | N=101 (%) | |
| 100 | 70 (43%) | 61 (60%) | .005 |
| 86-99 | 49 (30%) | 21 (21%) | Ref. |
| ≤85 | 45 (27%) | 19 (19%) | Ref. |
| % Donor myeloid chimerism | N=179 (%) | N=107 (%) | |
| 100 | 120 (67%) | 80 (75%) | .2 |
| 99 | 44 (25%) | 13 (12%) | Ref. |
| ≤98 | 15 (8%) | 14 (13%) | Ref. |
Ref. indicates reference.
Age, donor type, and a history of grade ≥ 2 acute GVHD, and cytogenetics risk group were comparable (P > 0.1) between the CR1/CR2 and non-CR1/CR2 groups (Table 1). Among CR1/CR2 patients with T-lymphocyte chimerism data available, 40% were greater than 50 years of age, 56% had a related donor transplant, 26% had a history of grade ≥ 2 acute GVHD, and 30% had poor risk, 57% intermediate risk, 10% good risk, and 2% unknown risk cytogenetics. The corresponding proportions in the non-CR1/CR2 group were 44%, 44%, 30%, 36%, 43%, 19%, and 3%.
Predictors of disease progression
CR1/CR2 cohort
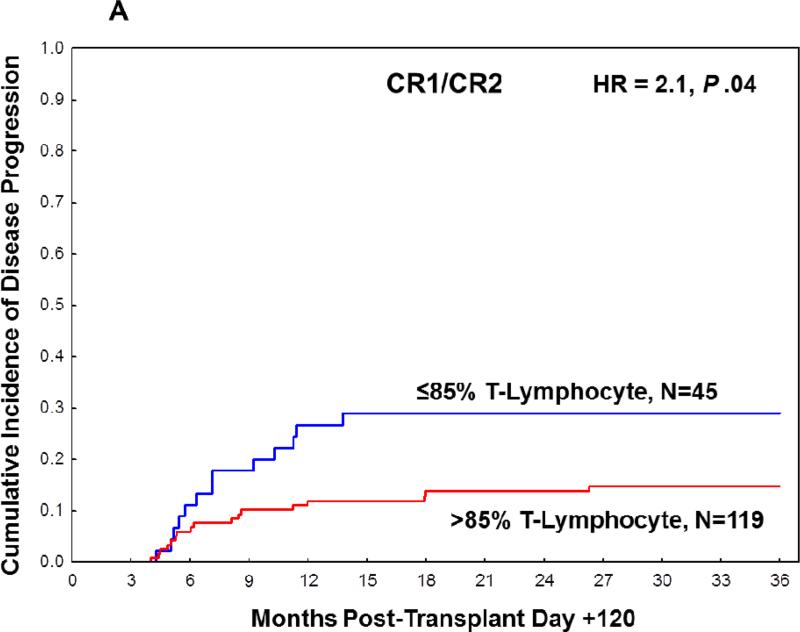
Based on the distribution of the percentages of donor T-lymphocyte chimerism in the CR1/CR2 cohort, T-lymphocyte chimerism was further subdivided by the following intervals: 100%, 96-99%, 86-95%, 76-85%, 51-75%, and ≤50%. The rate of disease progression was comparably low in patients with 100%, 96-99% or 86-95%, and comparably high in patients with 76-85%, 51-75%, or ≤50% (Table S2). Based on these findings, 85% was chosen as the cut-off for subsequent analyses of T-lymphocyte donor chimerism. In landmark analysis, the cumulative incidence of disease progression at 3 years post-transplant was significantly higher (29%; 95% CI 18%-46%) in the ≤ 85% group compared with the >85% group (15%; 95% CI 9%-23%, HR=2.1, P =.04, Table S4, Figure 1A). Patients classified as adverse according to the modified ELN risk stratification system had a significantly higher rate of disease progression (HR=3.0, P = .004). Nevertheless, on bivariate analysis, ≤ 85% donor T-lymphocyte chimerism at day +90 to +120 remained an independent predictor of disease progression irrespective of an adverse modified ELN risk (HR=2.4, P = .02). None of the remaining patient or transplant characteristics assessed (age, gender, cytogenetic risk group, donor type, stem cell source, and fixed or adjusted busulfan dose) were significantly associated with the rate of disease progression in the CR1/CR2 cohort (data not shown).
Figure 1.
