Abstract
High-risk transplantation-associated thrombotic microangiopathy (TMA) can present with multisystem involvement and is associated with a poor outcome after hematopoietic stem cell transplantation (HSCT), with < 20% 1-year survival. TMA may involve the intestinal vasculature and can present with bleeding and ischemic colitis. There are no established pathologic criteria for the diagnosis of intestinal TMA (iTMA). The goal of our study was to identify histologic features of iTMA and describe associated clinical features. We evaluated endoscopic samples from 50 consecutive HSCT patients for 8 histopathologic signs of iTMA and compared findings in 3 clinical groups based on the presence or absence of systemic high-risk TMA (hrTMA) and the presence or absence of clinically staged intestinal graft-versus-host disease (iGVHD): TMA/iGVHD, no TMA/iGVHD, and no TMA/no iGVHD. Thirty percent of the study subjects had a clinical diagnosis of systemic hrTMA. On histology, loss of glands, intraluminal schistocytes, intraluminal fibrin, intraluminal microthrombi, endothelial cell separation, and total denudation of mucosa were significantly more common in the hrTMA group (P < .05). Intravascular thrombi were seen exclusively in patients with hrTMA. Mucosal hemorrhages and endothelial cell swelling were more common in hrTMA patients but this difference did not reach statistical significance. Patients with hrTMA were more likely to experience significant abdominal pain and gastrointestinal bleeding requiring multiple blood transfusions (P < .05). Our study shows that HSCT patients with systemic hrTMA can have significant bowel vascular injury that can be identified using defined histologic criteria. Recognition of these histologic signs in post-transplantation patients with significant gastrointestinal symptoms may guide clinical decisions.
Keywords: Intestinal thrombotic microangiopathy, Thrombotic microangiopathy (TMA), Endothelial injury, Graft-versus-host disease TA-TMA
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT)–associated thrombotic microangiopathy (TMA) is a potentially life-threatening pathologic process that significantly contributes to nonrelapse morbidity and mortality. The majority of reports in the literature describe the effect of TMA on the kidney, with important long-term consequences, including end-stage renal disease [1–5]. However, little is known about how TMA involves other organs, including the gastrointestinal (GI) tract [6]. Our recent prospective study showed that severe life-threatening GI bleeding after HSCT was observed only in patients with systemic high-risk TMA (hrTMA) [7]. Although no other prospective studies are available, previous retrospective studies have found significant relationships between endoscopic evidence of intestinal TMA (iTMA) and extreme abdominal pain, GI bleeding, and diarrhea [8–10]. Histologic signs of iTMA reported in the literature include small blood vessel endothelial swelling and denudation, visible schistocytes within the vessel lumen, noninflammatory crypt degeneration, apoptosis and detachment of epithelial cells, wedge-shaped segmental injury, and/or interstitial edema with hemorrhage or fragmented red blood cells (RBC).
Our study objectives were to examine and delineate histologic features of iTMA in children and young adults using biopsy specimens and to associate the histologic findings with clinical features of disease.
METHODS
Study Subjects
Three hundred thirty-eight allogeneic HSCT recipients underwent transplantation at Cincinnati Children’s Hospital Medical Center between June 2008 and December 2012. Those patients who underwent diagnostic upper and lower GI endoscopies for any GI symptoms during the first year after HSCT were included in this study. The primary indication for endoscopy was to rule out gut graft-versus-host disease (GVHD). Secondary indications included investigation of ongoing infections or persistent GI symptoms other than prolonged diarrhea. This research was performed with institutional review board approval. Demographic and transplantation data collected included age, gender, diagnosis, stem cell source, HSCT conditioning regimen, clinical stage of acute intestinal GVHD (iGVHD), the day after HSCT when systemic hrTMA and iGVHD diagnoses were made, and the day after HSCT when endoscopy was performed. The clinical symptoms for all patients were acquired by rigorous review of the electronic medical record and included the presence or absence of bleeding, nausea or vomiting, diarrhea, the need for 3 or more blood product transfusions in 1 week’s time, documentation of pain requiring any narcotic medications, the presence of hypertension, and coinfections. Clinical symptoms were captured and documented 7 days before and 7 days after the date of GI endoscopy. Patients received transfusion based on clinical indications (eg, significant bleeding) or if their hemoglobin and platelet count were <7 g/dL and <10 × 109/L, respectively.
Definition of hrTMA and iGVHD
Systemic hrTMA was diagnosed using published risk stratification criteria that included evidence of terminal complement activation as measured by an elevated soluble C5b-9 level above normal in the blood and proteinuria > 30 mg/dL, in addition to hematologic TMA markers (elevated lactate dehydrogenase, haptoglobin below normal, schistocytes, RBC and platelet transfusion dependence) at the time of TMA diagnosis [7,11–14]. The clinical diagnosis of acute iGVHD was made using standard Glucksberg GVHD staging [15]. Maximum stage of iGVHD and the presence or absence of systemic hrTMA were reported for all patients who received a diagnostic endoscopy. All study subjects were divided into the following 3 clinical groups: (1) presence of systemic hrTMA and iGVHD (TMA/iGVHD), (2) absence of systemic hrTMA but presence of iGVHD (no TMA/iGVHD), or (3) absence of both systemic hrTMA and iGVHD (no TMA/no iGVHD). This division was performed and based on established clinical criteria to gauge the incidence of iTMA in patient populations with or without systemic hrTMA [7,15].
