Abstract
Hematopoietic chimerism established by allogeneic bone marrow transplantation is known to promote donor-specific organ allograft tolerance; however, clinical application is limited by the need for toxic host conditioning and “megadoses” of donor bone marrow cells. A potential solution to this problem has been suggested by the observation that recipient bone marrow mobilization by the CXCR4 antagonist AMD3100 promotes chimerism in congenic bone marrow transplantation experiments in mice. Here we report that a single subcutaneous dose of 10 mg/kg AMD3100 in recipient C57BL/6 mice was able to enhance hematopoietic chimerism when complete MHC-mismatched BALB/c donor bone marrow cells were transplanted one hour after drug dosing. However, levels of chimerism measured 30 days post-transplantation were not sustained when mice were reexamined on day 90 post transplantation. Moreover, transient chimerism induced by this protocol did not support robust donor-specific skin allograft tolerance. Using the same transient immunosuppression protocol, we confirmed that “megadoses” of donor bone marrow cells could induce durable chimerism associated with donor-specific skin allograft tolerance without AMD3100 pre-treatment. We conclude that in this protocol AMD3100 pretreatment may empty bone marrow niches that become reoccupied by allogeneic donor hematopoietic progenitor cells but not by true long-lived donor hematopoietic stem cells, resulting in short-lived chimerism and failure to support durable donor-specific allograft tolerance.
Keywords: AMD3100, CXCR4, bone marrow mobilization, mixed chimerism, tolerance
1. Introduction
It has been observed in numerous animal models and in humans that mixed hematopoietic chimerism after bone marrow allotransplantation can induce tolerance to any other tissue or organ from the same or isogenic donor [1]. However, standard preparative regimens for mixed chimerism induction generally are harsh, and include total body irradiation and immunosuppression [2] [3]. Aggressive myeloablative conditioning is thought to promote chimerism by depleting recipient bone marrow cells to “make space” for donor hematopoietic stem cells (HSC). However the significant morbidity and mortality associated with myeloablation makes it highly desirable to develop alternative approaches which are mild and efficient. An irradiation-free, transient and mild host immunosuppressive conditioning regimen composed of an anti-T cell antibody cocktail and rapamycin has been reported to induce durable mixed chimerism in mice, but only in the context of “megadose” donor bone marrow infusion or vascularized bone transplantation [4] [5] . Proof of concept for host bone marrow mobilization as a method to enhance mixed chimerism induction after bone marrow transplantation has been reported using the drugs AMD3100 and G-CSF, but only in a congenic mouse bone marrow transplantation system [6]; whether this might also work in the setting of allotransplantation has not yet been reported. The chemokine receptor CXCR4 antagonist AMD3100 (also named plerixafor or Mozobil™) has been approved by the FDA for use with G-CSF to mobilize and collect HSCs for transplantation in the treatment of non-Hodgkins’ lymphoma and multiple myeloma [7] [8]. It is also being developed for mechanism-based therapy for the rare primary immunodeficiency disease WHIM syndrome, which is caused by gain-of-function mutations in CXCR4. These mutations result in panleukopenia caused by leukocyte retention in bone marrow [9]. Both AMD3100 and G-CSF promote release of HSCs into peripheral blood by preventing CXCR4 interaction with its ligand CXCL12 in bone marrow niches. AMD3100 does this by binding to CXCR4, whereas G-CSF inhibits CXCL12 availability. In addition to mobilizing HSCs, subcutaneous injection of AMD3100 has been demonstrated to mobilize both mature myeloid and lymphoid cells into the peripheral blood [10]. In the present study, we tested our hypothesis that recipient bone marrow mobilization by AMD3100 can enhance mixed chimerism and donor-specific skin allograft tolerance using allogeneic donor bone marrow cells under mild, transient host immunosuppression.
