Abstract
Background
We describe the relative impact of the heptavalent pneumococcal conjugate vaccine (PCV7, introduced 2001) and antibiotic use on colonization by antibiotic resistant pneumococci in urban Alaskan children during 2000–2010.
Methods
We obtained nasopharyngeal swab specimens from a convenience sample of children aged <5 years at clinics annually during 2000–2004 and 2008–2010. PCV7 status and antibiotic use <90 days before enrollment was determined by interview/medical records review. Pneumococci were characterized by serotype and susceptibility to penicillin (PCN). Isolates with full PCN resistance (PCN-R) or intermediate PCN resistance (PCN-I) were classified as PCN-NS.
Results
We recruited 3,496 children (median: 452/year). During 2000–2010, a range of 18–29%/year of children used PCN/amoxicillin (p-value for trend [p] = 0.09); the proportion age-appropriately vaccinated with PCV7 increased (0%–90%; p <0.01). Among pneumococcal isolates, the PCV7-serotype proportion decreased (53%–<1%; p <0.01) and non-PCV7–serotype proportion increased (43%–95%; p <0.01). PCN-R pneumococcal colonization prevalence decreased (23%–9%, p <0.01) and PCN-I pneumococcal colonization prevalence increased (13%–24%, p <0.01); overall PCN-NS pneumococcal colonization prevalence was unchanged. PCN-NS among colonizing PCV7-type and non-PCV7-type pneumococci remained unchanged; a mean of 31%/year of PCV7-type and 10%/year of non-PCV7-type isolates were PCN-R, and 10%/year of PCV7 and 20%/year of non-PCV7-type isolates were PCN-I.
Conclusions
Overall PCN-NS pneumococcal colonization remained unchanged during 2000–2010 because increased colonization by predominantly PCN-I non-PCV7 serotypes offset decreased colonization by predominantly PCN-R PCV7 serotypes. Proportion PCN-NS did not increase within colonizing pneumococcal serotype-groups (PCV7 versus non-PCV7) despite stable penicillin use in our population.
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccine, antibiotic resistance, epidemiology
INTRODUCTION
Streptococcus pneumoniae is a leading cause of infections in children such as otitis media, pneumonia, and meningitis.1 Therefore, understanding and controlling pneumococcal resistance to antibiotics is an important clinical and public health challenge. Young children, especially those aged <2 years, are at highest risk for pneumococcal colonization because of their immature immune response.2, 3 Asymptomatically colonized children play an important role in pneumococcal transmission to other children in settings such as daycare centers and to household adults.4, 5 Antibiotic use by individuals is widely believed to select for resistant bacteria by eradicating susceptible bacteria and allowing for resistant bacteria to expand and fill the niche; subsequent transmission of resistant bacteria results in an increased prevalence of resistant bacteria in a population.6, 7
Prior to introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), the serotypes included in PCV7 accounted for the majority of antibiotic resistant pneumococci.8 In Alaska during the pre-PCV7–era, 60% of invasive pneumococcal isolates that were nonsusceptible to penicillin (PCN), trimethoprim-sulfamethoxazole (TMP-SMX), tetracycline, or erythromycin belonged to serotypes included in PCV7.9 The introduction of PCV7 was expected to reduce the prevalence of antibiotic resistant pneumococci. However, there was concern that sustained antibiotic use would result in increased resistance among nonvaccine pneumococcal serotypes.10
Previous studies have separately evaluated the impact of antibiotic use on the risk for resistant pneumococcal colonization or the impact of widespread PCV7 vaccination on the prevalence of resistant pneumococcal colonization.11–17 The objective of our study is to evaluate the combined influence of antibiotic use (penicllins and trimethoprim-sulfamethoxazole [TMP-SMX]) and PCV7 vaccination in determining the prevalence of colonization by pneumococci resistant to those antibiotics in urban Alaskan children during 2000–2010 (the period in which PCV7 was used in the United States).
METHODS
Participants
PCV7 was introduced in Alaska in January 2001. Children in Alaska received PCV7 according to the immunization schedule recommended by the Advisory Committee on Immunization Practices (ACIP).18 During 2000–2004 and 2008–2010, we conducted annual cross-sectional pneumococcal colonization surveys among children aged 3 months to 5 years. We recruited a convenience sample of children presenting for sick or well-child visits to general pediatric clinics in Anchorage, Alaska (3 clinics during 2000–2004, 2 clinics during 2008–2010 chosen by convenience). We excluded children living outside of the Anchorage metropolitan area (as defined by the Alaska State Department of Labor and Workforce) or if another child from the same household was already enrolled in the study.
The study was approved by the Institutional Review Boards of the Alaska Native Tribal Health Consortium in Anchorage, Alaska and the Centers for Disease Control and Prevention in Atlanta, Georgia. Written informed consent was obtained from each child’s parent.