Pathology Review- Definitions of Histologic Signs of iTMA and GVHD
The reviewing pathologist was blinded to the patients’ clinical history. For patients having multiple endoscopic evaluations after HSCT, routine hematoxylin and eosin–stained slides from the first diagnostic procedure were used for this study. Each available study sample from the esophagus, stomach, small intestine, and/or colon was evaluated for 8 histologic markers previously reported as signs possibly associated with iTMA. These included mucosal hemorrhage, loss of glands, intraluminal schistocytes, intraluminal fibrin, intraluminal microthrombi, endothelial cell swelling, endothelial cell separation, and total denudation of mucosa. Each histologic marker was listed as present or absent.
Mucosal hemorrhage was defined by RBC extravasation from capillaries into the lamina propria (LP). It is important to note that this can be differentiated from procedure-related hemorrhage, which is typically diffuse in distribution, located in the LP close to the surface epithelium, and contains fresh, more intact RBCs. Loss of glands was defined as an unequivocal count of >50% reduction in the number of observed glands; GI biopsies should contain approximately 50 to 60 glands, 20 to 30 glands, and 10 to 20 glands per high-power field (×400) in the gastric fundus, gastric antrum, and colon, respectively. Intraluminal schistocytes were defined by the presence of fragmented and/or degenerated RBCs within the capillaries. The fragmented RBCs had to represent >50% of total RBCs and in at least 50% of the capillaries within the LP. Intraluminal fibrin was defined by the presence of fibrin material in at least 1 capillary in the LP and/or submucosa. Intraluminal microthrombi was defined by the presence of thrombus, with and/or without organization, occluding at least 1 capillary or small arteriole in the LP and/or submucosa. Endothelial cell swelling was defined by nuclear enlargement more than 3 times wider than the normal width of endothelial cells in > 50% of the capillaries in the LP. Endothelial cell separation was defined by endothelial cell detachment from the basement membrane in at least 1 capillary in the LP and/or submucosa. Mucosal denudation was defined by epithelial denudation in at least 1 tissue fragment. Procedural artifacts were excluded. Capillaries from normal controls were available for comparison.
Histologic iGVHD features were identified and described as present or absent based upon the degree of crypt apoptosis, apoptosis with the development of crypt abscess, or crypt necrosis with loss of glands.
Statistical Analysis
Categorical and continuous data are described by frequencies (percent) and median (range), respectively. Fisher’s exact or Wilcoxon rank-sum test were used to determine the significance of categorical and continuous data. Statistical significance was assessed by a P value of < .05. One patient received an HSCT using stem cells from both bone marrow and cord blood. This patient was considered as receiving only bone marrow for the purposes of performing statistical analysis and calculating a P value for stem cell source versus clinical groups.
RESULTS
Study Cohort Characteristics
Fifty consecutive allogeneic transplant recipients undergoing endoscopy met study criteria and were included into analyses. The total number of patients who underwent transplantation during this study period was 338. Patients had biopsies of a median of 1 (range,1 to 4) anatomic site (eg, esophagus, stomach, intestine, or colon), depending upon the procedure (upper and/or lower endoscopy). There were a total of 81 biopsy sites (4 to 18 slides per site) for our cohort of 50 patients.
The demographic and transplantation characteristics for study subjects are summarized in Table 1. The majority of our cohort was male (72%) with a median age of 6.6 years (range, .6 to 28.7 years). Eighty percent of patients received HSCT for nonmalignant disorders, including hematophagocytic lymphohistiocytoses, other primary immune deficiencies, and bone marrow failure syndromes. The main stem cell source was bone marrow (88%) from a fully matched unrelated donor (50%). Forty-four percent and 56% of patients received myeloablative and reduced-intensity conditioning regimens, respectively. The majority (82%) of myeloablative regimens did not include total body radiation. There was no statistically significant difference in transplantation features between the 3 study groups.
Table 1.
Patient Demographics
| Feature | TMA/iGVHD | No TMA/iGVHD | No TMA/No iGVHD | P Value |
|---|---|---|---|---|
| No. patients | 15 | 21 | 14 | |
| Gender | ||||
| Male | 12 (80)% | 16 (76.2%) | 8 (57.1%) | .3827 |
| Female | 3 (20%) | 5 (23.8%) | 6 (42.9%) | |
| Age at HSCT, median (range), yr | 10.3 (.6–19.4) | 5.4 (.7–28.7) | 5.9 (.6–28.3) | .6062 |
| Diagnosis | ||||
| Malignancy | 2 (13.3%) | 7 (33.3%) | 1 (7.1%) | .1746 |
| Primary immune deficiency | 10 (66.7%) | 13 (61.9%) | 9 (64.3%) | |
| Bone marrow failure | 3 (20%) | 1 (4.8%) | 4 (28.6%) | |
| Stem cell source | ||||
| Bone marrow | 13 (86.7%) | 18 (85.7%) | 13 (92.9%) | .6603 |
| Peripheral blood | 1 (6.7%) | 3 (14.3%) | 1 (7.1%) | |
| Cord blood | 1 (6.7%) | 0 | 1* | |
| Donor source | ||||
| Related | 3 (20%) | 3 (14.3%) | 4 (28.6%) | .5478 |
| Unrelated | 12 (80%) | 18 (85.7%) | 10 (71.4%) | |
| HLA match status | ||||
| Fully matched | 10 (66.7%) | 12 (57.1%) | 11 (78.6%) | .484 |
| Mismatched | 5 (33.3%) | 9 (42.9%) | 3 (21.4%) | |
| Conditioning regimen | ||||
| Ablative (see below) | 6 (40%) | 9 (42.9%) | 7 (50%) | .8763 |
| Reduced intensity (fludarabine/melphalan) | 9 (60%) | 12 (57.1%) | 7 (50%) | |
| Ablative regimen | ||||
| Chemo only (busulfan/cyclophoshamide†) | 5 (83.3%) | 7 (77.8%) | 6 (85.7%) | 1.00 |
| TBI with cyclophosphamide | 1 (16.7%) | 2 (22.2%) | 1 (14.3%) | |
| Serotherapy | ||||
| Anti-thymocyte globulin | 6 (40%) | 8 (77.8%) | 7 (50%) | .8705 |
| Alemtuzumab | 9 (60%) | 9 (22.2%) | 7 (50%) | |
| GVHD prophylaxis | ||||
| Calcineurin inhibitor (tacrolimus, cyclosporine) + Steroid (methylprednisolone) | 14 (93.3%) | 14 (66.7%) | 10 (71.4%) | .4369 |
| Calcineurin inhibitor + mycophenolate | 0 | 3 (14.3%) | 0 | |
| Calcineurin inhibitor + methotrexate | 1 (6.7%) | 5 (23.8%) | 4 (28.6%) | |
| Clinical stage 3–4 iGVHD | 12 (80%) | 16 (76.2%) | 0 | n/a |
| Day after HSCT of GI scope, median (range) | +63 (27–323) | +58 (26–254) | +87 (35–341) | n/a |
| Day after HSCT of iGVHD diagnosis, median (range) | +53 (15–322) | +48 (18–251) | n/a | n/a |
| Day after HSCT of TMA diagnosis, median (range) | +39 (13–150) | n/a | n/a | n/a |
TBI indicated total body irradiation; n/a, not available.
One patient received stem cells using both cord blood and bone marrow.
These represent regimens that contain busulfan and cyclophosphamide. These regimens also included patients conditioned with busulfan, cyclophosphamide, and fludarabine.
Fifteen of 50 patients (30%) undergoing endoscopic evaluation in our cohort were identified as having systemic hrTMA (4.4%, or 15 of 338, of all patients undergoing transplantation). The median time to TMA diagnosis was 39 days after HSCT (range, 13 to 150). Seventy-two percent of patients undergoing endoscopy had a clinical diagnosis of iGVHD at a median of 48 days after HSCT (range, 15 to 322). The incidence of clinical stage 3 and 4 intestinal GVHD was similar in the TMA/iGVHD and no TMA/iGVHD groups (80% versus 76%). Endoscopy for all patients was done at the time of GI symptom presentation, a median of 65 days (range, 26 to 341) after HSCT (Table 1).
Histologic Signs of iTMA and GVHD
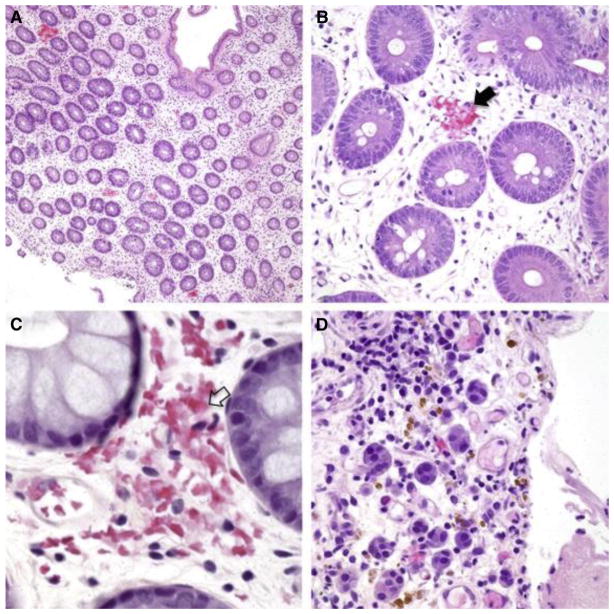
Six of 8 histologic features previously described as possibly associated with iTMA were more common in patients with systemic hrTMA compared with those with GVHD, reaching statistical significance (P < .05). These histologic features included loss of glands, endothelial cell separation, total denudation of mucosa, intraluminal schistocytes, intraluminal fibrin, and intraluminal microthrombi (Table 2). Mucosal hemorrhages were observed more commonly in patients with systemic hrTMA (80%) compared with the other groups (52.4% with no TMA/iGVHD and 36% with no TMA/no iGVHD), but this difference did not reach statistical significance (P = .057). Mucosal hemorrhages seen in the setting of iTMA contained fragmented extravasated and/or degenerated RBCs that were located at and/or close to disrupted capillaries (a patchy distribution, Figure 1A). At times, the capillaries were totally effaced and replaced by hemorrhage. These areas also