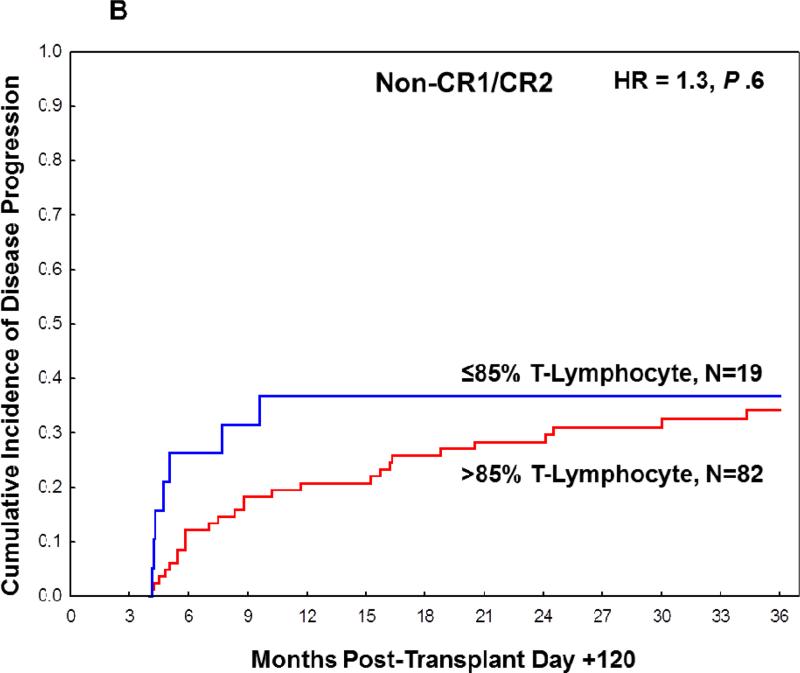
Cumulative incidence of disease progression at 3 years post-transplant based on T-lymphocyte chimerism in (A) CR1/CR2 patients at transplant and (B) non-CR1/CR2 patients at transplant.
Non-CR1/CR2 cohort
Using the same categorization of chimerism as described for the CR1/CR2 cohort, there was no significant association between mixed T-lymphocyte donor chimerism and the rate of disease progression by 3 years post-transplant in the non-CR1/CR2 cohort (Tables S3 and S4). In a landmark analysis, the cumulative incidence of disease progression at 3 years post-transplant was 37% (95% CI 20%-66%) in the ≤ 85%, and 34% (95% CI 25%-47%) in the >85% group (HR=1.3, P =.6, Figure 1B). When examined at earlier time points prior to the three-year landmark analysis, there was a trend towards increased rate of disease progression in patients with ≤ 85% T-lymphocyte chimerism at six months and one year, although this did not reach statistical significance (Table S5). Patients classified as having adverse risk under the modified ELN stratification system had higher rates of disease progression, although this did not reach statistical significance (HR=2.1, P = .06). On bivariate analysis, neither modified ELN risk stratification or day +90 to +120 T-lymphocyte chimerism were significant predictors of disease progression. Moreover, age, gender, cytogenetic risk group, donor type, cell type, and fixed or adjusted busulfan dose were not associated with the rate of disease progression in the non-CR1/CR2 cohort.
T-Lymphocyte chimerism, graft-versus-host-disease, and disease progression
There was no significant difference in the history of acute GVHD based on donor T-lymphocyte chimerism levels at day +90 to +120 when stratified at the 85% cut-off point (Table 3A). Grade II-IV acute GVHD occurred in 15 of 64 patients (23%) with ≤85% T-lymphocyte chimerism compared to 58 of 201 patients (29%) with >85% T-lymphocyte chimerism (P = .4). A history of grade II-IV or III-IV acute GVHD was not associated with the rate of disease progression (Table 4). Likewise, the frequency of chronic GVHD did not differ significantly based on T-lymphocyte chimerism levels at day +90-120. The prevalence of cGVHD by day +90 to +120 was 9% in ≤85% group and 11% in the >85% group (P =.7, Table 3A); and the incidence of chronic GVHD after day +90-120 was 52% and 59% (P =.9, Table 3B) in the two groups, respectively. Because the known associations between complete donor T-lymphocyte chimerism and GVHD26-28, which may influence relapse risk, we evaluated the impact of chronic GVHD on disease progression as time-dependent covariates. Indeed, chronic GVHD was associated with lower rate of disease progression irrespective of disease status at transplant. However, this association was only statistically significant in CR1/CR2 patients (P =.02), but not in the non-CR1/CR2 cohort (P =.2). On bivariate analysis taking into consideration both chronic GVHD as a time-dependent variable and mixed T-lymphocyte chimerism ≤ 85% (Table 4), both factors remained significant predictors of disease progression for the CR1/CR2 group (P =.02, P =.03, respectively), but not in the non-CR1/CR2 cohort (P =.2, P = .6, respectively).