Procedures
Data collection occurred in December and the following January of each year. We interviewed each child’s parent/guardian to obtain social and demographic information such as date of birth, sex, number of rooms, and persons in each household, and daycare/school attendance <30 days prior to study enrollment. In addition, we asked each child’s parent/guardian about antibiotic use <90 days prior to study enrollment, including antibiotics received elsewhere other than the study clinic. Each child’s medical record was reviewed to identify any PCN (including amoxicillin) or TMP-SMX use <90 days prior to study enrollment (i.e., recent antibiotic use) and to determine the dates of prior PCV7 vaccine doses. A sterile calcium-alginate tipped swab (Calgiswab type 1; Harwood Products) (2000–2004) or nylon fiber flocked swab (Copan Diagnostics, Corona, CA) (2008–2010) was inserted through the nose into the posterior nasopharynx of each child and immediately plated onto a gentamicin-blood agar medium (2000–2004) or media containing skim milk-tryptone-glucose-glycerin (STGG) (2008–2010) for transportation to our laboratory.19, 20
Streptococcus pneumoniae were identified in the laboratory by bile solubility and optochin sensitivity when plated on gentamicin-blood agar medium.21 Pneumococcal serotype was determined by latex agglutination and the Quellung reaction. The minimal inhibitory concentration (MIC) of pneumococcal isolates to PCN and TMP-SMX were determined by Etest (bioMérieux Clinical Diagnostics). Isolates were classified as susceptible to PCN (PCN-S) if MICs ≤ 0.064 mcg/mL, resistant to PCN (PCN-R) if MICs >1 mcg/mL, intermediate susceptibility to PCN (PCN-I) if MICs > 0.064–≤1 mcg/mL.22 Isolates sensitivity to TMP-SMX was determined by their sensitivity to trimethoprim and were classified as susceptible to TMP-SMX (TS-S) if MICs <1 mcg/mL, resistant to TMP-SMX (TS-R) if MICs ≥4 mcg/mL, and intermediate susceptibility to TMP-SMX (TS-I) if MICs = 1–4 mcg/mL. PCN-I/PCN-R isolates were defined as nonsusceptible to PCN (PCN-NS) and TS-I/TS-R isolates were defined as nonsusceptible to TMP-SMX (TS-NS).
Statistical analysis
In order to determine whether children were age-appropriately vaccinated with PCV7, we developed an algorithm based on ACIP guidelines (algorithm available from the authors).18 Children living in homes with ≥1.5 persons/room were defined as living in a crowded household. We evaluated for changes in the prevalence of key study outcomes over the study period by using the Cochran-Armitage test of trend. For analysis, the 7 serotypes included in PCV7 (4, 6B, 9V, 14, 18C, 19F, 23F) were grouped together and classified as PCV7-serotype pneumococci, the serotypes not included in the vaccine were classified as non-PCV7-serotype pneumococci, and pneumococcal isolates where a serotype could not be determined were classified as non-typeable pneumococci. We used the likelihood-ratio χ2 statistic to test for differences in pneumococcal colonization between children with and without antibiotic use and the Mantel-Haenszel estimator to adjust prevalence ratios for study year. We used log-binomial regression to adjust prevalence ratios for age, sex, day-care attendance, and PCV7 status. All p-values are two-sided and exact when sample sizes necessitated. Statistical tests were run in SAS version 9.3 (SAS Institute, Cary, NC) or StatXact version 9.0 (Cytel Software Corporation, Cambridge, MA).
RESULTS
We recruited a total of 3,496 children (median: 452 children/year) during 2000–2004 and 2008–2010 (Table 1). Among children recruited in our study, 51% were male over all study years and that proportion increased from 48% in 2000 to 56% in 2010 (p-value for trend = 0.05). By age, 51% of children recruited were aged <2 years; the proportion of children aged <2 years increased during the study period (p-value for trend = 0.02). The proportion of children attending daycare/school (35%), living in a crowded household (9%), using PCN or TMP-SMX <90 days of enrollment (24% and 4%, respectively) did not change significantly over the study period.
TABLE 1.
Demographic characteristics and antibiotic use among urban children — Alaska, 2000–2010
| Characteristics | Study year(Number of children) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 (451) | 2001 (450) | 2002 (452) | 2003 (452) | 2004 (452) | 2008 (337) | 2009 (449) | 2010 (453) | p-value for trend | All years (3496) | |
| Male | 48% | 48% | 51% | 52% | 50% | 50% | 51% | 56% | 0.05 | 51% |
| Age group | ||||||||||
| <2 years | 52% | 49% | 48% | 44% | 52% | 55% | 55% | 52% | 0.02 | 51% |