stained positive for hemosiderin deposits and hemosiderin-laden macrophages, indicating old bleeding not directly associated with the endoscopic procedure (Figure 1D). A representative picture showing loss of glands and mucosal denudation is displayed in Figure 2. The space between glands in patients with systemic hrTMA was markedly widened and the intervening stroma showed fibroplasia and/or hyalinization. True mucosal denudation was almost always associated with regenerative epithelium and fibrotic and/or hyalinized LP stroma. These features, likely representative of a subacute or chronic phase of ischemia, were also observed. Overt acute ischemic colitis was not observed in any patient specimen. Intraluminal schistocytes and endothelial cell separation, more prevalent in the systemic hrTMA group, are displayed in Figure 3. Intraluminal serum protein, which was a thinner, paler, pink and translucent material, was clearly defined from true intraluminal fibrin (Figure 3C,D). In iTMA cases, fibrin was also present in the space between the endothelial cells and the basement membrane (Figure 3C,D). Intraluminal microthrombi were seen in 26.5% of patients with systemic hrTMA, a feature seen exclusively in this group (Figure 3E,F). Endothelial cell swelling was seen more commonly in patients with hrTMA (86.6%) compared with the other groups (61.9% with no TMA/iGVHD and 50% with no TMA/no iGVHD), but this difference also did not reach statistical significance (P = .086) (Table 2). Patients without TMA and without iGVHD did not have any evidence of mucosal infarcts or total denudation of mucosa.
Table 2.
Histologic Signs and Clinical Features
| Feature | TMA/iGVHD (n = 15) | No TMA/iGVHD (n = 21) | No TMA/No iGVHD (n = 14) | P Value |
|---|---|---|---|---|
| Histologic sign | ||||
| Mucosal hemorrhages | 12 (80%) | 11 (52.4%) | 5 (36%) | .057 |
| Loss of glands | 11 (73.3%) | 8 (38%) | 0 (0%) | <.0001 |
| Intraluminal schistocytes | 10 (66.7%) | 5 (23.8%) | 3 (21.4%) | .016 |
| Intraluminal fibrin | 8 (53.3%) | 2 (9.6%) | 2 (14%) | .008 |
| Intraluminal microthrombi | 4 (26.5%) | 0 (0%) | 0 (0%) | .01 |
| Endothelial cell swelling | 13 (86.6%) | 13 (61.9%) | 7 (50%) | .086 |
| Endothelial cell separation | 8 (53.3%) | 3 (14.3%) | 1 (7%) | .007 |
| Total denudation of areas of mucosa | 7 (46.7%) | 3 (14.3%) | 0 (0%) | .005 |
| Clinical features | ||||
| Bleeding | 12 (80%) | 5 (23.8%) | 0 (0%) | <.0001 |
| Nausea/vomiting | 10 (66.67%) | 16 (76%) | 9 (64.3%) | .7346 |
| Diarrhea | 14 (93.3%) | 19 (90.5%) | 10 (71.4%) | .2602 |
| Transfusion for blood loss | 9 (60%) | 2 (9.5%) | 0 (0%) | <.0001 |
| Pain | 14 (93.3%) | 16 (76%) | 6 (40%) | .0124 |
| Hypertension | 6 (40%) | 8 (36.1%) | 8 (57.1%) | .5139 |
| Co-infections | 8 (53.3%) | 10 (47.6%) | 7 (50%) | 1.00 |
| Total deaths | 12 (80%) | 9 (42.9%) | 2 (14.3%) | .0015 |
| Nonrelapse deaths | 10 (66.7%) | 7 (33.3%) | 2 (14.3%) | .017 |
Figure 1.
Histologic samples showing mucosal hemorrhage (H&E, biopsies from 4 iTMA patients, A–C: colon, original magnification ×100, ×400, ×600, respectively, D: stomach, ×400). (A) Patchy distribution of fragmented RBC extravasation located at and/or close to the disrupted capillaries in the lamina propria. (B–C) Capillaries demonstrate fragmented RBC extravasation with partial (B, black arrow) and total (C, clear arrow) disruption of the wall. (D) Hemosiderin deposits (stained brown) and hemosiderin-laden macrophages represent remote mucosal hemorrhage. Nikon Eclipse 80i, 10x/0.30, 40x/0.75, 60x/0.85, Diagnostic Instruments 14.2 Color Mosaic, Spot software.
Figure 2.
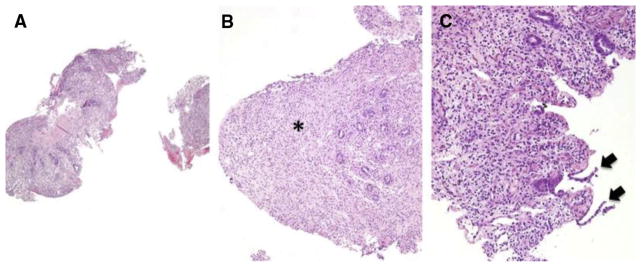
Histologic samples showing loss of glands and mucosal denudation (H&E, biopsies from 3 iTMA patients, A–B: colon, original magnification ×100, C: colon, original magnification ×200). (A–C) The surface epithelium is denuded. The lamina propria shows significant (> 50%) gland loss, appears fibrotic (B, asterisk), and is partially replaced by regenerated epithelium (C, black arrows). Nikon Eclipse 80i, 10x/0.30, 20/0.75, Diagnostic Instruments 14.2 Color Mosaic, Spot software.
Figure 3.
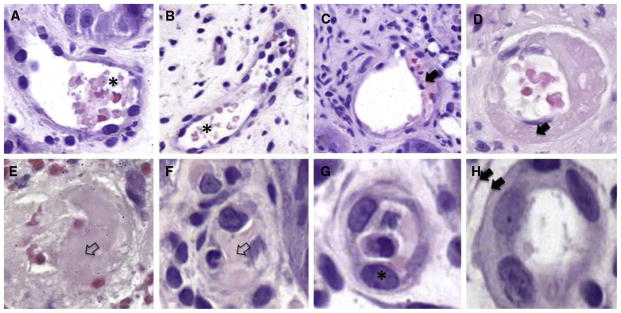
Histologic samples showing intraluminal schistocytes, fibrin and microthrombi as well as endothelial cell swelling and separation. (A–B) Intraluminal schistocytes (H&E, biopsies from 2 iTMA patients, colon, original magnification ×400). Fragmented and degenerated RBCs (asterisk) are present within the capillaries. (C–D) Intraluminal fibrin (H&E, biopsies from 2 iTMA patients, C: esophagus, original magnification ×400, D: colon, original magnification ×400). Capillaries demonstrate fibrin debris in the lumen. Note the fibrin is present in the space between separated endothelial cells (black arrows) and the basement membrane. (E–F) Intraluminal microthrombi (H&E, biopsies from 2 iTMA patients, colon, original magnification ×400). Capillaries are totally occluded by fibrin (clear arrow). (G) Endothelial cell swelling (H&E, biopsies from 2 iTMA patients, colon, original magnification ×400). Capillaries are lined by endothelial cells (asterisk) with enlarged nuclei > 3 times wider than the normal nuclei. (H) Endothelial cell separation (H&E, biopsies from 2 iTMA patients, colon, original magnification ×400). The endothelial cells are separated from the basement membrane (double arrow). Nikon Eclipse 80i, 40x/0.75, Diagnostic Instruments 14.2 Color Mosaic, Spot software.
Eighty-three percent of all patients with a clinical diagnosis of iGVHD (87% with hrTMA and 81% without hrTMA) and 28.6% without a clinical diagnosis of iGVHD were found to have histologic signs of crypt apoptosis or crypt necrosis with abscess formation consistent with gut GVHD.
Clinical Features
Clinically significant bleeding, defined as the need for more than 3 transfusions of blood products (RBC and/or platelets) per week and abdominal pain requiring narcotic administration, was more commonly seen in patients with systemic hrTMA (P < .05) (Table 2). There were no documented events of GI bleeding or the need for blood product support in subjects without systemic hrTMA and without iGVHD (no TMA/no iGVHD group). Fifty-three percent of patients with TMA/iGVHD also had stool positive for adenovirus, norovirus, herpes simplex virus, cytomegalovirus, Clostridium difficile, and/or yeast species based on analysis performed on stool. Forty-seven percent of patients with no TMA/iGVHD had stool positive for adenovirus, norovirus, enterovirus, C. difficile, and/or yeast species; and 50% of patients with no TMA/no iGVHD had stool positive for adenovirus, human herpes virus-6, Epstein Barr virus, and/or C. difficile. Overall survival was 20% (TMA/iGVHD), 57.1% (no TMA/iGHVD), and 85.7% (no TMA/no iGVHD). Nonrelapse mortality was higher (66.7%, P = .017) in patients with TMA compared with that in other clinical groups (33.3% in no TMA/iGVHD and 14.3% in no TMA/no GVHD). There was no statistically significant difference in the incidence of diarrhea, nausea/vomiting, presence of hypertension, or coinciding infections between these 3 groups.
DISCUSSION
To our knowledge, this is the largest retrospective analysis examining 8 histologic signs of iTMA in pediatric and young adult HSCT patients with GI symptoms who were phenotyped for systemic hrTMA using rigorous published diagnostic criteria. We have previously reported a TMA incidence of 39% in 100 prospectively monitored patients. In this previous study, 26 of these 39 patients (67%) were found to findings consistent with systemic hrTMA. Ten of 39 patients with systemic hrTMA (25.6%) or 10% of all transplantation patients were found to have significant GI bleeding [7]. In this current study, 30% of our patients requiring endoscopic evaluations had systemic hrTMA, constituting 4.4% of all patients who underwent transplantation during this study time. Future prospective studies will be required to examine true incidences of systemic and intestinal TMA.
Our study identified 6 histological features of the gut biopsies that were more common in children and young adults with systemic hrTMA, and we believe these findings can be used to help identify transplant recipients in whom TMA contributes to their clinical symptoms. It is important to note that GVHD and iTMA can occur together, and failure to recognize and treat concomitant iTMA might contribute to some cases of failure of GVHD therapy.
In our previous experience as well as reports in the literature, patients with systemic hrTMA at the time of autopsy or small bowel surgical resection have been shown to display marked angiopathic changes involving mesenteric vessels [16]. In the current study, > 60% of patients with systemic hrTMA had significant evidence of intestinal vascular endothelial injury, regardless of coincident clinical gut GVHD. The most significant histologic markers associated with iTMA were endothelial cell separation from the vascular wall, intraluminal schistocytes, intraluminal fibrin, intraluminal microthrombi, loss of glands, and total denudation of mucosa. Mucosal denudation and loss of glands are also histologic features commonly interpreted as severe GVHD but may likely also represent ischemic mucosal damage caused by microangiopathy, especially in our patients with systemic hrTMA. The other histologic signs of iTMA are specific to the vasculature and have not been well described in the context of GVHD. Some investigators have de-emphasized the importance of thrombus formation in patients with TMA [14]. However, intravascular thrombosis was exclusively seen in our patients with systemic hrTMA and likely also reflects severe endothelial injury. Mucosal hemorrhages and endothelial cell swelling were also observed more frequently in patients with systemic hrTMA, but this difference did not reach statistical difference. All histologic findings of iTMA must be evaluated in the context of other ongoing symptoms on a case-by-case basis.
Severe abdominal pain due to ischemia and GI bleeding were significant clinical signs of iTMA and not necessarily due to grade 3 and 4 iGVHD. Our patients with systemic hrTMA experienced symptoms that clearly delineated them from other patients who underwent endoscopy. Pain requiring narcotics, bleeding, and the need for transfusion were clearly more common among these patients. This has been a recurrent observation seen in the limited prior publications reporting on patients with histologic and clinical findings consistent with iTMA. Additionally, iTMA patients with coincident iGVHD have been shown to recover from voluminous diarrhea when treated with immunosuppressants but may continue to have clinically significant GI blood loss, extremely debilitating pain, and transfusion dependency due to ongoing microangiopathy in the bowel [8–10,17,18]. Continued bleeding is likely due to ongoing iTMA-induced ischemic colitis. These patients require treatment strategies that address both TMA and GVHD.
Our study data support prior observations by Innamoto et al. associating severe abdominal pain and intestinal bleeding with iTMA. The authors reviewed 87 endoscopic procedures done for chronic therapy-unresponsive diarrhea. In that study, patients with iTMA presented with intestinal bleeding and did not respond to immunosuppressive therapies targeting iGVHD. Ninety-two percent (80 of 87) of patients in the study cohort were found to have iTMA hallmarked by histologic features similar to those used in our study. Only 31% (27 of 87) and 12.6% (11 of 87) of patients were found to have clinical and/or laboratory evidence supporting iGVHD and systemic TMA diagnoses, respectively. The authors concluded that iTMA is underdiagnosed and might be more prevalent than iGVHD [8]. Other studies analyzing both endoscopic and postmortem biopsies of HSCT patients with GI symptoms have shown an equal prevalence of iTMA in patient cohorts also found to have iGVHD. These publications have illustrated the importance of coinfections possibly contributing to both iTMA and iGVHD [9,10,17]. In our cohort, a majority of patients (80% or 29 of 36) with a clinical diagnosis of gut GVHD also had histologic evidence of iGVHD. Additionally, about one half of patients in all 3 clinical groups also had infectious pathogens identified in stool samples. We screen all patients with diarrhea using a comprehensive infectious disease panel. This close monitoring may explain the increased detection and incidence. However, it is unlikely that these higher incidence rates of infection were the primary trigger of findings in our study, as the number of identified pathogens was similar in all 3 clinical groups. We did not see a significant association of infection and systemic TMA in our previously published prospective study [7]. A clearer association of these factors and iTMA may be answered in future studies.
Our data clearly show that intestinal vascular injury occurs more often in patients with systemic hrTMA and may not be solely from T cell–mediated tissue injury due to concurrent GVHD. Histologically proven iTMA has been documented in autologous stem cell or solid-organ transplantation where a concurrent diagnosis of GVHD is highly unlikely [19,20]. Luft et al. showed that HSCT recipients with TMA who have a genetic predisposition for endothelial injury often develop refractory GVHD, with both complications requiring aggressive intervention for an optimal outcome. Such patients often have elevated markers, such as angiopoietin-2, chemokine IL-8, hepatocyte growth factor, and soluble thrombomodulin indicative of endothelial stress, vulnerability and dysfunction [21,22]. Four of our patients with systemic hrTMA still had clinically significant GVHD at the time of their death. Furthermore, 9 of these patients still had clinically active TMA at the time of their death. It is unclear whether iTMA or GVHD are completely separate entities but our data indicate that patients with gut GVHD and microvascular injury have inferior outcomes. Additional prospective work should be performed to see if HSCT patients with systemic TMA develop iTMA in the absence of a clinical or pathologic diagnosis of GVHD. Furthermore, these studies could observe for whether or not such patients also suffer the same poor prognosis.
