Abstract
Adolescent and young adult oncology (AYAO, including patients 15-39 years of age) is an emerging discipline in the field of cancer treatment and research. Poorer survival outcomes for this population and characteristic age-related challenges in care have called attention to the need for increased AYAO research. This chapter outlines pressing questions and reviews recent progress in AYAO research within the current organizational structure of the federal clinical trials enterprise, emphasizing how the United States National Cancer Institute's National Clinical Trials Network (NCTN) has created novel opportunities for collaborative AYAO research among the pediatric and adult NCTN groups. Potential strategies for expanding collaborative AYAO research, both within the NCTN and with other partners in the federal and advocacy domains are identified.
INTRODUCTION
Historically, the process of identifying a patient's primary oncologist and establishing a management strategy has been largely dependent upon age, rather than cancer type. Newly-diagnosed patients less than 18 years of age are managed almost exclusively by pediatric oncologists, patients 21 years and older almost exclusively by medical oncologists, and those in between by one or the other, resulting in only minor overlap. For long-term survivors of childhood and adolescent cancer, health care transitions have been challenging, as patients encounter adult providers and facilities unfamiliar with their medical issues and treatment protocols.
Similarly, traditional incorporation of similar age parameters into clinical oncology trials has contributed to a lack of regular collaboration among the pediatric and adult cooperative groups. Study endpoints and therapeutic approach have been discernably different, though not necessarily based upon differences in cancer and host biology. Further, pediatric cancer centers are less ubiquitous than adult facilities, making geographic access to those sites more challenging.
AYAO became the focus of concern following seminal reports by Bleyer and colleagues documenting that over the past 30 years, patients 15-39 years of age have not experienced the same improvements in five-year survival observed for both younger and older age groups.1 Although the reasons for this are multifactorial and potential solutions complex, its recognition has led to increased AYA-focused oncology research, including its prioritization within the United States (US) National Cancer Institute (NCI).2 Signs of progress include publication of studies more fully characterizing AYA outcome disparities, development of clinical trials addressing cancers characteristic of AYAs, establishment of the NCI National Clinical Trials Network (NCTN), formation of AYA committees within the NCTN groups, and launching of AYA-focused initiatives supporting intergroup NCTN collaboration. Continued progress will require broader incorporation of AYAO research in the cancer community and increased collaboration across a research enterprise traditionally divided by age. The purpose of this article is to describe the importance of collaborative AYAO research for the NCTN groups, discuss recent progress made, and identify strategies for continued advancement.
RATIONALE: WHY INVOLVEMENT OF ADULT NCTN GROUPS IS ESSENTIAL FOR AYA ONCOLOGY
Confronting the AYAO population are several challenges that impede progress in improving survival. These include inferior geographic access to care,3 insurance,3 tolerance of therapy,4 and treatment adherence.5 There appear to be biological differences in some histologically similar malignancies presenting in pediatric, AYA, and older patients.6 In addition, there likely are substantial developmental differences in pharmacokinetics/pharmacodynamics that influence toxicity and efficacy.7
Critical for elucidating these outcome determinants is an effective, modern translational and clinical research infrastructure. The impact clinical and translational cancer trials have made on improvements in survival and quality of life is well documented.8-10 Unfortunately, enrollment of AYA patients onto clinical trials substantially lags behind that for younger and older age individuals.11 Barriers to enrollment exist in clinical trial infrastructure as well as care delivery.12 Until recently, clinical trials sponsored by the Children's Oncology Group (COG) restricted participation to patients to 21 years of age and younger. In fact, many COG institutions are free-standing children's hospitals that limit care to young people less than 18-21 years of age. Conversely, most medical oncologists and adult-focused clinical trials observe age thresholds of greater than 18, effectively excluding a subset of AYA patients. Depending on the primary provider designation and treating institution, oncologists may not even be aware of potential clinical trials for their AYA patients.
