Abstract
Myeloproliferative neoplasms (MPN) are chronic marrow disorders with variable prognosis. Most patients with Polycythemia Vera, Essential Thrombocythemia or even Primary Myelofibrosis (PMF) are successfully managed by conservative strategies for years or even decades, and recent data suggest that even in patients with high-risk disease, in particular those with PMF, life expectancy can be extended by treatment with JAK2 inhibitors. However, none of those modalities are curative, and once marrow failure develops, the disease “accelerates” or transforms to acute leukemia, the only treatment option able to effectively treat and, in fact, cure MPN is allogeneic hematopoietic cell transplantation (HCT). Outcome is superior if HCT is performed before leukemic transformation occurs. Several reports document survival in unmaintained remission beyond 10 years. The most recent analyses show reduced regimen-related mortality (less than 10% or even 5% at day 100), and progressively improved survival with both HLA-identical sibling and unrelated donors. The development of low/reduced intensity conditioning regimens has contributed to the improved success rate and has allowed to successfully carry out HCT in patients in the 7th and even 8th decade of life. We propose, therefore, that HCT should be offered to fit patients in these age groups and should be covered by their respective insurance carriers.
Introduction
Primary myelofibrosis (PMF) is a rare myeloproliferative neoplasm (MPN), that occurs primarily in older individuals, with a median age at diagnosis of 67 years [1]. Median life expectancy is 4–5 years. However, the prognosis varies considerably. Some patients show progression to marrow failure or transformation to acute myeloid leukemia (AML) within a year or two, while others have a course that may extend over a decade or more.
Patients who present originally with other MPNs such as Polycythemia Vera (PV) or Essential Thrombocythemia (ET), tend to be younger at diagnosis and typically show a more protracted course, with median life expectancies approaching two decades. However, a proportion of those patients will also experience an acceleration of their disease with marrow fibrosis and marrow failure or transformation to acute myeloid leukemia (AML).
Some patients are managed effectively with conservative strategies (e.g. hydroxyurea or interferon) for years, but management of more advanced disease is challenging. The prognosis is especially poor with transformation to AML, which is associated with a median survival (after transformation) of a few months. One-year overall survival, regardless of treatment intervention, is less than 15 % [2,3].
The use of recently introduced compounds that interfere with Janus kinase (JAK) function, so called JAK inhibitors, has resulted in profound symptomatic improvement and reduction in spleen size in patients with myelofibrosis and may modify the natural course of the disease. Jakafi® (ruxolitinib), an oral JAK1/JAK2 inhibitor, has been approved by the FDA for the treatment of patients with intermediate or high-risk myelofibrosis, including PMF, post-PV and post-ET MF, and has been shown to improve patients’ quality of life [4,5], although it may be associated with the development of anemia. In two phase III studies of patients with Dynamic International Prognostic Scoring System (DIPSS; see Table 1) intermediate-2 or high-risk myelofibrosis, ruxolitinib was associated with improvement in survival compared to placebo treated patients (COMFORT-I study) or patients who received “best available therapy” (COMFORT-II study) [4,6]. The COMFORT-II study showed a modest survival advantage for patients who had been treated for more than 3 years [6]. However, while recent data suggest that molecular remissions can be achieved with ruxolitinib [7], this compound is not curative and may not affect the risk for transformation to AML. At 3 years 60% of patients are off treatment because of toxicity or a lack of or loss of response. Therefore, while it is appropriate to offer symptomatic patients and those with morbid splenomegaly a trial of ruxolitinib, physicians and patients must remain alert to the possibility of disease progression (e.g., cytogenetic/clonal evolution, transformation to AML) while on such therapy. Particularly patients who are considered candidates for HCT must be monitored carefully in order not to miss the opportunity of receiving a transplant before prominent disease progression.
Table 1.