Our data suggest that iTMA can contribute to significant morbidity and mortality in HSCT patients and should be included in the differential diagnosis of GI pathology, especially in patients with intestinal bleeding and severe abdominal pain without voluminous diarrhea typical for iGVDH. Each intestinal biopsy should be carefully evaluated not only for GVHD and infection but also for vascular endothelial injury compatible with iTMA as described above. However, assessment might be limited in some biopsy specimens based on inadequate sampling of vessels because of operator variability. Histological changes are typically multifocal and may not be present in small representative biopsies. Previous reports and our personal experience examining autopsy and surgical bowel resection specimens showed that iTMA may affect mesenteric arterioles, causing ischemic bowel necrosis, and may not necessarily be visible in smaller submucosal vessels [6,7,19,23,24]. It is important to consider iTMA based on the highly suggestive clinical symptoms of intestinal bleeding and severe pain in patients with systemic hrTMA. These features may be present despite a lack of histologic evidence. Additionally, histologic sections that display denuded mucosa or loss of glands cannot be solely attributed to GVHD, especially in patients with symptoms of systemic hrTMA and bowel ischemia.
Recognition of iTMA may influence clinical management decisions that improve HSCT outcome. Careful consideration must be made for patients with symptoms consistent with iGVHD, iTMA, and other ongoing HSCT complications. Certain immunosuppressants, such as calcineurin inhibitors given for GVHD, may worsen TMA. Previous groups have showed that patients with iTMA who were misdiagnosed as having iGVHD had worse outcomes when calcineurin inhibitors were continued [8,10]. These agents have been shown to contribute to the development of systemic TMA and iTMA in both humans and animals after stem cell and solid-organ transplantation [25–30]. Some studies have shown that patients with iTMA had reversal of bleeding and abdominal pain complaints when these agents were stopped [8,10,17]. Fujino et al. were able to induce and then successfully reverse histologic and clinical signs of iTMA in rats exposed to tacrolimus [28]. The risk of exacerbating GVHD as a result of lowering or even discontinuing certain immunosuppressive agents underscores the need for careful consideration of the risks and benefits involved. Yamada-Fujiwara et al. successfully addressed these concerns by randomizing patients to different management schema based on the presence of iTMA, GVHD, or both [17]. Patients with iTMA and GVHD had an increase in steroid dose while calcineurin inhibitors were stopped. Despite small numbers, the strategy and outcomes were positive. Although controversial, total plasma exchange has also been shown to be effective when implemented early, along with the discontinuation of calcineurin inhibitors [16,31]. We recently showed that terminal complement blockade by eculizumab may be useful therapy in HSCT patients with TMA [19]. We have successfully treated iTMA and iGVHD using combined therapies including the above strategies, eculizumab as targeted therapy for TMA, and photopheresis for iGVHD. However, further studies evaluating the effectiveness of each strategy need to be performed.
In summary, we have shown that patients with systemic hrTMA experience more significant GI symptoms and have histologic evidence of intestinal vascular injury that require clinical intervention to reduce TMA-associated morbidity and mortality. Histologic GI tissue evaluation should include a careful examination of the small blood vessels of the gut wall using histologic markers associated with iTMA. After iTMA is identified, these patients will require closer monitoring and adjustment in treatment strategies based on coinciding pathology, such as iGVHD or infections.
We acknowledge that our study is limited by retrospective review of patient samples and potential sampling errors. Our study provides the incidence of systemic hrTMA with significant histologic and clinical evidence of iTMA in patients after HSCT. We were unable to show what proportion of patients without systemic hrTMA develop isolated iTMA. Furthermore, a large proportion of our pediatric patients (64%) had primary immune deficiencies with a smaller proportion (25%) receiving transplants for malignancy. It is unclear if our patients’ underlying primary disorders may have contributed to histologic findings in the gut. However, we prospectively observed a higher incidence of systemic TMA in patients with nonmalignant disorders [7]. Additionally, it is unclear how generalizable our findings may be to adult HSCT recipients who underwent transplantation predominantly for malignant diseases. Our findings will require additional validation with future prospective studies. However, our data deliver an important message of awareness about the significance of iTMA and provide a panel of histologic features strongly associated with intestinal microangiopathy.
Footnotes
Conflict of interest statement: S.J. has a United States provisional patent application for methods and compositions related to transplantation-associated thrombotic microangiopathy. No other authors have any conflicts to disclose.
Authorship contributions: J.E., M.W., and S.J. designed the study, performed research, and wrote the paper; C.D., K.C.M., G.W., and S.M.D. provided vital conceptual insights for study design, assisted with study subject accrual and data collection, and edited the manuscript. A.L. performed statistical analyses and edited the manuscript.
Financial disclosure statement: S.J.’s research is supported by CCHMC Center for Pediatric Genomics and Pediatric Center of Excellence in Nephrology (P50DK096418) grants.