Table 3A.
Correlation of day +90 to +120 T-lymphocyte chimerism with graft-versus-host disease
| T-lymphocyte chimerism ≤ 85% N=64(%) | T-lymphocyte chimerism > 85% N=201 (%) | P | |
|---|---|---|---|
| History of aGVHD | |||
| Grade 1 | 14 (22) | 55 (27) | |
| Grades 2-4 | 15 (23) | 58 (29) | 0.4 |
| Grade 2 | 13 (20) | 44 (22) | |
| Grades 3-4 | 2 (3) | 14 (7) | 0.2 |
| cGVHD before Day +120 | 6 (9) | 22 (11) | 0.7 |
aGVHD indicates acute graft-versus-host-disease; cGVHD, chronic graft-versus-host-disease
Table 4.
Impact of acute GVHD and chronic GVHD on disease progression
| CR1/CR2 N=164 | Non-CR1/CR2 N=101 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| History of aGVHD II-IV | 1.7 | 0.8-3.6 | 0.2 | 0.5 | 0.2-1.2 | 0.1 |
| History of aGVHD III-IV | No Events* | 0.3 | 1.2 | 0.3-5.0 | 0.8 | |
| cGVHD (as time-dependent variable) | 0.3 | 0.1-0.8 | 0.02 | 0.6 | 0.3-1.3 | 0.2 |
| Bivariate analysis cGVHD and T-lymphocyte chimerism | HR | 95% CI | P | HR | 95% CI | P |
|---|---|---|---|---|---|---|
| cGVHD (as time-dependent variable) | 0.3 | 0.1-0.8 | 0.02 | 0.6 | 0.3-1.3 | 0.2 |
| Chimerism ≤ 85% | 2.2 | 1.1-4.6 | 0.03 | 1.2 | 0.5-2.9 | 0.6 |
No events in 2 patients with grade III-IV aGVHD
Table 3B.
Correlation of day +90 to +120 T-lymphocyte chimerism with three-year cumulative incidence of chronic GVHD
| T-lymphocyte chimerism ≤ 85% | T-lymphocyte chimerism > 85% | ||||
|---|---|---|---|---|---|
| % 3-year cumulative incidence* (95% CI) | % 3-year cumulative incidence* (95% CI) | HR | 95% CI | P | |
| cGVHD overall | 52% (41-63) | 59% (53-66) | 0.8 | 0.6-1.2 | 0.4 |
| cGVHD in CR1/CR2 | 58% (45-74) | 61% (53-71) | 0.94 | 0.6-1.5 | 0.8 |
| cGVHD in non-CR1/CR2 | 37% (20-66) | 56% (46-68) | 0.6 | 0.2-1.4 | 0.2 |
Accounting for disease progression or death before cGVHD as competing risks
DISCUSSION
In this analysis, we report the largest study to-date to our knowledge investigating the role of chimerism testing between post-transplant day +90 to +120 in predicting disease progression in AML/MDS patients. Several studies have evaluated the role of chimerism analysis in predicting disease recurrence in AML/MDS post-transplant, yet these reports have often been limited by small patient cohorts29, 30, heterogeneous disease populations with variable kinetics of relapse10, 31, or lack of lineage specific analyses.32, 33 This study focused on patients receiving a uniform, widely used, busulfan-fludarabine myeloablative preparative regimen, which is known to have a relatively high rate of mixed lymphocyte chimerism.24 All patients received tacrolimus/methotrexate post-transplant immunosuppressive therapy. We hypothesized that a higher degree of mixed lymphocyte peripheral blood chimerism may be associated with an increased risk of leukemia relapse post-transplant.