| 2–4 years | 48% | 51% | 52% | 56% | 48% | 45% | 45% | 48% | 49% | |
| Daycare/school attendance <30 days* | 35% | 34% | 35% | 35% | 30% | 32% | 37% | 43% | 0.09 | 35% |
| In crowded household† | 14% | 9% | 8% | 8% | 8% | 8% | 11% | 6% | 0.08 | 9% |
| PCN use <90 days* | 25% | 22% | 26% | 29% | 24% | 27% | 18% | 22% | 0.09 | 24% |
| TMP-SMX use <90 days* | 6% | 4% | 4% | 2% | 2% | 6% | 4% | 3% | 0.45 | 4% |
Abbreviations: PCN, penicillin-class antibiotic; TMP-SMX, trimethoprim-sulfamethoxazole
Days before study enrollment
≥ 1.5 persons per room in the household
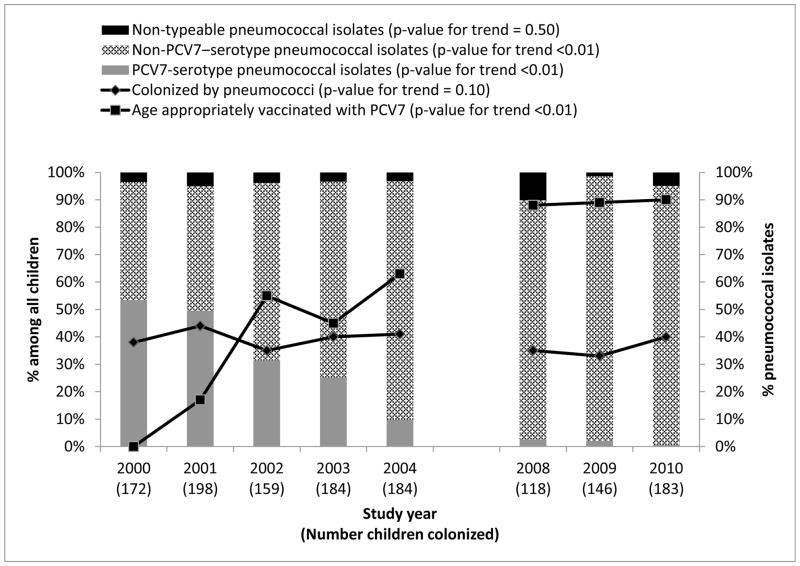
The proportion of children age-appropriately vaccinated with PCV7 increased from 0% in 2000 to 90% in 2010 (p-value for trend <0.01) (Figure 1). The prevalence of pneumococcal colonization remained stable between 33% and 44% during the study period (p-value for trend = 0.10). The proportion of PCV7-serotype pneumococci among pneumococcal isolates decreased from 53% to 1% and the proportion of isolates that were non-PCV7–serotype pneumococci increased from 43% to 95% (p-value for trend <0.01). The proportion of nontypeable pneumococcal isolates (i.e., isolates that were optochin sensitive and bile soluble but for which a serotype could not be determined by latex agglutination/Quellung reaction or multiplex PCR) remained stable at <10%/year over the study period (p-value for trend = 0.50).
FIGURE 1.
Prevalence of nasopharyngeal pneumococcal colonization, proportion age-appropriately vaccinated with PCV7, and proportion of PCV7-serotype pneumococcal isolates among urban children — Alaska, 2000–2010 (n=3,496)
Abbreviations: PCV7, 7-valent pneumococcal conjugate vaccine
Note: PCV7 introduced in 2001
The proportion of pneumococcal isolates that were PCN-R decreased from 22.7% to 9.4% (p-value for trend <0.01) and the proportion of PCN-I isolates increased from 12.8% to 23.8% (p-value for trend <0.01) from 2000 to 2010; overall, there was no change in the proportion of isolates that were PCN-NS (range: 28.0%–37.4%; p-value for trend = 0.48) (Table 2). During 2000–2004, there was no change in the proportion of PCV7-serotype pneumococcal isolates that were PCN-R (range: 24.4%–42.0%; p-value for trend = 0.71), PCN-I (range: 7.6%–13.3%; p-value for trend = 0.73), or PCN-NS (range: 37.8%–52.0%; p-value for trend = 0.57); there were only 7 PCV7-serotype isolates during 2008–2010 for analysis and therefore were excluded from this analysis. During the study period, there was no change in the proportion of non-PCV7–serotype pneumococcal isolates that were PCN-R (range: 6.8%–13.3%; p-value for trend = 0.84), PCN-I (range: 14.6%–23.7%; p-value for trend = 0.18), or PCN-NS (range: 21.4%–35.6%; p-value for trend = 0.19). Prior to introduction of PCV7 (2000 only), non-PCV7 serotypes were less likely to be PCN-R than PCV7 serotypes (10.8% vs. 31.5%, prevalence ratio [PR] = 0.3, 95% CI: 0.2–0.7) and with no difference in PCN-I prevalence (16.2% vs. 7.66%, PR = 2.1, 95% CI: 0.9, 5.1).
TABLE 2.
Penicillin nonsusceptibility among pneumococcal isolates — Alaska, 2000–2010 (N = 3,496)*
| % PCN-NS pneumococci among all pneumococcal isolates | % PCN-NS pneumococci among PCV7-serotype isolates† | % PNC-NS pneumococci among non-PCV7–serotype isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PCN-R | PCN-I | PCN-NS | N | PCN-R | PCN-I | PCN-NS | N | PCN-R | PCN-I | PCN-NS | |
| 2000 | 172 | 22.7% | 12.8% | 35.5% | 92 | 31.5% | 7.6% | 39.1% | 74 | 10.8% | 16.2% | 27.0% |
| 2001 | 198 | 18.7% | 18.7% | 37.4% | 98 | 25.5% | 13.3% | 38.8% | 90 | 13.3% | 21.1% | 34.4% |
| 2002 | 159 | 17.6% | 13.8% | 31.4% | 50 | 42.0% | 10.0% | 52.0% | 103 | 6.8% | 14.6% | 21.4% |
| 2003 | 184 | 11.2% | 17.9% | 29.1% | 45 | 24.4% | 13.3% | 37.8% | 128 | 7.0% | 18.0% | 25.0% |
| 2004 | 184 | 15.2% | 21.7% | 37.0% | 18 | 38.9% | 5.6% | 44.4% | 160 | 12.5% | 23.1% | 35.6% |
| 2008 | 118 | 11.9% | 16.1% | 28.0% | 103 | 11.7% | 14.6% | 26.2% | ||||
| 2009 | 146 | 11.1% | 23.6% | 34.7% | 139 | 10.8% | 23.7% | 34.5% | ||||
| 2010 | 183 | 9.4% | 23.8% | 33.1% | 171 | 9.9% | 23.4% | 33.3% | ||||
| p-value for trend | <0.01 | <0.01 | 0.48 | 0.71 | 0.73 | 0.57 | 0.84 | 0.18 | 0.19 | |||
Abbreviations: N, number of pneumococcal isolates; PCV7, 7-valent pneumococcal conjugate vaccine; PCN, penicillin-class antibiotic; PCN-I, intermediate penicillin resistance (MIC > 0.064 and ≤ 1 mcg/mL); PCN-NS, full, plus intermediate penicillin resistance (MIC > 0.064 mcg/mL); PCN-R, full penicillin resistance (MIC > 1 mcg/mL)
Nontypeable pneumococcal isolates not included in table.
During 2008–2010, there were insufficient PCV7-serotype isolates for analysis (N = 7).
A multivariate logistic regression model for the trend in PCN resistance among all isolates adjusted for age, sex, day-care attendance, household crowding, and PCN use confirmed the trend towards a decrease in the proportion of isolates that were PCN-R (p-value for trend = 0.01), an increase in the proportion of isolates that were PCN-I, and no change in isolates that were PCN-NS (p-value for trend = 0.31). Adding pneumococcal serotype (PCV7-serotype versus non-PCV7–serotype) to that multivariate model eliminated any trends in resistance among all isolates during our study period (p-value for trend for proportion of isolates PCN-R, PCN-I, PCN-NS was 0.98, 0.54, and 0.62, respectively).
The proportion of pneumococcal isolates that were TS-R decreased from 33.7% in 2000 to 12.7% in 2010 (p-value for trend <0.01) and the proportion TS-I remained unchanged (range: 4.4%–10.5%; p-value for trend = 0.95) during that time (Table 3). The proportion of TS-NS isolates declined during the study period from 44.2% to 19.3% (p-value for trend <0.01). There was no change from 2000 to 2004 in the proportion of PCV7-serotype pneumococcal isolates that were TS-R (range: 35.6%–46.0%; p-value for trend = 0.60), TS-I (range: 0%–14.1%; p-value for trend = 0.17), or TS-NS (range: 44.4%–60.0%; p-value for trend = 0.17). During 2000–2010, there was no change in the proportion of non-PCV7–serotype pneumococci that were TS-R (range: 8.8%–22.2%; p-value for trend = 0.53), TS-I (range: 1.1%–7.9%; p-value for trend = 0.06), or TS-NS (range: 13.2%–24.3%; p-value for trend = 0.57). Prior to introduction of PCV7 (2000 only), non-PCV7 serotypes were less likely to be TS-R than PCV7 serotypes (18.9% vs. 45.7%, PR = 0.4, 95% CI: 0.2–0.7) and with no difference in TS-I prevalence (5.4% vs. 14.1%, PR = 0.4, 95% CI: 0.1–1.1).
TABLE 3.
Trimethoprim-sulfamethoxazole nonsusceptibility among pneumococcal isolates — Alaska, 2000–2010 (N = 3,496)*
| % TS-NS pneumococci among all pneumococcal isolates | % TS-NS pneumococci among PCV7-serotype isolates† | % TS-NS pneumococci among non-PCV7–serotype isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | TS-R | TS-I | TS-NS | N | TS-R | TS-I | TS-NS | N | TS-R | TS-I | TS-NS | |
| 2000 | 172 | 33.7% | 10.5% | 44.2% | 92 | 45.7% | 14.1% | 59.8% | 74 | 18.9% | 5.4% | 24.3% |
| 2001 | 198 | 29.3% | 5.1% | 34.3% | 98 | 37.8% | 9.2% | 46.9% | 90 | 22.2% | 1.1% | 23.3% |
| 2002 | 159 | 22.0% | 8.2% | 30.2% | 50 | 46.0% | 14.0% | 60.0% | 103 | 10.7% | 4.9% | 15.5% |
| 2003 | 184 | 16.2% | 6.1% | 22.3% | 45 | 35.6% | 8.9% | 44.4% | 128 | 10.2% | 4.7% | 14.8% |
| 2004 | 184 | 12.0% | 4.4% | 16.4% | 18 | 44.4% | 0.0% | 44.4% | 160 | 8.8% | 4.4% | 13.2% |
| 2008 | 118 | 12.7% | 8.5% | 21.2% | 103 | 13.6% | 6.8% | 20.4% | ||||
| 2009 | 146 | 17.4% | 8.3% | 25.7% | 139 | 15.8% | 7.9% | 23.7% | ||||
| 2010 | 183 | 12.7% | 6.6% | 19.3% | 171 | 12.3% | 6.4% | 18.7% | ||||
| p-value for trend | <0.01 | 0.95 | <0.01 | 0.60 | 0.17 | 0.17 | 0.53 | 0.06 | 0.57 | |||
Abbreviations: N, number of pneumococcal isolates; PCV7, 7-valent pneumococcal conjugate vaccine; TS-I, intermediate trimethoprim-sulfamethoxazole resistance (MIC ≥ 1 – 2 mcg/mL); TS-NS, full/intermediate trimethoprim-sulfamethoxazole resistance (MIC ≥ 1 mcg/mL); TS-R, full trimethoprim-sulfamethoxazole resistance (MIC ≥ 4mcg/mL)
Nontypeable pneumococcal isolates not included in table.
During 2008–2010, there were insufficient PCV7-serotype isolates for analysis (N = 7).
A multivariate logistic regression model for the trend in TMP-SMX resistance among all isolates adjusted for age, sex, day-care attendance, household crowding, and TMP-SMX use confirmed the trend towards a decrease in the proportion of isolates that were TS-R (p-value for trend < 0.01) or TS-NS (p-value for trend <0.01), and no change in isolates that were TS-I (p-value for trend = 0.78). Adding pneumococcal serotype (PCV7-serotype versus non-PCV7–serotype) to that multivariate model eliminated any trends in resistance among all isolates during our study period (p-value for trend for proportion of isolates TS-R, TS-I, TS-NS was 0.57, 0.22, and 0.33, respectively).
Serotype 19A emerged as the most prevalent non-PCV7 pneumococcal serotype after the introduction of PCV.23 The proportion of pneumococcal isolates that were 19A increased from 6% during 2000–2004 to 15% during 2008–2010 (p-value for trend = <0.01). During that same time period, the proportion of serotype 19A isolates that were PCN-R increased from 11% to 38% (p-value for trend = p<0.01), PCN-I decreased from 85% to 53% (p-value for trend <0.01), TS-R increased from 27% to 60% (p-value for trend = <0.01), and TS-I decreased from 13% to 3% (p-value for trend = 0.05).
We examined the prevalence and risk of pneumococcal colonization stratified by time between date of first PCN use and study enrollment (Figure 2 and Table 4). We detected a time-dependent relationship between time since prior PCN use and colonization. Children with recent PCN use were more likely to be noncolonized by pneumococci, were less likely to have PCN-S pneumococcal colonization, and had a similar risk for PCN-NS pneumococcal colonization than children without PCN use. By comparison, results suggest no association between PCN use 61–90 days prior to study enrollment and risk for pneumococcal noncolonization, PCN-S colonization, or PCN-NS colonization. PRs adjusted by age, sex, daycare attendance, and PCV7 status differed from unadjusted PRs in all comparisons by < 10% (Table 4).
FIGURE 2.
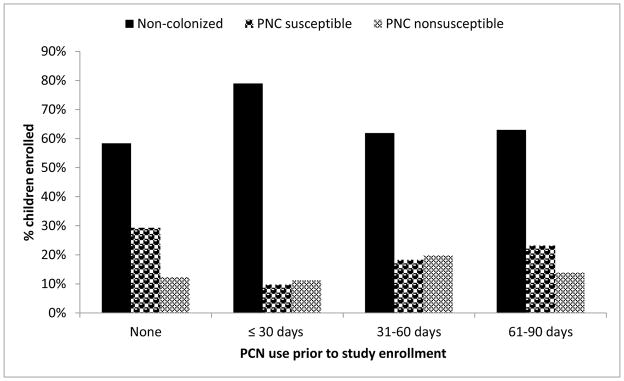
Prevalence of nasopharyngeal pneumococcal colonization among urban children by time from penicillin use to study enrollment — Alaska, 2000–2010 (n=3,496)
Abbreviations: PCN, penicillin
TABLE 4.
Prevalence ratio (PR) for nasopharyngeal pneumococcal colonization among urban children by time from penicillin use to study enrollment — Alaska, 2000–2010 (n=3,496)*
| Children using penicillin | Non-Colonized PR (95% CI) |
PCN-S Colonized PR (95% CI) |
PCN-NS Colonized PR (95% CI) |
|---|---|---|---|
| None ≤90 prior to enrollment | Reference | Reference | Reference |
| ≤ 30 days prior to enrollment | 1.37 (1.29–1.44) | 0.32 (0.24–0.43) | 0.84 (0.64–1.11) |
| 31–60 days prior to enrollment | 1.07 (0.97–1.18) | 0.62 (0.47–0.80) | 1.50 (1.16–1.95) |
| 61–90 days prior to enrollment | 1.11 (1.00–1.24) | 0.79 (0.61–1.02) | 1.03 (0.73–1.45) |
Abbreviations: PCN-NS, full, plus intermediate penicillin resistance (MIC > 0.064 mcg/mL); PCN-S, penicillin susceptible (MIC ≤ 0.064 mcg/mL)
PR adjusted for age, sex, day-care attendance, and 7-valent pneumococcal vaccination status
CONCLUSIONS
Our study examined together the selective pressures of PCN/TMP-SMX use and PCV7 vaccination in children on population-level antibiotic resistance among colonizing pneumococci during the PCV7-era from 2000–2010. Widespread PCV7 use caused a shift from PCV7 serotypes to non-PCV7 serotypes and was the dominant influence on the epidemiology of pneumococcal antibiotic resistance in our study population. Although PCN/TMP-SMX use likely exerted selection pressure, its contribution to the development of pneumococcal resistance to those antibiotics is less directly apparent in our epidemiologic data.