Parameters in current prognostic scoring systems for primary myelofibrosis
| DIPSS[21] | MIPSS [32] | |
|---|---|---|
| Age | ≥ 65 (1 Point) vs. <65 | > 60 (0.5 Points) |
| Leukocyte Count (× 109/L) | > 25 (1 point) vs. ≤ 25 | N/A |
| Hemoglobin (g/dL) | <10 (2 points) vs. ≥ 10 | <10 (0.5 points) |
| Constitutional Symptoms | Present (1 point) | Present (0.5 points) |
| Circulating Blasts | ≥ 1% (1 point) vs. absent | N/A |
| Platelets (× 10(9)/L) | N/A | <200 (1 point) |
| Triple Negative# | N/A | Present (1.5 points) |
| JAK2 V617F or MPL Mutatation | N/A | Present (0.5 points) |
| ASXL1 Mutation | N/A | Present (0.5 points) |
| SRSF2 Mutation | N/A | Present (0.5 points) |
Triple Negative = Wild type for JAK2 (V617F), MPL, and CALR mutations
N/A= not applicable.
Transplantation
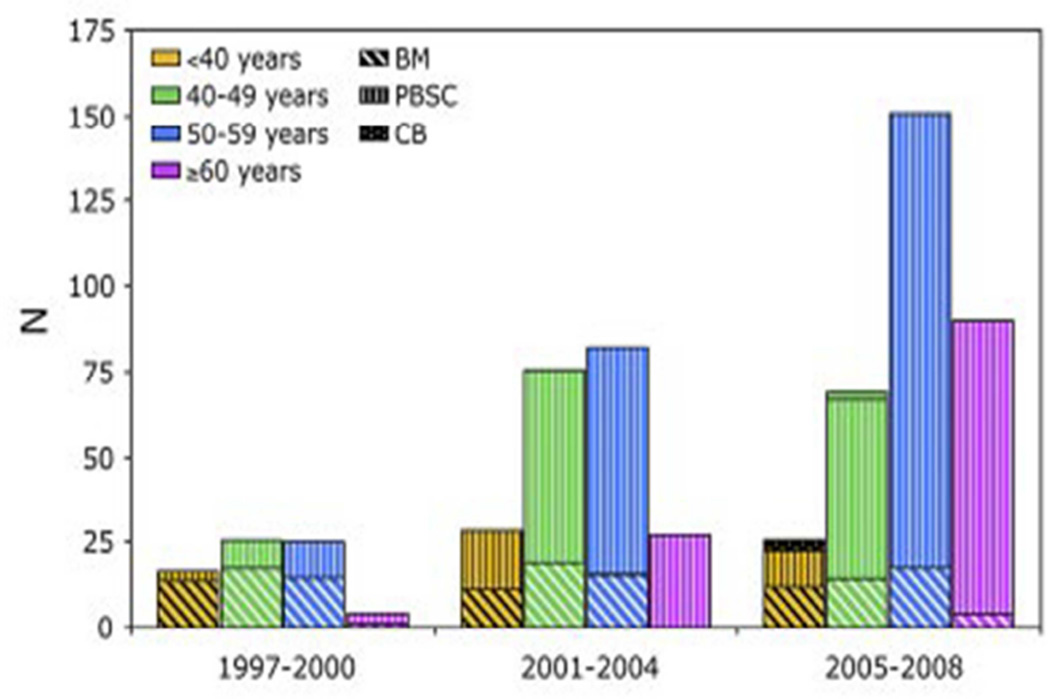
At present, allogeneic hematopoietic cell transplantation (HCT) is the only modality with proven curative potential, i.e HCT currently represents the only definitive therapy for MPN. Because of this, the number of patients undergoing HCT for MPN has increased progressively (Figure 1).
Figure 1. Increase in transplants for myelofibrosis since 1997 by patient age and source of stem cells.#.
Data provided by CIBMTR (Center for International Bone Marrow Transplant Research) BM= bone marrow; PBSC= mobilized peripheral blood stem cells; CB= cord blood cells.
Transplantation for hematopoietic failure
Numerous clinical trials have shown the efficacy of allogeneic HCT (Table 2). In a prospective multicenter phase II trial involving 66 patients, with a median follow-up of 2 years, Rondelli et al observed a survival of 75% after transplantation from HLA-identical siblings; results were inferior, with survival of 32%, in patients transplanted from unrelated donors [8]. In another prospective phase II multicenter trial that enrolled 103 patients, 5-year overall survival was 67% [9].
Table 2.
Selected reports of HCT outcomes in patients with myelofibrosis [33]
| Reference | Timeline of HCT |
N | Median age (range), years |
Conditioning regimen |
% of patients with RIC |
NRM | PFS | OS | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Rondelli [34] | NR | 21 | 54 (27–68) | Multiple | 100 | 10% at 1 y | 81% at 2.7 y | 85% at 2.7 y | Extensive cGVHD in 44%; 2 patients needed DLI for 100% donor chimerism; resolution of fibrosis and splenomegaly in majority |
| Kerbauy [35] | NR | 104 | 49 (18–70) | Multiple, Bu/Cy (62%) | 9 | 35% at 5 y | NR | 61% at 5 y | 3 syngeneic donors, 54 of the patients overlapped with a prior report10; targeted Bu improved OS; comorbidity score had impact on survival |
| Patriarca [36] | 1986–2006 | 100 | 49 (21–68) | Multiple, Bu/Cy 50% of full intensity; Thiotepa + Cy in 46% of RIC | 52 | 43% at 3 y | 35% at 3 y | 42% at 3 y | AHCT before 1995; unrelated donor and longer interval from diagnosis predicted worse outcome but not conditioning intensity; relapse at 2 y 41%, progressive decline in NRM over 20 y studied |
| Kroger [9] | 2002–2007 | 103 | 55 (32–68) | Flu-Bu (100%) | 100 | 16% at 1 y | 51% at 5 y | 67% at 5 y | First prospective study in MF, cGVHD in 43%; 12% NRM for fully matched donor AHCT; age > 55 y and HLA mismatch adversely affected OS; JAK2-positive recipients had better EFS and OS; splenectomy increased risk of relapse |
| Ballen [10] | 1989–2002 | 289 | 47 (18–73) | Multiple, Bu/Cy (43%) | 21 | 35% siblings 50% for URD at 5 y | 33% siblings 27% for URD at 5 y | 37% siblings 30% for URD at 5 y | Relapse at 5 y, 32% in sibling and 23% in URD; performance status, peripheral blasts sibling donor status impacted OS; RIC was similar in outcomes, except early NRM |
| Alchalby [31] | 1999–2009 | 162 | 56 (32–73) | Flu-Bu in 96% | 100 | 22% at 1 y | 46% at 5 y | 62% at 5 y | 82 patients reported previously54; age and HLA mismatch impacted NRM; 23% relapse at 3 y; clearance of mutated JAK2 at median of 96 days, and this reduced relapse risk |
| Bacigalupo [37] | 1994–2007 | 46 | 51 (24–67) | Thiotepa-Cy + melphalan | 100 | 24% at 5 y | NR | 45% at 5 y | A risk score based on transfusion history, spleen > 22 cm and alternative donor use predicted lower OS; no benefit for splenectomy |
| Robin [38] | 1997–2008 | 147 | 53 (20–68) | Multiple | 69 | 39% at 4 y | 32% at 4 y | 39% at 4 y | 19% patients had LT; poor outcome with mismatched donor |
| Samuelson [20] | 1999–2007 | 30 | 65 (60–78) | Multiple | 63 | 13% at day 100 | 40% at 3 y | 45% at 3 y | Studied outcomes in patients ≥ 60 y, 7 patients had preceding LT |
| Ditschkowski [13] | 1994–2010 | 76 | 50.5 (22–67) | Multiple | NR | 36% at 5 y | 50% at 5 y | 53% at 5 y | Significant high risk of relapse in patients without cGvHD; DIPSS was predictive of survival |
| Scott [16] | 1990–2009 | 170 | 51.5 (12–78) | Multiple | NR | 34% at 5 y | 57% at 5 y | 57% at 5 y | Post-HCT success was dependent on pre-HCT DIPSS scores |
MRD=HLA matched related donor; PFS = progression-free survival; OS = overall survival; TBI=, total body irradiation; Hb = hemoglobin; Bu= busulfan; Cy = cyclophosphamide; cGvHD= chronic GVHD; NR= not reported; DLI= donor lymphocyte infusion; AHCT= allogeneic HCT; PFS= event-free survival; NRM= non-relapse mortality; OS= overall survival; KPS= Karnofsky performance status; ATG= antithymocyte globulin.
In a retrospective registry analysis of results in 289 patients ranging in age from 18–73 years, long-term survival was observed in about one third of patients; however, the probability of survival varied dependent upon the transplant conditioning regimen and, similar to the observations of Rondelli et al., the source of stem cells used for the transplant [10]. Another retrospective registry analysis of data on 233 patients showed similar results, with 5-year survival ranging from 56% to 34%, dependent on donor type and stem cell source [11]. In that cohort, 27% of patients were older than 60 years, and after adjusting for other risk factors, multivariate analyses failed to show an association of age at HCT with overall survival or progression-free survival. The relative risk of overall survival for patients >60 years, compared to patients 41–60 years of age, was 0.77 (95% CI, 0.52–1.12; P=0.171), and the relative risk for progression-free survival was 1.02 (95% CI, 0.72–1.45; P=0.904). Additional similar results were reported by Lussana et al. [12] in a retrospective cohort of 250 patients, 22–75 (median 56) years of age, with post-PV or post-ET MF, showing a 3-year overall survival of 55%. Survival was affected negatively by older age, as also noted in other studies [13], by transformation of the disease to AML [14,15], and by the use of unrelated rather than sibling donors. However, these transplants were carried out as far back as 1994, and analysis of results in more recently transplanted patients does not show a significantly inferior outcome with HLA-matched unrelated donors [16,17].
Of course, HCT in older patients is subject to selection bias, by focusing on fit patients without comorbidities [18]. However, this limitation applies to all diagnoses (and all age groups) and needs to be considered in any discussion regarding suitability for HCT [19]. In a report of results in 30 patients, 60–78 years of age, Samuelson et al. [20] showed an overall survival of 45% and progression-free survival of 40% at 3 years. No significant differences in survival were noted between patients 60–65 years of age and those 66–78 years of age. Transplant-associated mortality was 13% at day 100 after HCT.
Scott et al. presented yet another retrospective analysis of HCT results in 170 patients, 12–78 years of age, with PMF, post-PV, or post-ET MF. These authors used the DIPSS [21] to prognosticate transplant outcome in dependence of the DIPSS score [16]. DIPSS considers patient age, the presence of symptoms (e.g. night sweats), anemia (Hgb<10g/L), leukocytosis (WBC ≥25 × 109 /L), and circulating myeloblasts (≥1%) (Table 1). Overall survival and progression-free survival after HCT were closely correlated with pre-HCT DIPSS classification (Table 3). The probability of survival was approximately 80% in patients in the lowest DIPSS risk group (score 0) and 40% in the highest risk group (score >4). The incidence of disease relapse was similar for all DIPSS categories; however, non-relapse mortality increased progressively with higher DIPSS risk, presumably related to disease manifestations, in particular fibrosis, in non-hematopoietic organs such as liver and lungs, providing another argument in favor of earlier HCT. In this particular cohort some patients have been followed now for two decades after HCT, and remain in remission.
Table 3.
Median survival by DIPSS, without and with transplantation $
| Median Survival (years) | ||
|---|---|---|
| DIPSS Risk* | No Transplant (at reporting) |
Transplant (median follow-up 5.9) |
| Low | Not reached | Not reached |
| Intermediate 1 | 14.2 | Not reached |
| Intermediate 2 | 4 | 7 |
| High | 1.5 | 2.5 |
Disease progression and timing of HCT
Based on data reported to date it is challenging to decide upon the ideal time for HCT. Therefore, a decision analysis was conducted, restricted to patients with PMF, based on data on 190 patients transplanted at European centers or the Fred Hutchinson Cancer Research Center in Seattle, and 248 patients, treated at European centers with conventional non-HCT modalities [22]. Results in patients followed for as long as 30 years showed a clear advantage of HCT for patients in DIPSS categories intermediate 2 (scores 3–4) and high (scores >4). The data also indicate that patients in DIPSS risk group “low” have superior life expectancy with conventional management. DIPSS group intermediate 1 comprises patients in whom recommendations should be made on an individual basis. Of course, as MPNs progress, extramedullary manifestations tend to become more prominent, and the patient may acquire new comorbidities, underscoring the need for close monitoring and sequential re-assessment of patient and disease risk parameters in order not to miss the optimum time for HCT. Patients who are “triple negative (non-mutated JAK2, MPL1 and CALR)” [23,24] and patients with ASXL1 mutations in the presence of wild type CALR [25] have a more aggressive disease course [26]. These observations are reflected in the modified DIPSS (MIPSS; Table 1) Those patients should probably be considered for early HCT.