We found a significant association between disease-status at transplant and the kinetics of T-lymphocyte engraftment, as a lower proportion of patients in CR1/CR2 at transplant had complete T-lymphocyte engraftment at day +90 to +120 compared with the non-CR1/CR2 cohort. This is likely explained by the greater pretransplant chemotherapy exposure in the non-CR1/CR2 patients, resulting in greater cumulative immunosuppression, which would facilitate donor engraftment. This is consistent with previous reports that demonstrated a significant association between the number of pretransplant chemotherapy regimens and T-lymphocyte engraftment kinetics.34-37 In contrast, nearly all patients who were alive and free of disease at the time of chimerism testing achieved complete donor myeloid chimerism by day +90 to +120 regardless of disease status at transplant. Overall, T-lymphocyte chimerism lagged behind the myeloid compartment, which is consistent with a prior study evaluating lineage-specific chimerism kinetics using a busulfan-fludarabine conditioning regimen.24 Some reports have also suggested that conditioning regimens with busulfan such as those used in this study may result in less lymphodepletion compared to melphalan-based regimens37, but a recent study reported no difference in T-lymphocyte engraftment kinetics between the two agents.38 Taken together, these studies imply that the rates of lymphocyte chimerism vary for different preparative regimens, and that the predictive value of mixed lymphocyte chimerism may also vary depending on the type and intensity of conditioning, use of anti-T-cell antibodies, or T-cell depletion of the graft.
As most patients achieved complete or near complete donor myeloid chimerism by post-transplant day +90 to +120, our analysis focused on the impact of mixed T-lymphocyte chimerism on disease progression. T-lymphocyte donor chimerism ≤ 85% was a significant predictor of disease progression in CR1/CR2 patients. In contrast, mixed T-lymphocyte chimerism did not significantly impact disease progression rates in the non-CR1/CR2 cohort at three years, although there was a trend towards increased risk of disease progression in this group when examined at earlier time points within the first year post-transplant. This may reflect that achieving complete donor T-lymphocyte chimerism and maximizing the graft-versus-leukemia effect has a larger impact in maintaining remission in CR1/CR2 patients. This is in contrast to non-CR1/CR2 patients where the diminished predictive value of T-lymphocyte chimerism suggests that the presence of active disease at the time of transplant poses a greater risk for relapse than delayed immune reconstitution with donor T-lymphocytes. The fact that chronic GVHD when analyzed as a time-dependent variable was a significant predictor of disease progression only in the CR1/CR2 patients lends further support to this idea.
The role of mixed T-lymphocyte donor chimerism in predicting AML relapse post transplant has been controversial, although no previous studies have stratified patients based on disease status at transplant as in the current analysis. Four studies have reported no association of mixed T-lymphocyte chimerism and disease recurrence, although three of these studies contained different preparative regimens and very heterogeneous disease populations including acute and chronic leukemias of both myeloid and lymphoid lineages.27, 35, 38 However, the potential importance of T-lymphocyte chimerism in predicting disease recurrence has been highlighted by several recent studies. In a large analysis by Koreth et al, mixed total donor cell and T-lymphocyte chimerism < 90% at day +30 and day +100 in patients who underwent allo-HSCT was predictive of increased risk of relapse, with day +100 total donor cell chimerism most predictive.39 Two additional studies have also described the significance of day +30 mixed T-lymphocyte chimerism in predicting disease relapse. Saito et al reported that T-lymphocyte chimerism < 90% at day +30 portended poor outcomes in a cohort of 117 patients with both myeloid and lymphoid malignancies who underwent busulfan-based reduced-intensity conditioning allo-HSCT.36 Finally, day +30 mixed whole blood and T-lymphocyte chimerism were predictive of early relapse and shorter overall survival in a cohort of 121 patients with myeloid and lymphoid malignancies when chimerism was assessed as a continuous variable.40 In this particular model, T-lymphocyte chimerism was a stronger predictor of relapse and overall survival compared to whole blood chimerism testing. Likewise, the current study also supports the value of T-lymphocyte chimerism in predicting relapse in AML/MDS post allo-HSCT, although its applicability is limited to patients who are alive and without disease progression at day +120. This represented 78% of all AML/MDS patients who initially underwent allo-HSCT in our study. However, assessing the predictive impact of day +90 to +120 chimerism analysis may have particular therapeutic relevance if prophylactic DLIs are considered, as the timing usually corresponds to the tapering of immunosuppression after allo-HSCT. As a result, prophylactic DLIs given during this time period may be more tolerable with lower rates of GVHD, compared to earlier administration.41
Given the emergence of multiparameter flow cytometry (MFC) and RT-qPCR as highly sensitive tools in assessing MRD, one limitation of this study is the lack of MRD data that was incorporated in this analysis.44, 45 The value of MRD testing to predict relapse post transplant is being actively studied. Relapse can occur if residual clonogenic leukemia cells are present in the absence of an effective graft-vs-leukemia immune effect. Indeed, integration of chimerism data with MFC or RT-qPCR MRD measurements to develop relapse risk models may represent the most practical use of such data.46 Chimerism data remains a potential useful tool in assessing relapse risk in subsets of patients who may not have specific molecular markers to monitor by RT-qPCR.44 Moreover, while almost all AML patients will have an identifiable leukemia-associated immunophenotype (LAIP) at the time of diagnosis with MFC,47 changes in LAIP expression patterns at the time of relapse can occur in up to 80-90% of patients,48, 49 which represents one limitation of such testing.