Age also plays a significant role in the types of malignancies encountered in AYAO. This reflects known or likely differences in host and disease biology, as well as where patients receive their diagnostic, therapeutic and survivorship management. Cancers that span the entire AYA age range include breast cancer, leukemia, lymphoma, soft tissue sarcoma, melanoma, germ cell tumor, and central nervous system tumors.13 Due to the age at which some of these malignancies commonly develop, clinical experience may be concentrated in either the medical or pediatric oncology discipline. Therefore, truly comprehensive care for the full AYA age spectrum requires collaboration that taps the expertise of both. For example, in precursor B-cell acute lymphoblastic leukemia, superior survival for AYA patients has been noted in several studies when pediatric-based treatment regimens are used.14-16 On the other hand, chronic myeloid leukemia is rare in pediatrics, where the significantly greater medical oncology experience with tyrosine kinase inhibitor therapy may be useful to the pediatric oncologist.17,18 Similarly, melanoma and breast cancer management in AYAs would benefit from medical oncology experience, whereas neuroblastoma and bone sarcoma would benefit from pediatric oncology input. In sum, a collaborative approach to the management of several AYA cancers is critical.
Geographic access appears to play a role in where AYAO patients are treated. Being relatively uncommon, pediatric cancer is generally treated at specialized children's hospitals or units in medium-large cities, many of which—though not all—are traditional academic centers. In contrast, cancer providers for adults are more common in all settings. Cancer care for adults is largely office- and community-based, which results in most young adults and many older adolescents being referred to community-based medical oncologists. The relative infrequency of research-oriented pediatric cancer centers compared with the abundance of clinically-oriented medical oncology practices probably contributes to limiting access of AYA patients to clinical trial opportunities.
AYA ONCOLOGY AND THE NCTN: RECENT PROGRESS
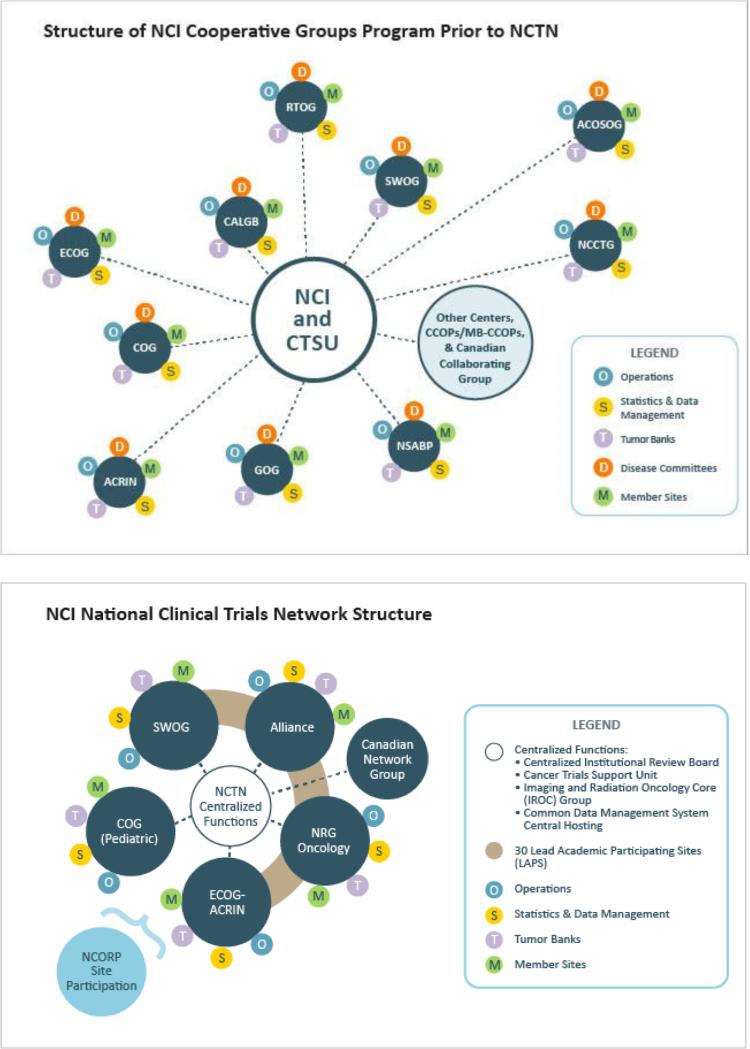
The recent restructuring of the NCI-supported cooperative group trials network has significant potential to advance AYAO research. In March 2014, following recommendations from the Institute of Medicine,19 the nine previous adult cooperative groups were merged into four (NRG Oncology, SWOG, Alliance Oncology, and ECOG-ACRIN). This reorganization helped form the NCI National Clinical Trials Network (NCTN) (Figure 1). Also included in this network are the Canadian adult group, NCIC Clinical Trials Group, and the COG, which itself represents an amalgamation of four legacy pediatric cancer research groups (Pediatric Oncology Group, Children's Cancer Group, National Wilms Tumor Study Group, and Intergroup Rhabdomyosarcoma Study Group). Community-based sites that conduct NCI-funded trials integrate with the NCTN through the new NCI Community Oncology Research Program (NCORP), which includes sites serving minorities and other populations characterized by health disparities. Under the NCTN structure, there is an opportunity for cancer clinical trials to be developed and conducted by the six NCTN groups functioning more as a single, coordinated research network.