As stated above, patients whose disease has accelerated (increase in blood or marrow myeloblast count to >10%, decline in platelet count to <50 × 109/L, abnormalities of chromosome 17) or progressed to frank AML have a median life expectancy of less than a year [27]. While HCT is the only promising treatment strategy, outcome tends to be inferior to that in patients with less advanced disease because of a higher risk of relapse as well as non-relapse mortality [16]. Nevertheless, Ciurea et al. have reported a long-term success rate of 40% in a small cohort of patients who received “debulking” treatment followed by HCT [28]. Similar results were reported by Kennedy et al. who showed a 2-year overall survival of 47% in patients responding to chemotherapy and undergoing HCT [3]. Alchalby et al. [29] reported a 1-year treatment–related mortality of 28% and a 3-year progression-free survival of 26% among 46 patients whose MPN had evolved to AML. The major cause of failure was disease relapse, which occurred in 47% of patients.
Based on a comprehensive evidence review, an international group of experts (European LeukemiaNet) recommends allogeneic HCT for patients with MPN with a projected median life expectancy of less than 5 years, which, therefore, includes patients in DIPSS categories intermediate-2 and high, as well as patients with accelerated disease and transformation to AML [30].
Patient age and transplantation
In patients with myelofibrosis up to 60 or 65 years of age, allogeneic HCT is considered standard therapy, both for those with PMF and those with post-PV and post-ET MF [30]. Published data on the use of allogeneic HCT for older patients with MPN, specifically for patients who are typically covered by Medicare, are limited. The analysis by Samuelson, presented above, shows a probability of long-term survival in the range of 30% [20]. Alchalby et al. suggested a model to predict survival after reduced-intensity conditioning (RIC) transplants in patients with myelofibrosis in which constitutional symptoms, non-mutated JAK2 and age >57 years predicted inferior outcome [31].
However, transplant outcomes have been improving. Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) on 254 patients, age ≥60 years, who received allogeneic HCT for myelofibrosis from 2008 to 2013, show a day-100 survival of 83%, and a 1-year survival rate of 61%. A report from the FHCRC on 22 patients up to 65 years of age, transplanted for MPN, showed a day-100 survival of 100% [17]. The oldest long-term survivor in the report by Scott et al. was aged 78 years at the time of HCT [16].
Summary and Conclusions
While ideally one would like to see results from a prospective trial comparing HCT with non-transplant therapy for patients with MPN, considering the rarity of these disorders and limited reources, it is unrealistic to expect that such a trial will be conducted. Thus, the present data, while not exhaustive, represent the best available evidence in support of HCT for patients with MPNs, including patients in the 7th or even 8th decade of life. Survival probabilities, certainly for patients in DIPSS risk groups intermediate 2 and high, are significantly higher after HCT than observed with conservative therapy. Comorbidities, in addition to disease classification (by DIPSS), significantly impact post-HCT outcome. As the probability of comorbidities increases with age, the selection of older individuals for HCT is profoundly affected by the presence of comorbidities. This is true for patients with MPN as it is for patients with other diagnoses. However, selection of the appropriate fit patients for HCT has led to rewarding results, and HCT for MPN should not be withheld solely on the basis of age.
Currently, “myelofibrosis” is not one of the indications listed in NCD 110.8.1 and, therefore, is not covered by Medicare insurance. This represents a major access barrier to effective treatment for patients with these diseases. It appears from available data, however, that it is time to reassess this scenario and, in view of progressively improving results with HCT, provide insurance coverage for appropriately selected older patients with MPN.
Acknowledgments
We thank Helen Crawford and Bonnie Larson for help with manuscript preparation.