Identifying AML/MDS patients at high risk for impending disease relapse in the post-transplant setting remains a challenge. In this large analysis, we demonstrate that mixed T-lymphocyte chimerism at day +90 to +120 post-transplant is a promising approach to detect patients at higher risk for disease recurrence, and its interpretation depends on disease status at transplant. Chimerism assessment should be considered with the disease risk index, minimal residual disease assessment, and potentially other factors in identifying patients at high risk of relapse. Strategies to enhance early full donor T-cell chimerism post-transplant and early preemptive therapies need assessment to prevent relapse in high-risk patients, such as immune modulation, donor-lymphocyte infusion, or initiation of hypomethylating agents or tyrosine kinase inhibitors. These approaches warrant study in prospective clinical trials.
Supplementary Material
Highlights.
“Mixed T-lymphocyte chimerism after allogeneic hematopoietic transplantation is predictive for relapse of AML/MDS”
■ T-lymphocyte chimerism was evaluated at day +90-120 after allo-HSCT for AML/MDS.
■ T-lymphocyte chimerism ≤ 85% predicted relapse in CR1/CR2 patients at transplant.
■ T-lymphocyte chimerism ≤ 85% was not predictive of relapse in non-CR1/CR2 patients.
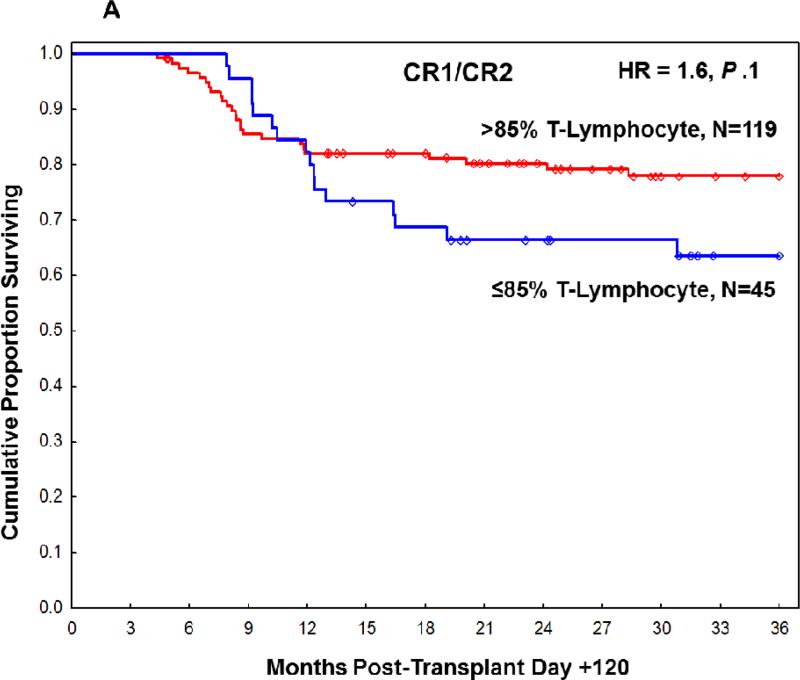
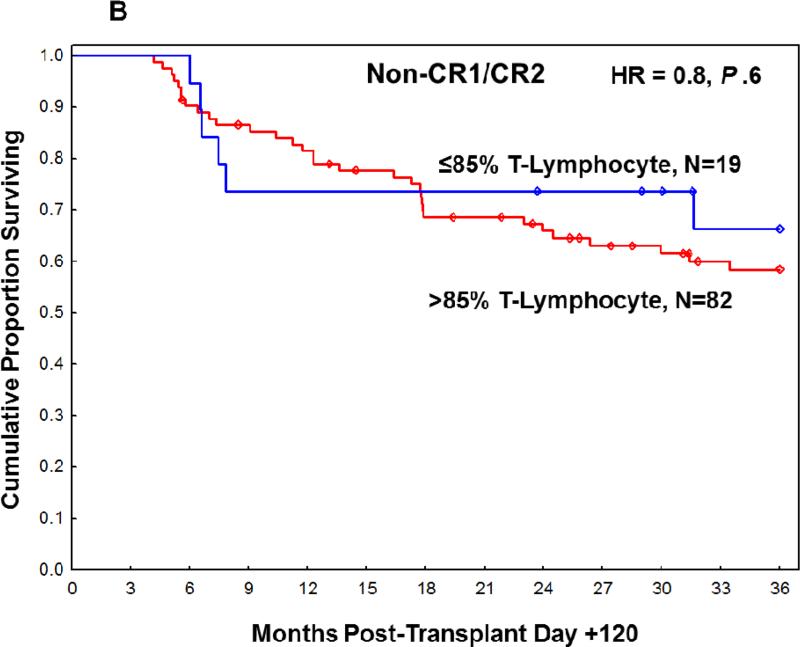
Figure 2.
Overall survival at 3 years post-transplant based on T-lymphocyte chimerism in (A) CR1/CR2 patients at transplant and (B) non-CR1/CR2 patients at transplant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest
R.C, B.A., and U.P. received research support from Otsuka Pharmaceutical, Inc.
Authorship Contributions
R.C. designed the research. J.C. provided database support. R.M.S. performed statistical analyses. L.J.M. performed the chimerism analysis. H.C.L, R.M.S, G.A., U.P., R.C. analyzed and interpreted data. H.C.L, R.M.S., R.C. wrote the manuscript. All authors reviewed and edited the manuscript and approved the final version.
REFERENCES
- 1.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubbert M, Bertz H, Wasch R, et al. Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed after allografting. Bone Marrow Transplant. 2010;45:627–632. doi: 10.1038/bmt.2009.222. [DOI] [PubMed] [Google Scholar]
- 4.Graef T, Kuendgen A, Fenk R, Zohren F, Haas R, Kobbe G. Successful treatment of relapsed AML after allogeneic stem cell transplantation with azacitidine. Leukemia research. 2007;31:257–259. doi: 10.1016/j.leukres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Solomon SR, Sizemore CA, Zhang X, et al. Preemptive DLI without withdrawal of immunosuppression to promote complete donor T-cell chimerism results in favorable outcomes for high-risk older recipients of alemtuzumab-containing reduced-intensity unrelated donor allogeneic transplant: a prospective phase II trial. Bone Marrow Transplant. 2014;49:616–621. doi: 10.1038/bmt.2014.2. [DOI] [PubMed] [Google Scholar]
- 6.Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26:381–389. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiede C, Bornhauser M, Ehninger G. Strategies and clinical implications of chimerism diagnostics after allogeneic hematopoietic stem cell transplantation. Acta Haematol. 2004;112:16–23. doi: 10.1159/000077555. [DOI] [PubMed] [Google Scholar]
- 8.Lassaletta A, Ramirez M, Montero JM, et al. Full donor chimerism by day 30 after allogeneic peripheral blood progenitor cell transplantation is associated with a low risk of relapse in pediatric patients with hematological malignancies. Leukemia. 2005;19:504–506. doi: 10.1038/sj.leu.2403692. [DOI] [PubMed] [Google Scholar]
- 9.Bornhauser M, Oelschlaegel U, Platzbecker U, et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica. 2009;94:1613–1617. doi: 10.3324/haematol.2009.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba R, Abella E, Kukuruga D, et al. Mixed hematopoietic chimerism at day 90 following allogenic myeloablative stem cell transplantation is a predictor of relapse and survival. Leukemia. 2004;18:1681–1686. doi: 10.1038/sj.leu.2403468. [DOI] [PubMed] [Google Scholar]
- 11.Bader P, Beck J, Frey A, et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant. 1998;21:487–495. doi: 10.1038/sj.bmt.1701119. [DOI] [PubMed] [Google Scholar]
- 12.Huisman C, de Weger RA, de Vries L, Tilanus MG, Verdonck LF. Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant. 2007;39:285–291. doi: 10.1038/sj.bmt.1705582. [DOI] [PubMed] [Google Scholar]
- 13.Lim ZY, Pearce L, Ingram W, Ho AY, Mufti GJ, Pagliuca A. Chimerism does not predict for outcome after alemtuzumab-based conditioning: lineage-specific analysis of chimerism of specific diseases may be more informative. Bone Marrow Transplant. 2008;41:587–588. doi: 10.1038/sj.bmt.1705937. [DOI] [PubMed] [Google Scholar]
- 14.Doney K, Loken M, Bryant E, Smith A, Appelbaum F. Lack of utility of chimerism studies obtained 2-3 months after myeloablative hematopoietic cell transplantation for ALL. Bone Marrow Transplant. 2008;42:271–274. doi: 10.1038/bmt.2008.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaap N, Schattenberg A, Mensink E, et al. Long-term follow-up of persisting mixed chimerism after partially T cell-depleted allogeneic stem cell transplantation. Leukemia. 2002;16:13–21. doi: 10.1038/sj.leu.2402343. [DOI] [PubMed] [Google Scholar]
- 16.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 17.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 18.Iravani M, Evazi MR, Mousavi SA, et al. Fludarabine and busulfan as a myeloablative conditioning regimen for allogeneic stem cell transplantation in high- and standard-risk leukemic patients. Bone Marrow Transplant. 2007;40:105–110. doi: 10.1038/sj.bmt.1705685. [DOI] [PubMed] [Google Scholar]
- 19.Andersson BS, de Lima M, Thall PF, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bredeson CN, Zhang MJ, Agovi MA, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell JA, Duan Q, Chaudhry MA, et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant. 2008;14:888–895. doi: 10.1016/j.bbmt.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Joo YD, Kim H, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31:701–709. doi: 10.1200/JCO.2011.40.2362. [DOI] [PubMed] [Google Scholar]