2. Materials & Methods
2.1 Biodistribution and pharmacokinetics of AMD3100
To determine the biodistribution pattern and pharmacokinetics of AMD3100 in immune organs, mice were injected intravenously with 10 ng AMD3100 labelled with 64Cu (100 μCi) in a total volume of 100 μl PBS. Mice were sacrificed at 2, 4, 6, 8, 10 and 12 hours after injection, and radioactivity was assayed in blood, spleen, bone marrow and thymus using a gamma counter, as described [11]. The results are corrected for the decay of 64Cu and presented as percent of injected dose per gram of tissue (%ID/g). Each group at each time-point contained 5 mice. In order to determine if the detected radioactivity was dependent on AMD3100, mice were injected intravenously with unlabeled AMD3100 (250 μg) 1 hour prior to sacrifice.
2.2 Skin and bone marrow transplantation
Heterotopic skin transplantation was performed in 10–16 week old male C57BL/6 (H2-Kb) mice with age matched male BALB/c (H2-Kd) or CBA/Ca (H2-Kk) mice as donors (The Jackson Laboratory, Bar Harbor, Maine). All care and handling of animals was carried out in accordance with guidelines provided in the Guide for the Care and Use of Laboratory Animals published by the U.S. Department of Health and Human Services. Full thickness skin from BALB/c donors (for primary grafts) or BALB/c donors and CBA/Ca third party donors (for secondary grafts) was transplanted onto the lateral thoracic wall of recipient mice using standard techniques [5]. After removal of the bandage on day 7 post-transplantation, grafts were monitored daily, and rejection was defined as complete loss of viable donor epithelium. All recipient mice except negative controls received a transient course of immunosuppression composed of an antibody cocktail: 1 mg of YTS177 (anti-CD4, non-depleting), YTS169 (anti-CD8, depleting), MR1 (anti-CD154, non-depleting) from BioXcell, (West Lebanon, NH) on days 0, 2 and 4 relative to skin grafting; plus rapamycin (12 mg/kg, LC Laboratories, Woburn, MA), prepared as previously described [5], on days 6 and 30 relative to skin grafting. For induction of mixed hematopoietic chimerism, mice also received a single dose of donor bone marrow (20, 50 or 100 million cells) on the day of skin grafting. Recipient bone marrow was mobilized by AMD3100 (Sigma Aldrich, St. Louis, MO) and/or G-CSF (PeproTech, Rocky Hill, NJ) pretreatment before bone marrow infusion according to the following protocol: AMD3100 1, 5 or 10 mg/kg, subcutaneous injection at 1, 3 or 10 hours before bone marrow infusion, and/or G-CSF 2.5 μg/mouse, subcutaneous injection twice per day, at 1 and 2 days before bone marrow infusion.
2.3 Mixed chimerism analysis
Mixed chimerism was assessed at 30 & 90 days after bone marrow and primary skin transplantation by flow cytometric analysis of peripheral blood stained with the following antibodies from Biolegend (San Diego, CA): FITC-conjugated anti-H-2Kd (SF1-1.1), APC-Cy7-conjugated anti-CD19 (6D5), Pacific blue- or APC-conjugated anti-CD4 (RM4-4), and PE-conjugated anti-CD8 (53–6.7). The level of mixed chimerism was determined by calculating the percentage of cells expressing donor BALB/c MHC class I among all blood-circulating leukocytes; where indicated, the lineage distribution of donor-derived cells was also analyzed.
2.4 Statistical analysis
All %ID/g values or mixed chimerism percentages were reported as mean ± SEM and comparison was performed between mice with or without co-injection of unlabeled AMD3100 or among mice with different levels of mixed chimerism, using the Student’s t-test; statistical significance was defined as P < 0.05.