The results indicate that introduction of PCV7 into the Alaska childhood immunization schedule had a substantial impact on the epidemiology of pneumococcal antibiotic resistance. In our study, the proportion of children who were age-appropriately vaccinated with PCV7 is comparable to PCV7 coverage nationally. According to the National Immunization Survey, 73% of children in Alaska and 80% of children nationally aged 19–35 months had received 4 doses of PCV7 in 2009.24 High coverage with PCV7 led to the near eradication of predominantly PCN-R PCV7-serotype pneumococci in our population by 2010, and the emergence of predominantly PCN-I non-PCV7 serotype pneumococci. The proportion of PCV7-serotype and non-PCV7 serotype pneumococcal isolates that were PCN-R or PCN-I did not change during the study period. Consequently, the proportion of all pneumococcal carriage isolates that were PCN-R declined and the PCN-I pneumococcal proportion increased such that the overall proportion of PCN-NS isolates remained stable. The trends in proportion of all pneumococcal isolates that were PCN-R or PCN-I persisted after adjusting for confounding factors (age, sex, day-care attendance, household crowding, and PCN use) in a multivariate logistic regression model. However, those trends became nonsignificant after also adjusting for pneumococcal serotype (PCV7-serotype versus non-PCV7–serotype), indicating that changes in the prevalence of PCN-resistant pneumococci can be accounted for by shifts in the distribution of pneumococcal serotypes.
During our study period, the proportion of all pneumococcal isolates that were TS-NS or TS-R decreased because of an increase in non-PCV7-serotype pneumococci, which were less likely to be TS-R than PCV7-serotype pneumococci that decreased in prevalence. Even though non-PCV7-serotype pneumococci were also less likely to be TS-I than PCV7–serotype pneumococci, the emergence of non-PCV7-serotype pneumococci was not associated with a corresponding decrease in the proportion of TS-I pneumococci among all isolates. That observation might be partly explained by a small increase over time in the proportion of non-PCV7–serotype pneumococci that were TS-I. Additionally, the magnitude of the difference between PCV7 and non-PCV7-serotype pneumococci in the proportion of isolates that were TS-R was greater than the difference in the proportion of isolates that were TS-I. Thus, the emergence of non-PCV7-serotype pneumococci had less impact on the trend in TS-I prevalence than the TS-R prevalence among all isolates. Similar to what we observed with pneumococcal PCN susceptibility, the multivariable logistic regression model adjusting for age, sex, day-care attendance, household crowding, and TMP-SMX use with and without adjustment for pneumococcal serotype confirmed that the changes in the proportion of all pneumococcal isolates that were TS-R or TS-NS over our study period were driven primarily by shifts in pneumococcal serotypes after widespread PCV7 use.
We were unable to demonstrate that penicillin use was associated with an increased prevalence of resistant pneumococci in our study population. Our analysis of pneumococcal colonization among children stratified by time between PCN use and study enrollment provides insights into the dynamics of antibiotic selection pressure. PCN use <30 days prior to study enrollment was associated with reduced colonization by PCN-S pneumococci, but the prevalence of PCN-NS pneumococci was not elevated. Consequently, the overall prevalence of pneumococcal colonization was lower among children with PCN use than among children with no PCN use. However, the overall prevalence of colonization and the prevalence of PCN-S and PCN-NS colonization were similar in children with PCN use 60–90 days prior to study enrollment compared to children with no PCN use. This pattern of reduced PCN-S/stable PCN-NS pneumococcal colonization <30 days after PCN use and similar PCN-S/stable PCN-NS pneumococcal colonization 60–90 days after PCN use is similar to a pattern predicted by a mathematical model on the effect of PCN use on colonization.25 Our results are also consistent with other previous pneumococcal colonization studies in children demonstrating that the primary effect of PCN use is transient eradication of susceptible pneumococci.11–14 Furthermore, it is unlikely that PCN use by itself selected for or resulted in enhanced transmission of resistant pneumococci in our population because the absolute prevalence of PCN-NS pneumococcal colonization did not increase among children using PCN compared with children not using PCN. Thus, penicillin does not appear to directly select for resistant pneumococcal carriage in children using it.
We did not present the results of a similar analysis for TMP-SMX among children stratified by time between TMP-SMX use and study enrollment for a couple of reasons. First, the analysis was limited by insufficient statistical power because only 131 children had received TMP-SMX <90 days prior to study enrollment (by comparison, 970 children had received PCN). Additionally, TMP-SMX might not concentrate in the nasopharynx at levels sufficient to eradicate colonizing pneumococci, so it might not be possible to evaluate for selection pressure from TMP-SMX use in nasopharyngeal colonization isolates.26, 27
The mechanisms by which pneumococcal serotypes acquire antibiotic resistance could explain why we were unable to demonstrate selection for resistant pneumococci associated with antibiotic use in our epidemiologic data. Pneumococcal resistance to PCN is mediated by alterations in penicillin binding proteins, to trimethoprim by mutations to dihydrofolate reductase, and to sulfonamides by mutations in dihydropteroate synthase; these alterations are hypothesized to occur by homologous recombination with other streptococcal species rather than by sequential point mutations.28–30 In addition, a pneumococcal serotype can acquire resistance to antibiotics through mutations at the cps locus. The cps gene codes for the capsular polysaccharides that define pneumococcal serotypes. Thus, capsular switching could allow a highly antibiotic-resistant PCV7-serotype pneumococci to switch to a non-PCV7–serotype and persist in the population.31 Genetic recombination events that lead to the acquisition of resistance traits or to capsular switching occur independent of antibiotic use by individuals and could explain why antibiotic use was not directly associated with increased colonization by resistant pneumococci among children in our study.32
A previous study describing the epidemiology of invasive serotype 19A pneumococcal isolates in Alaska suggests a potential indirect role for antibiotic use in selecting for resistance.33 Of the 12 sequence types (ST) identified by multilocus sequence typing among invasive pneumococcal isolates in Alaska from 2001–2010, ST199 and related STs (i.e., clonal complex CC199) accounted for 63% of isolates during 2001–2005 and 55% during 2006–2010. However, ST320 was not present in Alaska prior to 2006 but CC320 accounted for 13% of pneumococcal isolates during 2006–2010. ST320 was previously associated with the globally distributed, highly antibiotic-resistant PCV7-related serotypes 19F and 23F. ST320 likely became associated with serotype 19A because of capsular switching by serotype 19F or 23F. The abrupt appearance of ST320 in 2006 indicates that this ST was likely introduced into Alaska from outside the state. Although antibiotic use did not play a role in the introduction of CC320 into Alaska, selection pressure from a stable prevalence of antibiotic use could have facilitated the successful expansion of this antibiotic resistant clone after introduction. A higher proportion of CC320 pneumococcal isolates tend to be PCN-NS and TS-NS compared with CC199 isolates; the expansion of CC320 in Alaska helps explain the increased prevalence of antibiotic nonsusceptibility among our serotype 19A pneumococcal colonization isolates.
This study has limitations. First, we conducted a cross-sectional survey that enrolled unique children for the study each year. Therefore, despite our efforts to control for unmeasured confounders (e.g., conducting the study during the same calendar period and in the same clinics annually), it is still possible that the associations we observed resulted from factors that we were unable to measure/account for analytically. Additionally, we enrolled a convenience sample of children so our results might not be generalizable to other settings. It is notable, however, that our colonization prevalence is comparable with that reported from a colonization study conducted using similar methods in another urban setting.17 Further, we have no reason to suspect that the antibiotic prescribing practices in our study sites differ substantially from providers at clinics in similar urban settings (e.g., differences in antibiotic prescribing guidelines).
Our study also has strengths. First, we were able to establish the temporal association between antibiotic use/PCV7 vaccination and subsequent colonization by determining the dates of those events prior to study enrollment, thus enabling us to overcome a key limitation of the cross-sectional study design where the timing of the exposure in relation to the outcome might not be known. Additionally, the long-term, prospective nature of our study allows us to evaluate the effect of PCV7 from implementation to maximal impact.
The results of our study demonstrate how global trends, such as vaccination induced changes in colonizing pneumococcal serotypes, can interact with antibiotic use to shape the prevalence of antibiotic-resistant pneumococci in a population. Elucidating other intermediate pathways by which antibiotic use leads to the increased prevalence of resistant pneumococci can help identify more effective interventions to reduce resistance. Molecular laboratory techniques, such as whole genome sequencing of pneumococci and phylogenetic analysis, can offer deeper insights into pneumococcal transmission dynamics.34 Additional research is needed to determine whether our results are unique to colonizing pneumococci or whether similar results are observed among other bacteria colonizing the nasopharynx and among commensal enteric bacteria.
Supplementary Material
Acknowledgments
Funding source: Research supported by an Investigator Initiated Research grant from Wyeth Pharmaceuticals (now Pfizer Vaccines) and in-kind support by the Centers for Disease Control and Prevention
We thank the members of CDC’s Arctic Investigations Program for their efforts to recruit study participants (Henry Baggett, Laura Hammitt, James Keck, Jay Wenger, Helen Peters, Jim Gove, Catherine Dentinger, Lisa Chiou, Ken Petersen, Kim Boyd Hummel, Sassa Kitka, Cindy Hamlin), enter the data into and manage the data in the data base (Richard Baum, Jennie Lee, Tony Kretz, Debbie Parks, Rita Tangiegak), and conduct the laboratory analysis (Carolynn DeByle, Marcella Harker-Jones, Karen Miernyk, Julie Morris, Alisa Reasonover, Maria Warnke, Carolyn Zanis).
Footnotes
Conflict of interest: None declared by any of the authors.