- 23.Bartelink IH, van Reij EM, Gerhardt CE, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20:345–353. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Niiya H, Kanda Y, Saito T, et al. Early full donor myeloid chimerism after reduced-intensity stem cell transplantation using a combination of fludarabine and busulfan. Haematologica. 2001;86:1071–1074. [PubMed] [Google Scholar]
- 25.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minculescu L, Madsen HO, Sengelov H. T-cell chimerism is valuable in predicting early mortality in steroid-resistant acute graft-versus-host disease after myeloablative allogeneic cell transplantation. Acta Haematol. 2014;132:187–192. doi: 10.1159/000357728. [DOI] [PubMed] [Google Scholar]
- 27.Nikolousis E, Robinson S, Nagra S, et al. Post-transplant T cell chimerism predicts graft versus host disease but not disease relapse in patients undergoing an alemtuzumab based reduced intensity conditioned allogeneic transplant. Leuk Res. 2013;37:561–565. doi: 10.1016/j.leukres.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Balon J, Halaburda K, Bieniaszewska M, et al. Early complete donor hematopoietic chimerism in peripheral blood indicates the risk of extensive graft-versus-host disease. Bone Marrow Transplant. 2005;35:1083–1088. doi: 10.1038/sj.bmt.1704962. [DOI] [PubMed] [Google Scholar]
- 29.Mattsson J, Uzunel M, Tammik L, Aschan J, Ringden O. Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia. 2001;15:1976–1985. doi: 10.1038/sj.leu.2402311. [DOI] [PubMed] [Google Scholar]
- 30.Scheffold C, Kroeger M, Zuehlsdorf M, et al. Prediction of relapse of acute myeloid leukemia in allogeneic transplant recipients by marrow CD34+ donor cell chimerism analysis. Leukemia. 2004;18:2048–2050. doi: 10.1038/sj.leu.2403507. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Simon JA, Caballero D, Diez-Campelo M, et al. Chimerism and minimal residual disease monitoring after reduced intensity conditioning (RIC) allogeneic transplantation. Leukemia. 2002;16:1423–1431. doi: 10.1038/sj.leu.2402550. [DOI] [PubMed] [Google Scholar]
- 32.Barrios M, Jimenez-Velasco A, Roman-Gomez J, et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica. 2003;88:801–810. [PubMed] [Google Scholar]
- 33.Choi SJ, Lee KH, Lee JH, et al. Prognostic value of hematopoietic chimerism in patients with acute leukemia after allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 2000;26:327–332. doi: 10.1038/sj.bmt.1702504. [DOI] [PubMed] [Google Scholar]
- 34.Carvallo C, Geller N, Kurlander R, et al. Prior chemotherapy and allograft CD34+ dose impact donor engraftment following nonmyeloablative allogeneic stem cell transplantation in patients with solid tumors. Blood. 2004;103:1560–1563. doi: 10.1182/blood-2003-04-1170. [DOI] [PubMed] [Google Scholar]
- 35.Montero A, Savani BN, Kurlander R, et al. Lineage-specific engraftment and outcomes after T-cell-depleted peripheral blood stem cell transplant with Flu/Cy/TBI conditioning. Br J Haematol. 2005;130:733–739. doi: 10.1111/j.1365-2141.2005.05665.x. [DOI] [PubMed] [Google Scholar]