3. Results
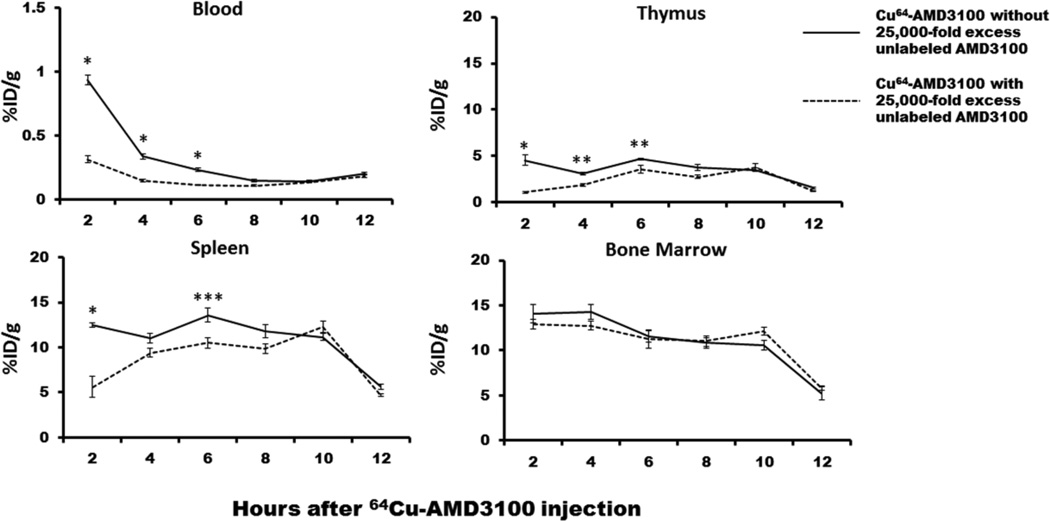
AMD3100 is known to be rapidly cleared from blood after injection [12], but detailed pharmacokinetics in immune organs have not been defined. To optimize an AMD3100 dosing schedule for our transplantation experiments, we measured radioactivity in mouse immune organs harvested at different time points after injection IV with 64Cu-labelled AMD3100. As shown in Figure 1, tissue 64Cu activity 2 hours after injection was 12.5 ± 0.26% in spleen, 14.08 ± 0.99% in bone marrow, 4.56 ± 0.56% in thymus and 0.94 ± 0.04% blood. The activity in blood declined quickly after injection, consistent with the reported rapid clearance of the drug from the blood in humans [12]. On the other hand, the administered 64Cu/64Cu-plerixafor that accumulated in spleen, bone marrow and thymus remained unchanged until 10 hours after injection, after which it declined rapidly (Fig. 1). Injection of excess unlabeled AMD3100 resulted in significantly reduced 64Cu activity (55 ~ 75% at 2 hours) in blood, spleen and thymus, suggesting specific tissue uptake of 64Cu-AMD3100; a similar but weaker competitive effect was observed until 6 hours after injection. However, in bone marrow, unlabeled AMD3100 did not reduce 64Cu activity at any time-point after injection (Fig. 1). The reason for this is unknown but possible explanations include: 1) 64Cu-AMD3100 rapidly binds to CXCR4 on the cell surface and becomes internalized, thus becoming inaccessible to unlabeled AMD3100 injected later; and 2) the 64Cu-AMD3100 compound becomes degraded so that the 64Cu is no longer bound to AMD3100 or to CXCR4. The overall result indicates that bone marrow transplantation should optimally be performed from 1 to 10 hours after AMD3100 pretreatment.
Figure 1. Biodistribution and pharmacokinetics of AMD3100 in immune organs.
Radioactivity associated with 64Cu-labelled AMD3100 was monitored in the blood and indicated immune organs after injection in the presence and absence of excess unlabeled AMD3100 injected one hour prior to sacrifice. Data are from a single experiment with 5 mice at each data point. * P < 0.0001, ** P < 0.01, * P < 0.05.
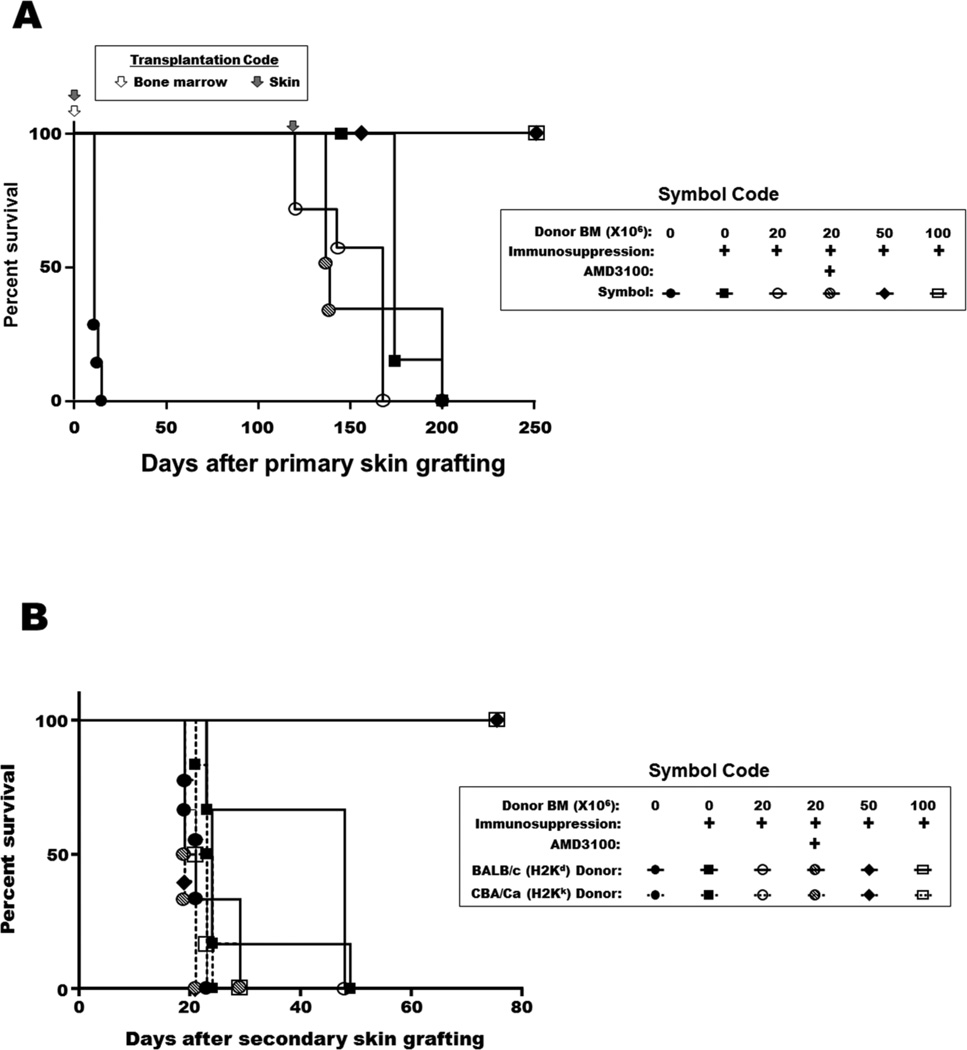
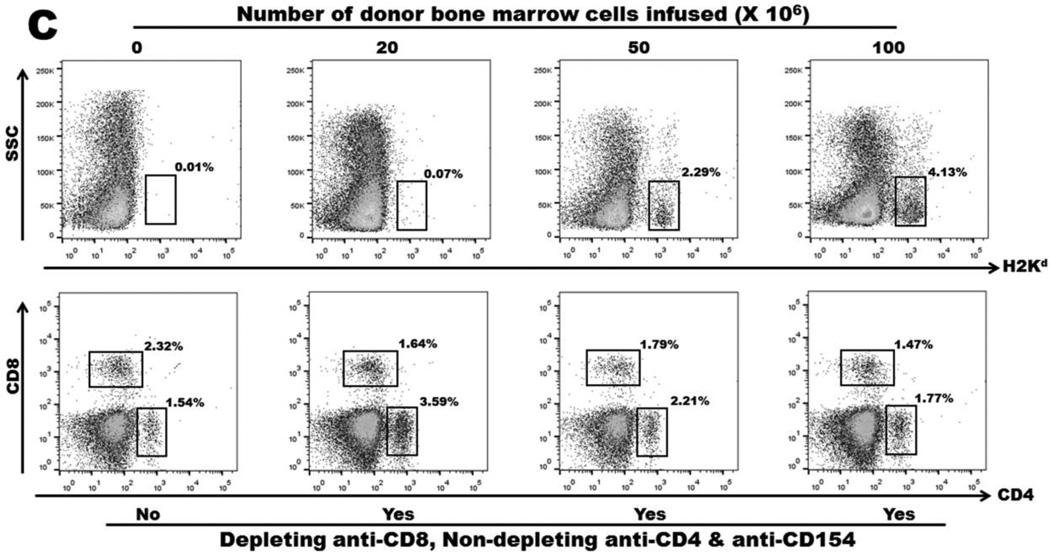
A transient course of CD4 T cell inhibition, CD8 T cell depletion and costimulation blockade combined with two doses of rapamycin permitted long-term BALB/c skin allograft survival in C57BL/6 recipients without the need for donor bone marrow infusion. Nevertheless, tolerance was not durable since the grafts were rejected if a second set of skin allografts were transplanted more than 100 days after the primary skin transplant had been performed (Fig 2A). Infusion of 50 or 100 million donor bone marrow cells at the time of primary skin transplantation induced durable mixed chimerism as well as durable skin allograft tolerance (Fig 2A), and tolerance under these conditions was donor-specific (Fig 2B). In contrast, infusion of 20 million donor bone marrow cells did not result in either mixed hematopoietic chimerism or donor-specific skin allograft tolerance (Fig 2C, Table 1). The levels of circulating CD4+ or CD8+ T lymphocytes were similar in mice receiving transient anti-T cell antibody cocktail or in control mice (Fig. 2C).
Figure 2. Skin allograft survival and mixed chimerism after donor bone marrow infusion with or without transient immunosuppression.
A: Survival of primary skin allografts. C57BL/6 mice were grafted with BALB/c skin at the same time they received different doses of donor bone marrow with or without immunosuppression. Filled circles: no bone marrow transplantation and no immunosuppression (negative control); filled squares: transient immunosuppression without bone marrow transplantation; open circles: transient immunosuppression with transfer of 20 million bone marrow cells; slashed circles: transient immunosuppression with transfer of 20 million bone marrow cells one hour after AMD3100 injection; filled diamonds: transient immunosuppression with transfer of 50 million bone marrow cells; open squares: transient immunosuppression with transfer of 100 million bone marrow cells. B: Survival of secondary skin allografts. Recipient C57BL/6 mice of BALB/c primary skin allografts were re-grafted with BALB/c donor-type and CBA/Ca third-party skin allografts from 120 days after primary skin grafting. Dashed lines: CBA/Ca allografts. Solid lines: BALB/c allografts. Filled circles: no bone marrow transplantation and no immunosuppression (negative control); filled squares: transient immunosuppression without bone marrow transplantation; open circles: transient immunosuppression with transfer of 20 million bone marrow cells; slashed circles: transient immunosuppression with transfer of 20 million bone marrow cells one hour after AMD3100 injection; filled diamonds: transient immunosuppression with transfer of 50 million bone marrow cells; open squares: transient immunosuppression with transfer of 100 million bone marrow cells. C: Mixed hematopoietic chimerism and circulating T lymphocyte levels in peripheral blood 90 days after mice received 0, 20, 50 and 100 million donor bone marrow cells with or without transient immunosuppression.
Table 1.