Note: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, Sluijter M, Toom NL, et al. Dynamics of pneumococcal colonization in healthy Dutch children. Microbiology. 2006;152:377–385. doi: 10.1099/mic.0.28394-0. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg K, Freijd A, Rynnel-Dagoo B, Hammarstrom L. Anti pneumococcal antibody activity in nasopharyngeal secretions in healthy adults and children. Acta oto-laryngologica. 1993;113:673–678. doi: 10.3109/00016489309135883. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert D, Engelen MN, Timmers-Reker AJ, et al. Pneumococcal carriage in children in The Netherlands: a molecular epidemiological study. J Clin Microbiol. 2001;39:3316–3320. doi: 10.1128/JCM.39.9.3316-3320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain M, Melegaro A, Pebody RG, et al. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiology and infection. 2005;133:891–898. doi: 10.1017/S0950268805004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 7.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP Active Bacterial Core Surveillance T. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631–639. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 8.McGee L, McDougal L, Zhou J, et al. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J Clin Microbiol. 2001;39:2565–2571. doi: 10.1128/JCM.39.7.2565-2571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudolph KM, Parkinson AJ, Reasonover AL, Bulkow LR, Parks DJ, Butler JC. Serotype distribution and antimicrobial resistance patterns of invasive isolates of Streptococcus pneumoniae: Alaska, 1991–1998. J Infect Dis. 2000;182:490–496. doi: 10.1086/315716. [DOI] [PubMed] [Google Scholar]
- 10.Spratt BG, Greenwood BM. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet. 2000;356:1210–1211. doi: 10.1016/S0140-6736(00)02779-3. [DOI] [PubMed] [Google Scholar]
- 11.Arason VA, Kristinsson KG, Sigurdsson JA, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ. 1996;313:387–391. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrag SJ, Pena C, Fernandez J, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001;286:49–56. doi: 10.1001/jama.286.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Ghaffar F, Muniz LS, Katz K, et al. Effects of large dosages of amoxicillin/clavulanate or azithromycin on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, nonpneumococcal alpha-hemolytic streptococci, and Staphylococcus aureus in children with acute otitis media. Clin Infect Dis. 2002;34:1301–1309. doi: 10.1086/340054. [DOI] [PubMed] [Google Scholar]
- 14.Cohen R, Bingen E, Varon E, et al. Change in nasopharyngeal carriage of Streptococcus pneumoniae resulting from antibiotic therapy for acute otitis media in children. Pediatr Infect Dis J. 1997;16:555–560. doi: 10.1097/00006454-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hare KM, Singleton RJ, Grimwood K, et al. Longitudinal nasopharyngeal carriage and antibiotic resistance of respiratory bacteria in indigenous Australian and Alaska native children with bronchiectasis. PLoS One. 2013;8:e70478. doi: 10.1371/journal.pone.0070478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennessy TW, Singleton RJ, Bulkow LR, et al. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine. 2005;23:5464–5473. doi: 10.1016/j.vaccine.2005.08.100. [DOI] [PubMed] [Google Scholar]
- 17.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–35. [PubMed] [Google Scholar]
- 19.O’Brien KL, Bronsdon MA, Dagan R, et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J Clin Microbiol. 2001;39:1021–1024. doi: 10.1128/JCM.39.3.1021-1024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennessy TW, Petersen KM, Bruden D, et al. Changes in antibiotic-prescribing practices and carriage of penicillin-resistant Streptococcus pneumoniae: A controlled intervention trial in rural Alaska. Clin Infect Dis. 2002;34:1543–1550. doi: 10.1086/340534. [DOI] [PubMed] [Google Scholar]
- 21.Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9. Washington, DC: American Society for Microbiology; 2007. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. Seventeenth informational supplement M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 23.Gounder PP, Bruce MG, Bruden DJ, et al. Effect of the 13-Valent Pneumococcal Conjugate Vaccine on Nasopharyngeal Colonization by Streptococcus pneumoniae--Alaska, 2008–2012. J Infect Dis. 2014;209:1251–1258. doi: 10.1093/infdis/jit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National state, and local area vaccination coverage among children aged 19–35 months --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1171–1177. [PubMed] [Google Scholar]
- 25.Lipsitch M. Measuring and interpreting associations between antibiotic use and penicillin resistance in Streptococcus pneumoniae. Clin Infect Dis. 2001;32:1044–1054. doi: 10.1086/319604. [DOI] [PubMed] [Google Scholar]
- 26.Wong GA, Hoeprich PD, Barry AL, Peirce TH, Rausch DC. Tracheobronchial Flora in Chronic Obstructive Pulmonary Disease: Preliminary Results with Authentic Specimens and Trial of Trimethoprim-Sulfamethoxazole. The Journal of Infectious Diseases. 1973;128:S719–S722. doi: 10.1093/infdis/128.supplement_3.s719. [DOI] [PubMed] [Google Scholar]
- 27.Feikin DR, Dowell SF, Nwanyanwu OC, et al. Increased carriage of trimethoprim/sulfamethoxazole-resistant Streptococcus pneumoniae in Malawian children after treatment for malaria with sulfadoxine/pyrimethamine. J Infect Dis. 2000;181:1501–1505. doi: 10.1086/315382. [DOI] [PubMed] [Google Scholar]
- 28.Crook DW, Spratt BG. Multiple antibiotic resistance in Streptococcus pneumoniae. British medical bulletin. 1998;54:595–610. doi: 10.1093/oxfordjournals.bmb.a011713. [DOI] [PubMed] [Google Scholar]
- 29.Adrian PV, Klugman KP. Mutations in the dihydrofolate reductase gene of trimethoprim-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2406–2413. doi: 10.1128/aac.41.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maskell JP, Sefton AM, Hall LM. Mechanism of sulfonamide resistance in clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2121–2126. doi: 10.1128/aac.41.10.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coffey TJ, Enright MC, Daniels M, et al. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Molecular microbiology. 1998;27:73–83. doi: 10.1046/j.1365-2958.1998.00658.x. [DOI] [PubMed] [Google Scholar]
- 32.Wyres KL, Lambertsen LM, Croucher NJ, et al. Pneumococcal Capsular Switching: A Historical Perspective. Journal of Infectious Diseases. 2013;207:439–449. doi: 10.1093/infdis/jis703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph K, Bruce MG, Bulkow L, et al. Molecular epidemiology of serotype 19A Streptococcus pneumoniae among invasive isolates from Alaska, 1986–2010. International journal of circumpolar health. 2013:72. doi: 10.3402/ijch.v72i0.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Croucher NJ, Finkelstein JA, Pelton SI, et al. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet. 2013;45:656–663. doi: 10.1038/ng.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.