- 36.Saito B, Fukuda T, Yokoyama H, et al. Impact of T cell chimerism on clinical outcome in 117 patients who underwent allogeneic stem cell transplantation with a busulfan-containing reduced-intensity conditioning regimen. Biol Blood Marrow Transplant. 2008;14:1148–1155. doi: 10.1016/j.bbmt.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Valcarcel D, Martino R, Caballero D, et al. Chimerism analysis following allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning. Bone Marrow Transplant. 2003;31:387–392. doi: 10.1038/sj.bmt.1703846. [DOI] [PubMed] [Google Scholar]
- 38.van Besien K, Dew A, Lin S, et al. Patterns and kinetics of T-cell chimerism after allo transplant with alemtuzumab-based conditioning: mixed chimerism protects from GVHD, but does not portend disease recurrence. Leukemia & lymphoma. 2009;50:1809–1817. doi: 10.3109/10428190903200790. [DOI] [PubMed] [Google Scholar]
- 39.Koreth J, Kim HT, Nikiforow S, et al. Donor chimerism early after reduced-intensity conditioning hematopoietic stem cell transplantation predicts relapse and survival. Biol Blood Marrow Transplant. 2014;20:1516–1521. doi: 10.1016/j.bbmt.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reshef R, Hexner EO, Loren AW, et al. Early Donor Chimerism Levels Predict Relapse and Survival after Allogeneic Stem Cell Transplantation with Reduced-Intensity Conditioning. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5675–5687. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann JC, Stabla K, Burchert A, et al. Monitoring of acute myeloid leukemia patients after allogeneic stem cell transplantation employing semi-automated CD34+ donor cell chimerism analysis. Ann Hematol. 2014;93:279–285. doi: 10.1007/s00277-013-1961-4. [DOI] [PubMed] [Google Scholar]
- 43.Tang X, Alatrash G, Ning J, et al. Increasing chimerism after allogeneic stem cell transplantation is associated with longer survival time. Biol Blood Marrow Transplant. 2014;20:1139–1144. doi: 10.1016/j.bbmt.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nature reviews. Clinical oncology. 2013;10:460–471. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hokland P, Cotter F. Readying the minimal residual disease concept in acute myeloid leukaemia for prime time - the American way. Br J Haematol. 2013;162:429–430. doi: 10.1111/bjh.12419. [DOI] [PubMed] [Google Scholar]
- 46.Lange T, Hubmann M, Burkhardt R, et al. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia. 2011;25:498–505. doi: 10.1038/leu.2010.283. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mawali A, Gillis D, Hissaria P, Lewis I. Incidence, sensitivity, and specificity of leukemia-associated phenotypes in acute myeloid leukemia using specific five-color multiparameter flow cytometry. Am J Clin Pathol. 2008;129:934–945. doi: 10.1309/FY0UMAMM91VPMR2W. [DOI] [PubMed] [Google Scholar]
- 48.Baer MR, Stewart CC, Dodge RK, et al. High frequency of immunophenotype changes in acute myeloid leukemia at relapse: implications for residual disease detection (Cancer and Leukemia Group B Study 8361). Blood. 2001;97:3574–3580. doi: 10.1182/blood.v97.11.3574. [DOI] [PubMed] [Google Scholar]
- 49.Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T, Kern W. Stability of leukemia-associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: comparison with cytomorphologic, cytogenetic, and molecular genetic findings. Cytometry B Clin Cytom. 2004;62:25–38. doi: 10.1002/cyto.b.20025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.