Effect of pretransplant BM cell-mobilization on mixed chimerism levels after BM allotransplantation
| Group | Number of Animals |
IS | BMT (million) |
BM mobilization pretreatment | Mixed Chimerism after BMT | ||||
|---|---|---|---|---|---|---|---|---|---|
| AMD3100* | G-CSF | 30 days | p value | 90 days | p value | ||||
| 1 | 3 | None | 0 | None | None | 0.17± 0.05 | 0.11± 0.02 | ||
| 2 | 3 | Yes | 0 | None | None | 0.27± 0.07 | 0.16± 0.03 | ||
| 3 | 6 | Yes | 20 | None | None | 0.35± 0.08 | 0.02 (vs group1) | 0.15± 0.02 | 0.21 (vs group1) |
| 4 | 5 | Yes | 20 | 1 mg/kg, 1Hr | None | 0.15± 0.05 | N/A | ||
| 5 | 5 | Yes | 20 | 5 mg/kg, 1 Hr | None | 0.13± 0.04 | N/A | ||
| 6 | 8 | Yes | 20 | 10 mg/kg,1Hr | None | 1.52± 0.18 | 0.0007 (vs group 1) 0.0001 (vs group 3) 0.3 (vs group 12) |
0.18± 0.02 | 0.06 (vs group 1) 0.42(vs group 3) 0.005 (vs group 12) |
| 7 | 6 | Yes | 20 | 10 mg/kg, 3 Hrs | None | 0.25± 0.01 | 0.13± 0.01 | ||
| 8 | 4 | Yes | 20 | 10 mg/kg, 10 Hrs | None | 0.56± 0.11 | 0.18± 0.02 | ||
| 9 | 5 | Yes | 20 | 10 mg/kg, 1 & 10 Hrs | None | 1.49± 0.17 | 0.13± 0.03 | ||
| 10 | 5 | Yes | 20 | 10 mg/kg,1 Hr | Yes | 1.06± 0.13 | 0.15± 0.04 | ||
| 11 | 4 | Yes | 20 | None | Yes | 0.45± 0.14 | 0.25± 0.06 | ||
| 12 | 6 | Yes | 50 | None | None | 1.28± 0.08 | 0.02 (vs group 13) | 1.39± 0.42 | 0.03 (vs group 13) |
| 13 | 5 | Yes | 100 | None | None | 2.47± 0.36 | 3.34± 0.71 | ||
BM: bone marrow; IS: immunosuppression; BMT: bone marrow transplantation; N/A: not performed;
hours (Hr) before BMT
To test if mixed chimerism could be enhanced by recipient bone marrow mobilization before allogeneic bone marrow transplantation, various AMD3100 and G-CSF mobilization protocols were screened (Table 1). Infusion of 20 million allogeneic donor bone marrow cells without recipient bone marrow mobilization induced background levels of mixed chimerism 30 and 90 days post-infusion, that is, levels similar to the level in mice that had received no donor bone marrow; whereas infusion of 50 and 100 million donor bone marrow cells induced significant and durable mixed chimerism (Table 1). Among the AMD3100 mobilization regimens, only 10 mg/kg pretreatment one hour before bone marrow infusion induced significant mixed chimerism 30 days later; no chimerism was observed if the mice were pretreated 3 or 10 hours before bone marrow infusion or when lower doses of AMD3100 were administered. However, the chimerism induced by one hour pre-treatment was not durable, since it was not observed at 90 days after bone marrow infusion (Table 1). AMD3100 administered at 1, 5 or 10 mg/kg one hour before transplantation of 50 million donor bone marrow cells neither enhanced nor inhibited donor cell engraftment at 30 days post transplantation of recipient mice treated with immunosuppression (data not shown).
Skin allografts were rejected in the AMD3100-pretreated group receiving 20 million total donor bone marrow cells, in which transient hematopoietic chimerism was observed, indicating a failure to induce robust donor-specific allograft tolerance (Fig. 2A & B). In the absence of immunosuppression and donor bone marrow transplantation, donor type allografts were rejected rapidly, whereas in the presence of immunosuppression without donor bone marrow transplantation the grafts survived until a secondary donor type allograft was applied, at which time both the primary and secondary donor type allografts were rapidly rejected (within 3 weeks). Third party allografts were rapidly rejected under all conditions. Robust donor specific skin allograft tolerance was only achieved for recipient mice given immunosuppression and 50 or 100 million total donor bone marrow cells, conditions that supported durable mixed hematopoietic chimerism. We did not test whether AMD3100 could affect donor specific skin allograft tolerance in these mice. Recipient bone marrow mobilization with G-CSF, either alone or in combination with AMD3100, did not induce mixed hematopoietic chimerism under the conditions tested (Table 1).
4. Discussion
In the present study, we tested the hypothesis that recipient bone marrow mobilization before allogeneic bone marrow infusion could serve as a non-toxic method to “make space” in bone marrow niches to facilitate establishment of stable mixed chimerism using clinically feasible doses of allogeneic donor bone marrow cells under mild and transient irradiation-free host conditioning. In our model, transient host conditioning with an anti-T cell antibody cocktail and rapamycin was able to induce long-term skin allograft survival, although tolerance was not durable since it could be abolished by a second set of skin allotransplantation applied at a later time. The factors required for ‘non-durable’ tolerance are poorly understood, and whether transitioning to ‘robust’ tolerance simply involves a quantitative change in these factors versus recruitment of a distinct toleragenic mechanism is not known. In our study, robust donor-specific tolerance was only achieved when durable donor mixed chimerism was established, which was only observed in mice receiving infusion of 50 million donor bone marrow cells or more, but not in those receiving 20 million cells, establishing a critical threshold. These findings were consistent with previous observations using similar or different regimens [4] [13].
To test if recipient bone marrow mobilization could enhance mixed chimerism induction, we performed bone marrow transplantation at three different time-points after AMD3100 pretreatment: one hour after pretreatment, when AMD3100 levels peaked in the peripheral blood; 3 hours after pretreatment, when the number of mobilized leukocytes has been reported to peak in the peripheral blood; 10 hours after pretreatment, when 64Cu radioactivity levels in immune organs was no longer responsive to inhibition by non-labelled AMD3100, possibly due to receptor ligation and internalization of AMD3100. The results revealed that among all the pretreatment schedules tested, only AMD3100 pretreatment one hour before bone marrow transplantation was able to induce mixed chimerism. Nevertheless, chimerism was transient, and not associated with robust tolerance to skin allografts.
The preparative regimen in mixed chimerism induction traditionally involves chemotherapy and/or irradiation to all or part of the body, to eliminate immunologic barriers of allogeneic bone marrow cell engraftment. Conditioning regimens also damage (or destroy) endogenous stem cells and provide a competitive advantage to the donor cells. However, impairment of host immune function and significant morbidity and mortality associated with these regimens has limited their clinical application, especially for non-malignant indications [14]. Chen et al. have reported that AMD3100 and cytokine mobilization of recipient bone marrow used as a preparative regimen in congenic bone marrow transplantation and parabiotic mice enhanced donor cell engraftment [6]. Kang et al. also observed selectively enhanced donor bone marrow cell engraftment in irradiated niches of mouse [15]. In the study by Chen et al. enhanced engraftment was observed when bone marrow was infused as late as 6 hours after AMD3100 mobilization, which was consistent with our 64Cu-AMD3100 biodistribution results. However, our mobilization results indicate that in an allogeneic setting, enhanced donor bone marrow cell engraftment by AMD3100 mobilization was not durable. The level of mixed chimerism achieved in our study is rather low (2–3% after transferring 100 million donor bone marrow cells). Therefore it is extremely hard to trace and investigate the fate of donor hematopoietic progenitor cells after they are transferred. Although the mechanism underlying the low and transient levels of mixed chimerism is not known, possibilities include 1) the alloimmune response destroys donor hematopoietic stem cells and 2) the vacated niches engraft short-lived hematopoietic progenitor cells but not the rare long-lived hematopoietic stem cells. Ablation of the host immune response is critical for donor cell engraftment; the immunosuppression in our model provides transient, specific targeting of host immune elements that may resist donor cell engraftment and/or GVHD. It has been demonstrated to be sufficient for inducing durable mixed chimerism when “megadoses” of bone marrow cells are transferred or when vascularized bone is transplanted [5] [13]. Additional work will be needed to evaluate whether more extended or intensive host immunosuppression can synergize with bone marrow mobilization to increase the efficiency of allogeneic bone marrow engraftment.