Grant support: N/A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deadmond MA, Smith-Gagen JA. Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973–2011. J Cancer Res Clin Oncol. 2015 doi: 10.1007/s00432-015-1983-5. [Epub ahead of print 2015 May 13]-Reference ID:47907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005;105:973–977. doi: 10.1182/blood-2004-07-2864. Reference ID: 39864. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy JA, Atenafu EG, Messner HA, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013;121:2725–2733. doi: 10.1182/blood-2012-10-464248. Reference ID: 47906. [DOI] [PubMed] [Google Scholar]
- 4.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. Reference ID: 42225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison C, Kiladjian JJ, Al Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. Reference ID: 42226. [DOI] [PubMed] [Google Scholar]
- 6.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. doi: 10.1182/blood-2013-02-485888. Reference ID: 47682. [DOI] [PubMed] [Google Scholar]
- 7.Pieri L, Pancrazzi A, Pacilli A, et al. JAK2V617F complete molecular remission in polycythemia vera/essential thrombocythemia patients treated with ruxolitinib. Blood. 2015;125:3352–3353. doi: 10.1182/blood-2015-01-624536. Reference ID: 47905. [DOI] [PubMed] [Google Scholar]
- 8.Rondelli D, Goldberg JD, Isola L, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–1191. doi: 10.1182/blood-2014-04-572545. Reference ID: 47593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kröger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:5264–5270. doi: 10.1182/blood-2009-07-234880. Reference ID: 38606. [DOI] [PubMed] [Google Scholar]
- 10.Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16:358–367. doi: 10.1016/j.bbmt.2009.10.025. Reference ID: 38599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta V, Malone AK, Hari PN, et al. Reduced intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2013;20:89–97. doi: 10.1016/j.bbmt.2013.10.018. Reference ID: 45761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lussana F, Rambaldi A, Finazzi MC, et al. Allogeneic hematopoietic stem cell transplantation in patients with polycythemia vera or essential thrombocythemia transformed to myelofibrosis or acute myeloid leukemia: a report from the MPN Subcommittee of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99:916–921. doi: 10.3324/haematol.2013.094284. Reference ID: 47685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ditschkowski M, Elmaagacli AH, Trenschel R, et al. DIPSS scores, pre-transplant therapy and chronic GVHD determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica. 2012;97:1574–1581. doi: 10.3324/haematol.2011.061168. Reference ID: 42295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciurea SO, de Lima M, Giralt S, et al. Allogeneic stem cell transplantation for myelofibrosis with leukemic transformation. Biol Blood Marrow Transplant. 2010;16:555–559. doi: 10.1016/j.bbmt.2009.12.004. Reference ID: 38633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116:2857–2858. doi: 10.1182/blood-2010-06-293415. Reference ID: 42227. [DOI] [PubMed] [Google Scholar]
- 16.Scott BL, Gooley TA, Sorror ML, et al. The Dynamic International Prognostic Scoring System for myelofibrosis predicts outcomes after hematopoietic cell transplantation. Blood. 2012;119:2657–2664. doi: 10.1182/blood-2011-08-372904. Reference ID: 41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezvani AR, McCune JS, Storer BE, et al. Cyclophosphamide followed by intravenous targeted busulfan for allogeneic hematopoietic cell transplantation: pharmacokinetics and clinical outcomes. Biol Blood Marrow Transplant. 2013;19:1033–1039. doi: 10.1016/j.bbmt.2013.04.005. Reference ID: 44179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection (Review) J Intern Med. 2011;270:550–560. doi: 10.1111/j.1365-2796.2011.02457.x. Reference ID: 43382. [DOI] [PubMed] [Google Scholar]
- 19.Deeg HJ, Sandmaier BM. Who is fit for allogeneic transplantation? Blood. 2010;116:4762–4770. doi: 10.1182/blood-2010-07-259358. Reference ID: 38931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuelson S, Sandmaier BM, Heslop HE, et al. Allogeneic haematopoeietic cell transplantation for myelofibrosis in 30 patients 60–78 years of age. Br J Haematol. 2011;153:76–82. doi: 10.1111/j.1365-2141.2011.08582.x. Reference ID: 39559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. Reference ID: 39860. [DOI] [PubMed] [Google Scholar]
- 22.Kröger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less then 65 years of age with primary myelofibrosis. Blood. 2015;125:3347–3350. doi: 10.1182/blood-2014-10-608315. Reference ID: 47505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panagiota V, Thol F, Markus B, et al. Prognostic effect of calreticulin mutations in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation. Leukemia. 2014;28:1552–1555. doi: 10.1038/leu.2014.66. Reference ID: 47683. [DOI] [PubMed] [Google Scholar]
- 24.Tefferi A, Lasho TL, Finke CM, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014;28:1472–1477. doi: 10.1038/leu.2014.3. Reference ID: 47699. [DOI] [PubMed] [Google Scholar]
- 25.Tefferi A, Guglielmelli P, Lasho TL, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28:1494–1500. doi: 10.1038/leu.2014.57. Reference ID: 47698. [DOI] [PubMed] [Google Scholar]
- 26.Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–2390. doi: 10.1056/NEJMoa1311347. Reference ID: 45994. [DOI] [PubMed] [Google Scholar]
- 27.Tam CS, Kantarjian H, Cortes J, et al. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. J Clin Oncol. 2009;27:5587–5593. doi: 10.1200/JCO.2009.22.8833. Reference ID: 39893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weston A, Perrin LS, Forrester K, et al. Allelic frequency of a p53 polymorphism in human lung cancer. Cancer Epidemiology, Biomarkers & Prevention. 1992;1:481–483. Reference ID: 28633. [PubMed] [Google Scholar]
- 29.Alchalby H, Zabelina T, Stubig T, et al. Allogeneic stem cell transplantation for myelofibrosis with leukemic transformation: a study from the Myeloproliferative Neoplasm Subcommittee of the CMWP of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014;20:279–281. doi: 10.1016/j.bbmt.2013.10.027. Reference ID: 47684. [DOI] [PubMed] [Google Scholar]
- 30.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29:761–770. doi: 10.1200/JCO.2010.31.8436. Reference ID: 47681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alchalby H, Badbaran A, Zabelina T, et al. Impact of JAK2V617F mutation status, allele burden, and clearance after allogeneic stem cell transplantation for myelofibrosis. Blood. 2010;116:3572–3581. doi: 10.1182/blood-2009-12-260588. Reference ID: 39859. [DOI] [PubMed] [Google Scholar]
- 32.Vannucchi AM, Guglielmelli P, Rotunno G, et al. Mutation-Enhanced International Prognostic Scoring System (MIPSS) for primary myelofibrosis: an AGIMM & IWG-MRT project. Blood. 2014;124:405. [abstr.] Reference ID: 47908. [Google Scholar]
- 33.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors (Review) Blood. 2012;120:1367–1379. doi: 10.1182/blood-2012-05-399048. Reference ID: 45819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rondelli D, Barosi G, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate- or high-risk patients with myelofibrosis with myeloid metaplasia. Blood. 2005;105:4115–4119. doi: 10.1182/blood-2004-11-4299. Reference ID: 29591. [DOI] [PubMed] [Google Scholar]
- 35.Kerbauy DMB, Gooley TA, Sale GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant. 2007;13:355–365. doi: 10.1016/j.bbmt.2006.11.004. Reference ID: 30445. [DOI] [PubMed] [Google Scholar]
- 36.Patriarca F, Bacigalupo A, Sperotto A, et al. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Haematologica. 2008;93:1514–1522. doi: 10.3324/haematol.12828. Reference ID: 37638. [DOI] [PubMed] [Google Scholar]
- 37.Bacigalupo A, Soraru M, Dominietto A, et al. Allogeneic hemopoietic SCT for patients with primary myelofibrosis: a predictive transplant score based on transfusion requirement, spleen size and donor type. Bone Marrow Transplant. 2010;45:458–463. doi: 10.1038/bmt.2009.188. Reference ID: 42228. [DOI] [PubMed] [Google Scholar]
- 38.Robin M, Tabrizi R, Mohty M, et al. Allogeneic haematopoietic stem cell transplantation for myelofibrosis: a report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) Br J Haematol. 2011;152:331–339. doi: 10.1111/j.1365-2141.2010.08417.x. Reference ID: 42293. [DOI] [PubMed] [Google Scholar]