Figure 1.
The NCI Cooperative Groups Program structure prior to (top) and following (bottom) the creation of the National Clinical Trials Network (NCTN).25
This new NCTN structure should result in significant benefits for AYAO research. For one, this network should expand clinical trial access for AYA patients. As discussed further below, this is achieved through an established cross-group enrollment process for patients deemed eligible for a particular clinical trial. For example, a 30 year old patient with recurrent osteosarcoma at an Alliance institution can be cross-enrolled onto the current COG study for refractory or recurrent osteosarcoma in patients up to 50 years old, AOST1322. With the NCTN, the typically burdensome process of study activation is streamlined with the required utilization of the NCI Central Institutional Review Board (CIRB), which should enable participating institutions to open more studies on a shorter timeline at lower cost. The CIRB mechanism also obviates the common historical barrier of needing separate local IRB approvals for use of pediatric protocols at adult hospitals. Additionally, the NCTN should foster co-development of AYA-focused intergroup clinical trials across pediatric and adult study groups through greater integration of scientific aims and resources. This scientific integration needs to extend to the new study concept review process within the NCI Cancer Therapy Evaluation Program (CTEP), which involves Steering Committees reflecting both disease- and age-specific expertise.11
The NCI Cancer Trials Support Unit (CTSU) is designed to facilitate access to NCTN clinical trials and to support their management and conduct. The CTSU serves as the platform for cross-group enrollment of AYA patients onto NCTN trials. Currently, there are several clinical trials on the CTSU that are pertinent to AYA patients (Table 1). Two COG-initiated studies for patients with localized (AEWS1031) and metastatic (AEWS1221) Ewing sarcoma allow enrollment of patients up to 50 years of age. AEWS1031 and AOST1322 were opened as intergroup NCTN phase II studies with the participation of NRG Oncology, Alliance Oncology, ECOG-ACRIN and SWOG. Although a trial opened by one NCTN adult cooperative group is available to all the adult groups, in order for a pediatric trial to be activated at both COG and adult cooperative group sites, it must receive NCI approval for this. Similarly, an adult group-sponsored trial must be specially approved for activation at COG sites. Overall, the new NCTN platform should facilitate intergroup enrollment of AYA patients by making more study protocols available with broader age eligibility.
Table 1.