Highlights.
“Megadose” bone marrow allotransplantation induced durable mixed chimerism in mice
Allotransplantation of clinically feasible doses failed to induce durable chimerism
CXCR4 blockade enhanced chimerism for at least 30 days after allotransplantation
CXCR4 blockade did not support durable chimerism or robust skin allograft tolerance
Acknowledgments
This works was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH.
The authors thank Dr. Ana Paula Marino of Laboratory of Molecular Immunology, NIAID, NIH for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4(1):a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169(2):493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storb R, Yu C, Barnett T, Wagner JL, Deeg HJ, Nash RA, et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen-identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation. Blood. 1999;94(3):1131–1136. [PubMed] [Google Scholar]
- 4.Anam K, Akpinar E, Craighead N, Black AT, Hale DA. Targeted T-cell depletion or CD154 blockade generates mixed hemopoietic chimerism and donor-specific tolerance in mice treated with sirolimus and donor bone marrow. Transplantation. 2004;78(9):1290–1298. doi: 10.1097/01.tp.0000138097.08050.d7. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Benghiat FS, Charbonnier LM, Kubjak C, Rivas MN, Cobbold SP, et al. CD8+ T-Cell depletion and rapamycin synergize with combined coreceptor/stimulation blockade to induce robust limb allograft tolerance in mice. Am J Transplant. 2008;8(12):2527–2536. doi: 10.1111/j.1600-6143.2008.02419.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Larochelle A, Fricker S, Bridger G, Dunbar CE, Abkowitz JL. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood. 2006;107(9):3764–3771. doi: 10.1182/blood-2005-09-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 8.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 9.McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O'Brien S, Penzak SR, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118(18):4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian Liu ZL, Gao Ji-Liang, Wan Wuzhou, Ganesan Sundar, McDermott David H, Murphy Philip M. Bone Marrow is the Major Source of Both Myeloid and Lymphoid Cells Mobilized to Blood by CXCR4 Antagonist AMD3100. Eur J Immunol. doi: 10.1002/eji.201445245. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson O, Weiss ID, Szajek L, Farber JM, Kiesewetter DO. 64Cu-AMD3100--a novel imaging agent for targeting chemokine receptor CXCR4. Bioorg Med Chem. 2009;17(4):1486–1493. doi: 10.1016/j.bmc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lack NA, Green B, Dale DC, Calandra GB, Lee H, MacFarland RT, et al. A pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100. Clin Pharmacol Ther. 2005;77(5):427–436. doi: 10.1016/j.clpt.2004.12.268. [DOI] [PubMed] [Google Scholar]
- 13.Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone marrow transplantation induces either clonal deletion or infectious tolerance depending on the dose. J Immunol. 1998;160(6):2645–2648. [PubMed] [Google Scholar]
- 14.Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23(3):165–173. doi: 10.1016/j.smim.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang Y, Chen BJ, Deoliveira D, Mito J, Chao NJ. Selective enhancement of donor hematopoietic cell engraftment by the CXCR4 antagonist AMD3100 in a mouse transplantation model. PLoS One. 2010;5(6):e11316. doi: 10.1371/journal.pone.0011316. [DOI] [PMC free article] [PubMed] [Google Scholar]