References
- 1.Hingorani S. Chronic kidney disease after pediatric hematopoietic cell transplant. Biol Blood Marrow Transplant. 2008;14:84–87. doi: 10.1016/j.bbmt.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995–2005. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani S, Guthrie KA, Schoch G, et al. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39:223–229. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Hudson MM, Landier W. Survivorship: childhood cancer survivors. Prim Care. 2009;36:743–780. doi: 10.1016/j.pop.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29:191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jodele S, Davies SM, Lane A, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124:645–653. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inamoto Y, Ito M, Suzuki R, et al. Clinicopathological manifestations and treatment of intestinal transplant-associated microangiopathy. Bone Marrow Transplant. 2009;44:43–49. doi: 10.1038/bmt.2008.419. [DOI] [PubMed] [Google Scholar]
- 9.Narimatsu H, Kami M, Hara S, et al. Intestinal thrombotic microangiopathy following reduced-intensity umbilical cord blood transplantation. Bone Marrow Transplant. 2005;36:517–523. doi: 10.1038/sj.bmt.1705099. [DOI] [PubMed] [Google Scholar]
- 10.Nishida T, Hamaguchi M, Hirabayashi N, et al. Intestinal thrombotic microangiopathy after allogeneic bone marrow transplantation: a clinical imitator of acute enteric graft-versus-host disease. Bone Marrow Transplant. 2004;33:1143–1150. doi: 10.1038/sj.bmt.1704512. [DOI] [PubMed] [Google Scholar]
- 11.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Cho BS, Min CK, Eom KS, et al. Clinical impact of thrombotic microangiopathy on the outcome of patients with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:813–820. doi: 10.1038/sj.bmt.1705976. [DOI] [PubMed] [Google Scholar]
- 13.Cho BS, Yahng SA, Lee SE, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90:918–926. doi: 10.1097/TP.0b013e3181f24e8d. [DOI] [PubMed] [Google Scholar]
- 14.Ruutu T, Barosi G, Benjamin RJ, et al. Diagnostic criteria for hematopoietic stem cell transplant-associated microangiopathy: results of a consensus process by an International Working Group. Haematologica. 2007;92:95–100. doi: 10.3324/haematol.10699. [DOI] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 17.Yamada-Fujiwara M, Miyamura K, Fujiwara T, et al. Diagnosis of intestinal graft-versus-host disease and thrombotic microangiopathy after allogeneic stem cell transplantation. Tohoku J Exp Med. 2012;227:31–37. doi: 10.1620/tjem.227.31. [DOI] [PubMed] [Google Scholar]
- 18.Komeno Y, Ogawa S, Ishida T, et al. Ischemic colitis as a manifestation of thrombotic microangiopathy following bone marrow transplantation. Intern Med. 2003;42:1228–1232. doi: 10.2169/internalmedicine.42.1228. [DOI] [PubMed] [Google Scholar]
- 19.Jodele S, Fukuda T, Vinks A, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20:518–525. doi: 10.1016/j.bbmt.2013.12.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laskin BL, Goebel J, Davies SM, et al. Early clinical indicators of transplant-associated thrombotic microangiopathy in pediatric neuroblastoma patients undergoing auto-SCT. Bone Marrow Transplant. 2011;46:682–689. doi: 10.1038/bmt.2010.182. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich S, Falk CS, Benner A, et al. Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol Blood Marrow Transplant. 2013;19:22–27. doi: 10.1016/j.bbmt.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Luft T, Dietrich S, Falk C, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 23.Jodele S, Hirsch R, Laskin B, et al. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19:202–207. doi: 10.1016/j.bbmt.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Dandoy CE, Davies SM, Hirsch R, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21:113–118. doi: 10.1016/j.bbmt.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holler E, Kolb HJ, Hiller E, et al. Microangiopathy in patients on cyclosporine prophylaxis who developed acute graft-versus-host disease after HLA-identical bone marrow transplantation. Blood. 1989;73:2018–2024. [PubMed] [Google Scholar]
- 26.Tezcan H, Zimmer W, Fenstermaker R, et al. Severe cerebellar swelling and thrombotic thrombocytopenic purpura associated with FK506. Bone Marrow Transplant. 1998;21:105–109. doi: 10.1038/sj.bmt.1701047. [DOI] [PubMed] [Google Scholar]
- 27.Paquette RL, Tran L, Landaw EM. Thrombotic microangiopathy following allogeneic bone marrow transplantation is associated with intensive graft-versus-host disease prophylaxis. Bone Marrow Transplant. 1998;22:351–357. doi: 10.1038/sj.bmt.1701359. [DOI] [PubMed] [Google Scholar]
- 28.Fujino M, Kim Y, Ito M. Intestinal thrombotic microangiopathy induced by FK506 in rats. Bone Marrow Transplant. 2007;39:367–372. doi: 10.1038/sj.bmt.1705588. [DOI] [PubMed] [Google Scholar]
- 29.Piscitelli D, Fiore MG, Rossi R, et al. Unusual case report of thrombotic microangiopathy of the small bowel following liver transplantation, a possible immunosuppressant-induced disease with histological and ultrastructural findings. Scientific World Journal. 2009;9:1031–1034. doi: 10.1100/tsw.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dierickx D, Monbaliu D, De Rycke A, et al. Thrombotic microangiopathy following intestinal transplantation: a single center experience. Transplant Proc. 2010;42:79–81. doi: 10.1016/j.transproceed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Worel N, Greinix HT, Leitner G, et al. ABO-incompatible allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning: close association with transplant-associated microangiopathy. Transfus Apher Sci. 2007;36:297–304. doi: 10.1016/j.transci.2007.03.004. [DOI] [PubMed] [Google Scholar]