Current AYA Trials Available through the NCI Cancer Trials Support Unit1
| Study No. (Current Status) | Title | Year Opened | Lead Group(s) | Age Range (Years) | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| AEWS 1031 (Available) | A Phase III Randomized Trial of Adding Vincristine-Topotecan-Cyclophosphamide to Standard Chemotherapy in Initial Treatment of Non-Metastatic Ewing Sarcoma | 2010 | COG and NRG | 1-50 | NCT01231906 |
| AEWS 1221 (Available) | Randomized Phase II Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479) to Multiagent Chemotherapy for Patients with Newly Diagnosed Metastatic Ewing Sarcoma | 2014 | COG | ≤ 50 | NCT02306161 |
| ARST 1321 (Available) | Pazopanib Neoadjuvant Trial in Non-Rhabdomyosarcoma Soft Tissue Sarcomas (PAZNTIS): A Phase II/III Randomized Trial of Preoperative Chemoradiation or Preoperative Radiation Plus or Minus Pazopanib | 2014 | COG and NRG | ≥ 2 | NCT02180867 |
| AOST1322 (Available) | Phase II Study of Eribulin in Recurrent or Refractory Osteosarcoma | 2014 | COG | 16-50 | NCT02097238 |
| E1609 (Available) | A Phase III Randomized Study of Adjuvant Ipilimumab Anti-CTLA4 Therapy Versus High-Dose Interferon Alpha-2b for Resected High-Risk Melanoma | 2015 | ECOG- ACRIN | ≥ 12 | NCT01274338 |
| A031102 (In Development) | A Randomized Phase III Trial Comparing-Dose Chemotherapy Using Paclitaxel, Ifosfamide, and Cisplatin (TIP) with High-Dose Chemotherapy Using Mobilizing Paclitaxel Plus Ifosfamide Followed by High-Dose Carboplatin and Etoposide (TI-CE) as First Salvage Treatment in Relapsed or Refractory Germ Cell Tumors | Alliance | ≥ 14 | ||
| AOST 1321 (In Development) | Phase II Study of Denosumab, A RANK Ligand Antibody, for Recurrent Osteosarcoma | COG | 12-49 | ||
| ARST 1431 (In Development) | A Randomized Phase III Study of Vincristine, Dactinomycin, Cyclophosphamide (VAC) Alternating with Vincristine and Irinotecan (VI) versus VAC/VI plus Temsirolimus (TEM) in Patients with Intermediate Risk Rhabdomyosarcoma | COG | < 40 | ||
| A091401 (In Development) | Randomized Phase II Study of Nivolumab With or Without Ipilimumab in Patients with Metastatic or Unresectable Sarcoma | Alliance | ≥ 16 | ||
| AOST1521 (In Development) | Phase II Study of GPNMB-Targeted Antibody Drug Conjugate CDX-011 (Glembatumumab Vedotin) in Recurrent Osteosarcoma | COG | ≥ 1 to < 50 |
Used with kind permission from Springer Science and Business Media (Reference 11).
However, simply expanding age parameters on existing trials is not enough to address the scientific gaps. A more strategic approach is needed to ensure availability of clinical trials across the AYA age spectrum for high-priority cancers. These include cancers associated with poor survival, or where opportunities exist for reducing treatment burden (e.g., excessive acute toxicity, late effects, or cost). This will require an unprecedented level of scientific integration across NCTN groups. In approaching this task, three categories of AYA studies could be considered to guide study development (see Table 2). Studies addressing typical pediatric cancers that occur less commonly in adults could be the primary responsibility of COG, whereas studies addressing typical adult cancers less common in adolescents could be the primary responsibility of an adult group. Studies addressing cancers that are distributed broadly across the AYA age range, where considerable expertise resides in both groups, may be amenable for development as true intergroup efforts. One of the greatest potential benefits of the NCTN is facilitating genesis of AYA-focused intergroup clinical trials among the adult groups and COG.
Table 2.
Potential Categories of AYA-focused Clinical Trials for Development within the NCI National Clinical Trials Network
| AYA Cancer Classification | Examples | Primary Study Population | Contributing Study Population | Potential Coordinating Group |
|---|---|---|---|---|
| “Pediatric type” | Acute Lymphoblastic Leukemia, Osteosarcoma, Ewing Sarcoma | Pediatric | Adult | COG |
| “Adult type” | