Abstract
Background
The impact of early adequate empirical antibiotic therapy on outcomes of infants in the neonatal intensive care unit (NICU) who develop Staphylococcus aureus bloodstream infection (BSI) is unknown.
Methods
Infants with S. aureus BSI discharged in 1997–2012 from 348 NICUs managed by the Pediatrix Medical Group were identified. Early adequate empirical antibiotic therapy was defined as exposure to ≥1 antibiotic with anti-staphylococcal activity on the day the first positive blood culture was obtained. All other cases were defined as inadequate empirical antibiotic therapy. We evaluated the association between inadequate empirical antibiotic therapy on outcomes controlling for gestational age, small-for-gestational-age status, gender, discharge year, mechanical ventilation, inotropic support, and use of supplemental oxygen. The primary outcome was 30-day mortality. Secondary outcomes were 7-day mortality, death before hospital discharge, and length of bacteremia.
Results
Of the 3339 infants with S. aureus BSI, 2492 (75%) had methicillin-susceptible S. aureus (MSSA) BSI and 847 (25%) had methicillin-resistant S. aureus (MRSA) BSI. Inadequate empirical antibiotic therapy was administered in 725 (22%) cases. Inadequate empirical antibiotic therapy was associated with increased 30-day mortality (odds ratio, 2.03 [95% confidence interval, 1.08, 3.82]) among infants with MRSA BSI. Inadequate empirical antibiotic therapy was not associated with increases in mortality among infants with MSSA BSI.
Conclusions
After controlling for confounders, inadequate empirical antibiotic therapy was associated with a modestly increased mortality at 30 days for infants with MRSA BSI.
Keywords: infants, early empirical antibiotic therapy, Staphylococcus aureus bloodstream infection, NICU
Staphylococcus aureus bloodstream infections (BSI) cause significant morbidity and mortality among infants in neonatal intensive care units (NICUs).1-3 Several risk factors for S. aureus BSI have been identified, including intravascular catheters,1,4-7 implanted foreign bodies,8 nasal colonization,9 and premature birth.1 The use of early adequate empirical antimicrobial therapy for S. aureus BSI has been studied in adults with mixed results.10-13 In infants, the effectiveness of early adequate empirical antimicrobial therapy has been limited to small studies that addressed the effects of limited antibiotic combinations.14-16 The true effect of early adequate empirical antibiotic therapy in infants is still unknown. This is an important unmet need as clinicians attempt to balance the necessity of early adequate empirical antibiotic coverage with antibiotic stewardship concerns, including the risk of increased incidence of antibiotic resistant bacteria.
Using information on a large cohort of infants with S. aureus BSI from NICUs in the United States, we sought to determine whether early adequate empirical antibiotic therapy affected outcomes in infants with S. aureus BSI.
METHODS
We identified all infants diagnosed with S. aureus BSI in the first 120 days of life who were discharged between 1997 and 2012 from 348 NICUs managed by the Pediatrix Medical Group. Data were obtained from a database that prospectively captured information from electronic medical records generated by clinicians.17 The Pediatrix Medical Group coordinates prospective data collection from NICUs using a computer-assisted tool that generates daily clinical progress notes. Data are extracted, de-identified, and stored in the Pediatrix Clinical Data Warehouse. Information collected from infants included maternal history, demographics, medications, laboratory results, microbiology results, diagnoses, and procedures. We excluded infants with major congenital anomalies.
Definitions
We included the first episode of S. aureus BSI for each infant. All positive cultures obtained within 21 days of each other were considered as single infectious episodes. We categorized S. aureus positive blood cultures as either methicillin-susceptible S. aureus (MSSA) or methicillin-resistant S. aureus (MRSA). We defined duration of bacteremia as the time from the first positive culture to the last positive culture within a single episode. We defined early adequate empirical antibiotic therapy as exposure to at least 1 antibiotic with anti-staphylococcal activity on the day the first positive culture was obtained (day 0). All other cases (anti-staphylococcal antibiotics started on day 1 to 3) were defined as inadequate empirical antibiotic therapy. Adequate empirical antibiotics for MRSA included vancomycin, daptomycin, linezolid, trimethoprim-sulfamethoxazole, and clindamycin. Adequate empirical antibiotics for MSSA included all aminoglycosides, carbapenems, fluoroquinolones, cephalosporins (with the exception of ceftazidime), penicillins combined with beta-lactamase inhibitors, nafcillin, oxacillin, dicloxacillin, methicillin, clarithromycin, clindamycin, trimethoprim-sulfamethoxazole, vancomycin, linezolid, daptomycin, and rifampin. Antibiotics not accepted as adequate empirical therapy for MSSA, either for lack of effectiveness or variable effectiveness, were ampicillin, amoxicillin, penicillin G, penicillin V, aztreonam, piperacillin, ticarcillin, metronidazole, ceftazidime, chloramphenicol, and actinomycin. Besides methicillin susceptibility, no further antibiotic susceptibility data were available for the S. aureus isolates. Delay in therapy was defined as the number of days between a positive blood culture and exposure to adequate antibiotic therapy. We excluded infants in whom therapy was delayed for >3 days.
We defined small-for-gestational-age (SGA) status as previously described.18 Inotropic support was defined as exposure to dopamine, dobutamine, epinephrine, norepinephrine, or milrinone on the day of the first positive culture. Mechanical ventilation was defined as exposure to any invasive mechanical ventilation on the day of the first positive culture. Oxygen supplementation was defined as the administration of any fraction of inspired oxygen >21% on the day of the first positive culture. We defined 7-day mortality and 30-day mortality as death within 7 and 30 days of the first positive culture, respectively.
Statistical Analysis
The primary outcome of our study was 30-day mortality. Secondary outcomes included 7-day mortality, mortality prior to hospital discharge, and length of bacteremia. The unit of observation for this study was the infant. We used standard summary statistics including medians (interquartile ranges) and counts (proportions) to describe the study variables. We compared the distribution of variables between infants with MSSA and MRSA bacteremia and between infants with adequate versus inadequate empirical antibiotic therapy using Wilcoxon rank sum and chi-square tests of association where appropriate.
We used separate multivariable logistic regression models with random effects for NICU site to evaluate the association between mortality and early adequate empirical antibiotic therapy in infants with MRSA and MSSA bacteremia. Standard assumption diagnostics and graphing techniques were used to evaluate model assumptions, outliers, and collinearity. All biologically plausible covariates significantly associated with mortality in a univariable analysis were included in the final model, and we tested all biologically plausible interactions. The final models included mortality (30-day, 7-day, or prior to hospital discharge) as the dependent variable and delay in adequate antibiotic therapy, gestational age, SGA status, gender, discharge year, mechanical ventilation on the day of culture, inotropic support on the day of culture, and use of supplemental oxygen on the day of culture as the covariates.
To examine the effect of delay in adequate antibiotic therapy on 30-day mortality, we used multivariable logistic regression controlling for the above covariates to evaluate the effect of 1-, 2-, and 3-day delays in adequate antibiotic therapy. In this analysis, the adjusted odds ratio represents the mortality for infants who initially received the antibiotics of interest on day 1, 2, or 3 relative to infants who received the antibiotics of interest on day 0 (i.e., same day as positive blood culture). For infants with MSSA BSIs, we examined 3 groups: (1) vancomycin and/or a beta-lactam antibiotic (nafcillin or oxacillin), (2) vancomycin only, or (3) beta-lactam only (nafcillin or oxacillin). For infants with MRSA BSI, we examined the effect of delay in vancomycin therapy only. For this analysis, we excluded infants treated with any other antibiotic with potential efficacy against MSSA or MRSA.
We used Stata version 13.1 (College Station, TX) for all statistical analyses and considered P<0.05 statistically significant. The study was approved by the Duke Institutional Review Board.
RESULTS
Epidemiology of S. aureus BSI
Out of a total cohort of 887,910 hospitalized infants, we identified 3339 (0.4%) infants with S. aureus BSI. The majority of isolates were MSSA (2492/3339, 75%). The median age at the initial positive blood culture was 16 days (interquartile range [IQR], 10-29), and the median length of bacteremia was 1 day (IQR, 1-4). Infants with MRSA infection were younger at the time of first positive culture (15 days [IQR, 9-26] vs. 17 days [IQR, 10-30], P=0.002) and had longer duration of bacteremia compared to those with MSSA infection (2 days [IQR, 1-4] vs. 1 day [IQR, 1-3], P<0.001).
Most infants received early adequate empirical antibiotic therapy (2614/3339, 78%) (Table 1). Infants receiving early adequate empirical antibiotic therapy had a lower gestational age (28 weeks [IQR, 25-30] vs. 28 weeks [IQR, 26-33], P<0.001) and birth weight (970 g [IQR, 740-1360] vs. 1060 g [IQR, 800-1860], P<0.001) than those who received inadequate empirical antibiotic therapy.
TABLE 1.
Demographic characteristics of infants with S. aureus bloodstream infection who received early adequate or inadequate empirical antibiotic therapy
| Inadequate Empirical Antibiotics N = 725 (%) |
Adequate Empirical Antibiotics N = 2614 (%) |
P-value | |
|---|---|---|---|
| Gestational age, weeks | |||
| <26 | 151 (21) | 659 (25) | <0.01 |
| 26-28 | 229 (32) | 932 (36) | |
| 29-32 | 158 (22) | 629 (24) | |
| 33-36 | 86 (12) | 220 (8) | |
| >37 | 101 (14) | 172 (7) | |
| Birth weight, g | |||
| <1000 | 324 (45) | 1383 (53) | <0.01 |
| 1000-1499 | 179 (25) | 678 (26) | |
| 1500-2499 | 97 (13) | 343 (13) | |
| 2500-3499 | 84 (12) | 157 (6) | |
| >3500 | 41 (6) | 52 (2) | |
| Age at positive blood culture, days | |||
| <7 | 123 (17) | 235 (9) | <0.01 |
| 7-14 | 220 (30) | 766 (29) | |
| 15-28 | 186 (26) | 913 (35) | |
| >28 | 196 (27) | 700 (27) | |
| Race/ethnicity | |||
| White | 328 (47) | 1241 (49) | 0.44 |
| African-American | 182 (26) | 673 (27) | |
| Hispanic | 154 (22) | 487 (19) | |
| Other | 36 (5) | 131 (5) | |
| 5-min Apgar | |||
| 0-3 | 34 (5) | 123 (5) | 0.43 |
| 4-6 | 119 (17) | 484 (19) | |
| 7-10 | 553 (78) | 1943 (76) | |
| Male | 389 (54) | 1447 (55) | 0.45 |
| Inborn | 549 (76) | 2084 (81) | <0.01 |
| Cesarean section | 488 (68) | 1897 (73) | 0.01 |
| Small for gestational age | 118 (16) | 533 (20) | 0.01 |
| Inotrope support on day of culture | 19 (3) | 220 (8) | <0.01 |
| Oxygen support on day of culture | 365 (50) | 1684 (64) | <0.01 |
| Ventilator support on day of culture | 229 (32) | 1295 (50) | <0.01 |
| Infection with methicillin-resistant S. aureus |
280 (39) | 567 (22) | <0.01 |
Outcomes
The database contained mortality data for 2946 of the total 3339 (88%) infants with S. aureus BSI. The 393 infants with missing mortality data were included in the epidemiological results described above, but are not included in the outcomes results described here. The overall 30-day mortality was 7% (211/2946), and this outcome was similar between infants with MRSA and infants with MSSA BSIs (63/748 [8%] vs. 148/2198 [7%], respectively, P=0.12) (Table 2). The 30-day mortality was similar for infants receiving early adequate empirical antibiotic therapy and those receiving inadequate empirical antibiotic therapy (176/2298 [8%] vs. 35/648 [5%], respectively, P=0.05). In the subset of infants with MRSA BSIs, the mortality was similar for those treated with early adequate empirical antibiotic therapy and inadequate empirical antibiotic therapy (39/493 [8%] vs. 24/255 [9%], P=0.48). In the subset of infants with MSSA bacteremia, mortality was higher in infants who received early adequate empirical antibiotic therapy compared to those who did not (137/1805 [8%] vs. 11/393 [3%], P=0.001).
TABLE 2.
Impact of inadequate empirical antibiotics on clinical outcomes
| MSSA | MRSA | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Deaths with Inadequate Empirical Antibiotics n (%) |
Deaths with Adequate Empirical Antibiotics n (%) |
Mortality Adjusted Odds Ratio (95% CI) |
Deaths with Inadequate Empirical Antibiotics n (%) |
Deaths with Adequate Empirical Antibiotics n (%) |
Mortality Adjusted Odds Ratio (95% CI) |
|
| 30-day mortality | 11 (3) | 137 (8) | 0.52 (0.26, 1.06) |
24 (9) | 39 (8) | 2.03 (1.08, 3.82) |
| 7-day mortality | 6 (2) | 76 (4) | 0.55 (0.21, 1.46) |
11 (4) | 16 (3) | 2.51 (0.93, 6.74) |
| In-hospital mortality | 17 (4) | 175 (10) | 0.64 (0.36, 1.14) |
30 (12) | 56 (11) | 1.76 (0.99, 3.14) |
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
On multivariable analysis, 30-day mortality was higher for infants with MRSA who received inadequate empirical antibiotic therapy versus those who received early adequate empirical antibiotic therapy (odds ratio [OR], 2.03 [95% CI, 1.08-3.82]). There were also trends toward higher 7-day mortality (OR, 2.51 [95% CI, 0.93-76.74]) and mortality prior to hospital discharge (OR, 1.76 [95% CI, 0.99-3.14]), though both failed to reach statistical significance (Table 2). Among infants with MSSA BSI, there was no association between inadequate empirical antibiotic therapy on mortality at 30 days (OR, 0.52 [95% CI, 0.26-1.06]), at 7 days (OR, 0.55 [95% CI, 0.21-1.46], or at hospital discharge (OR, 0.64 [0.36-1.14] on multivariable analysis. Early adequate empirical antibiotic therapy did not influence the length of bacteremia in infants with either MRSA (regression coefficient [RC], −0.04 [95% CI, −0.57 - 0.49]) or MSSA (RC, −0.13 [95% CI, −0.45 - 0.19]) BSI.
Among infants with MSSA BSI who received either vancomycin or a beta-lactam therapy, no increase in 30-day mortality was observed if therapy was delayed 1 day (Table 3). Temporal analyses involving the effects of individual antibiotic classes on mortality in infants with MSSA BSI were limited as infants generally received multiple antibiotic classes during their treatment course. For example, no infants with MSSA BSI who received inadequate empirical antibiotic therapy were treated with beta-lactams only starting on day 2 or 3 (Table 3). Among infants with MRSA BSI, delays in vancomycin therapy of 1, 2, or 3 days were not associated with increased mortality. When we included infants with MRSA BSIs who received alternative adequate antibiotics (e.g., linezolid, daptomycin, clindamycin, or trimethoprim-sulfamethoxazole), no significant difference in results relative to those who received vancomycin alone was observed (data not shown).
TABLE 3.
30-day mortality for methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) bloodstream infections by delay in therapy
| Antibiotic Administered | Day of Therapy* |
Died /Total (%) | 30-Day Mortality Adjusted Odds Ratio (95% CI) |
|---|---|---|---|
| MSSA | |||
| Vancomycin or beta-lactam | 0 | 119/1562 (8) | REFERENCE |
| Vancomycin or beta-lactam | 1 | 9/262 (3) | 0.61 (0.29, 1.29) |
| Vancomycin or beta-lactam | 2 | 1/64 (2) | N/A† |
| Vancomycin or beta-lactam | 3 | 1/12 (8) | 3.64 (0.39, 33.76) |
| Vancomycin only | 0 | 101/1341 (8) | REFERENCE |
| Vancomycin only | 1 | 5/219 (2) | 0.44 (0.17, 1.15) |
| Vancomycin only | 2 | 1/50 (2) | N/A† |
| Vancomycin only | 3 | 1/6 (17) | 9.42 (0.83, 106.51) |
| Beta-lactam only | 0 | 12/182 (7) | REFERENCE |
| Beta-lactam only | 1 | 1/29 (4) | 0.46 (0.04, 5.09) |
| Beta-lactam only | 2 | 0/10 (0) | N/A† |
| Beta-lactam only | 3 | 0/4 (0) | N/A† |
| MRSA | |||
| Vancomycin | 0 | 39/487 (8) | REFERENCE |
| Vancomycin | 1 | 13/159 (8) | 1.62 (0.76, 3.43) |
| Vancomycin | 2 | 2/40 (4) | 1.31 (0.28, 6.05) |
| Vancomycin | 3 | 2/32 (6) | 1.38 (0.29, 6.51) |
Number of days after first positive culture (i.e., day 0) that particular antibiotic therapy was first received.
Mortality odds ratios could not be generated due to either lack of infants or missing covariate data for the single infant.
Variation by Discharge Year
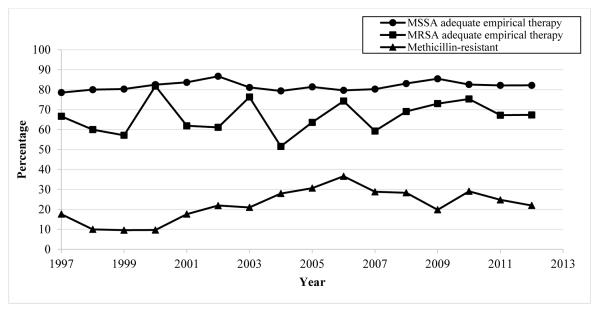
The proportion of BSIs caused by MRSA increased from 1997 (3/17, 18%) to 2006 (105/287, 37%) and decreased through 2012 (49/223, 22%) (Figure 1). The proportion of S. aureus BSIs treated with early adequate empirical antibiotic therapy increased (13/17 [76%] in 1997 vs. 176/223 [79%] in 2012). This was also the case for MSSA alone (11/14 [79%] in 1997 vs. 143/174 [82%] in 2012), while the proportion remained stable for MRSA (2/3 [67%] in 1997 vs. 33/49 [67%] in 2012) (Figure 1).
FIGURE 1.
Percent methicillin-resistance in S. aureus bloodstream infections and adequate empirical antibiotic therapy by year of discharge. Abbreviations: MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
DISCUSSION
We identified a large, contemporary cohort of hospitalized infants with S. aureus BSI and described the epidemiology, effect of early adequate empirical antibiotic therapy, and changing resistance patterns in S. aureus BSI. Mortality prior to hospital discharge was 10% in our study, which is consistent with previous studies that have reported S. aureus BSI mortality between 1% and 17%.1,2,19-21 Higher mortality (26%) has been noted in very low birth weight (<1500 g) infants.22
We found that infants with MRSA BSI treated with early adequate empirical antibiotic therapy had decreased 30-day mortality. This is despite the fact that infants with S. aureus BSI receiving early adequate empirical antibiotic therapy were both younger and more acutely ill, as demonstrated in Table 1. There was no clear progressive increase in mortality with delays of 1, 2, or 3 days. However, this temporal analysis was limited by the low number of infants with MRSA BSI who received vancomycin therapy with 2- and 3-day delays. Future studies are needed to more completely explore the effects of particular delays (e.g., 2 or 3 days) on clinical outcomes.
In adults, several studies have demonstrated efficacy of early adequate empirical antibiotic therapy on MRSA BSI outcomes,13,23-25 including a recent meta-analysis that demonstrated increased odds of survival with early adequate empirical therapy (OR, 1.84 [95% CI, 1.25-2.71]).26 However, other studies have not demonstrated a benefit of early adequate empirical antibiotic therapy on MRSA BSI outcomes.11,27-30 In infants with MSSA BSI, no clear effect of early adequate empirical antibiotic therapy on mortality was observed, regardless of the therapeutic drug class. This stands in contrast to adults, for whom multiple studies have demonstrated the superiority of beta-lactam therapy when treating MSSA BSI.31-36
There was an increase in the proportion of S. aureus BSI due to MRSA from 1998 to 2006. This finding has been noted in other NICU studies.2,37 The proportion of MRSA subsequently decreased from 2006 to 2012, however. Again, this has been demonstrated previously in both the pediatric38 and adult populations.39,40 Although the reasons for the observed changes in MRSA epidemiology are unknown, it may be that the widespread adoption of MRSA prevention practices in hospitals and other acute-care settings has been successful in decreasing MRSA transmission among both adults and children.
The strengths of this study are the large cohort size and multiple sites, which allowed us to identify trends that are broadly generalizable. Study weaknesses include a lack of data on antibiotic dosing, antimicrobial sensitivities other than methicillin, mortality information for all infants, exact timing of positive culture relative to antibiotic administration, the clinical circumstances (e.g., fever, hypotension) that led to the sepsis evaluation, the effects of individual antibiotic classes on mortality in infants with MSSA BSI, and the overall low number of infants with prolonged (e.g., 2-3 days) delays in adequate therapy, which limited our temporal analyses.
In conclusion, inadequate empirical antibiotic therapy was associated with a modestly increased mortality in infants with MRSA BSI at 30 days after controlling for confounders. Antimicrobials with MRSA activity should be considered when MRSA BSI is suspected. NICUs with a high incidence of MRSA should also consider use of these antimicrobials as empirical therapy for septic infants.
Acknowledgments
Source of funding This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number UM1AI104681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. Ericson receives support from the National Institute of Child Health and Human Development (NICHD) of the NIH under award number 5T32HD060558. Dr. Fowler receives salary support for research from the NIH (K24-AI093969, 2R01-AI068804, and NO1-AI90023); he also has served as Chair of V710 Scientific Advisory Committee (Merck), has received grant support from Cerexa, Pfizer, Advanced Liquid Logic, MedImmune, and Cubist, has been a paid consultant for Merck, Astellas, Affinium, Theravance, Bayer, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, Medicines Company, Biosynexus, MedImmune, and Inimex, and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis. Dr. Benjamin receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-05, 1K24HD058735-05, and UL1TR001117, and NICHD contract number HHSN275201000003I) and the nonprofit organization Thrasher Research Fund for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). Dr. Smith receives salary support for research from the NIH, the U.S. Department of Health and Human Services, and the National Center for Advancing Translational Sciences of the NIH (HHSN267200700051C, HHSN275201000003I, and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
Footnotes
conflicts of interest:
The other authors report no conflicts.
REFERENCES
- 1.Burke RE, Halpern MS, Baron EJ, Gutierrez K. Pediatric and neonatal Staphylococcus aureus bacteremia: epidemiology, risk factors, and outcome. Infect Cont Hosp Epidemiol. 2009;30:636–644. doi: 10.1086/597521. [DOI] [PubMed] [Google Scholar]
- 2.Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. J Perinatol. 2010;30:135–139. doi: 10.1038/jp.2009.119. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 4.Carrillo-Marquez MA, Hulten KG, Mason EO, Kaplan SL. Clinical and molecular epidemiology of Staphylococcus aureus catheter-related bacteremia in children. Ped Infect Dis J. 2010;29:410–414. doi: 10.1097/INF.0b013e3181c767b6. [DOI] [PubMed] [Google Scholar]
- 5.Gray JW. A 7-year study of bloodstream infections in an English children’s hospital. Eur J Ped. 2004;163:530–535. doi: 10.1007/s00431-004-1489-7. [DOI] [PubMed] [Google Scholar]
- 6.Hultén KG, Kaplan SL, Lamberth LB, et al. Hospital-acquired Staphylococcus aureus infections at Texas Children’s Hospital, 2001-2007. Infect Cont Hosp Epidemiol. 2010;31:183–190. doi: 10.1086/649793. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg JP, Clark CC, Hackman BO. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin Infect Dis. 1996;23:255–259. doi: 10.1093/clinids/23.2.255. [DOI] [PubMed] [Google Scholar]
- 8.Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man; a study of the problems of wound infection. Br J Exp Path. 1957;38:573–586. [PMC free article] [PubMed] [Google Scholar]
- 9.Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 10.Herzke CA, Chen LF, Anderson DJ, Choi Y, Sexton DJ, Kaye KS. Empirical antimicrobial therapy for bloodstream infection due to methicillin-resistant Staphylococcus aureus: no better than a coin toss. Infect Control Hosp Epidemiol. 2009;30:1057–1061. doi: 10.1086/606163. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Park WB, Lee KD, et al. Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemoth. 2004;54:489–497. doi: 10.1093/jac/dkh366. [DOI] [PubMed] [Google Scholar]
- 12.Lee CC, Hong MY, Chan TY, Hsu HC, Ko WC. The impact of appropriateness of antimicrobial therapy in adults with occult bacteraemia. Emerg Med J. 2014;31:53–58. doi: 10.1136/emermed-2012-201941. [DOI] [PubMed] [Google Scholar]
- 13.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36:1418–1423. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 14.Metsvaht T, Ilmoja ML, Parm U, Maipuu L, Merila M, Lutsar I. Comparison of ampicillin plus gentamicin vs. penicillin plus gentamicin in empiric treatment of neonates at risk of early onset sepsis. Acta Paediatrica. 2010;99:665–672. doi: 10.1111/j.1651-2227.2010.01687.x. [DOI] [PubMed] [Google Scholar]
- 15.Miall-Allen VM, Whitelaw AG, Darrell JH. Ticarcillin plus clavulanic acid (Timentin) compared with standard antibiotic regimes in the treatment of early and late neonatal infections. Br J Clin Pract. 1988;42:273–279. [PubMed] [Google Scholar]
- 16.Snelling S, Hart CA, Cooke RWI. Ceftazidime or gentamicin plus benzylpenicillin in neonates less than forty-eight hours old. J Antimicrob Chemoth. 1983;12:353–356. doi: 10.1093/jac/12.suppl_a.353. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system—tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 19.Frederiksen MS, Espersen F, Frimodt-Møller N, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J. 2007;26:398–405. doi: 10.1097/01.inf.0000261112.53035.4c. [DOI] [PubMed] [Google Scholar]
- 20.Suryati BA, Watson M. Staphylococcus aureus bacteraemia in children: a 5-year retrospective review. J Paediatr Child Health. 2002;38:290–294. doi: 10.1046/j.1440-1754.2002.00787.x. [DOI] [PubMed] [Google Scholar]
- 21.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Resp Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 22.Shane AL, Hansen NI, Stoll BJ, et al. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129:e914–e922. doi: 10.1542/peds.2011-0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchaim D, Kaye KS, Fowler VG, et al. Case–control study to identify factors associated with mortality among patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2010;16:747–752. doi: 10.1111/j.1469-0691.2009.02934.x. [DOI] [PubMed] [Google Scholar]
- 24.Schramm GE, Johnson JA, Doherty JA, Micek ST, Kollef MH. Methicillin-resistant Staphylococcus aureus sterile-site infection: The importance of appropriate initial antimicrobial treatment. Crit Care Med. 2006;34:2069–2074. doi: 10.1097/01.CCM.0000227655.41566.3E. [DOI] [PubMed] [Google Scholar]
- 25.Soriano A, Martinez JA, Mensa J, et al. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2000;30:368–373. doi: 10.1086/313650. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Kariv G, Goldberg E, et al. Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. Journal Antimicrobial Chemother. 2010;65:2658–2665. doi: 10.1093/jac/dkq373. [DOI] [PubMed] [Google Scholar]
- 27.Allard C. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin Microbiol Infect. 2008;14:421–428. doi: 10.1111/j.1469-0691.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 28.Ammerlaan H. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 2009;49:997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 29.Libert M. Risk factors for methicillin resistance and outcome of Staphylococcus aureus bloodstream infection in a Belgian university hospital. J Hosp Infect. 2008;68:17–24. doi: 10.1016/j.jhin.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Roghmann MC. Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch Intern Med. 2000;160:1001–1004. doi: 10.1001/archinte.160.7.1001. [DOI] [PubMed] [Google Scholar]
- 31.Chan KE, Warren HS, Thadhani RI, et al. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol. 2012;23:1551–1559. doi: 10.1681/ASN.2012010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang FY, Peacock JE, Jr., Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine. 2003;82:333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 33.Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006;38:7–14. doi: 10.1080/00365540500372846. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:192–197. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schweizer ML, Furuno JP, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11:279. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stryjewski ME, Szczech LA, Benjamin DK, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44:190–196. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 37.Rana D, Abughali N, Kumar D, Super DM, Jacobs MR, Kumar ML. Staphylococcus aureus, including community-acquired methicillin-resistant S. aureus, in a level III NICU: 2001 to 2008. Am J Perinatol. 2012;29:401–408. doi: 10.1055/s-0032-1304819. [DOI] [PubMed] [Google Scholar]
- 38.Day CT, Kaplan SL, Mason EO, Hulten KG. Community-associated Staphylococcus aureus infections in otherwise healthy infants less than 60 days old. Pediatr Infect Dis J. 2014;33:98–100. doi: 10.1097/INF.0b013e3182a5f9a8. [DOI] [PubMed] [Google Scholar]
- 39.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Int Med. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kallen AJ, Mu Y, Bulens S, et al. Health care-associated invasive MRSA infections, 2005-2008. JAMA. 2010;304:641–648. doi: 10.1001/jama.2010.1115. [DOI] [PubMed] [Google Scholar]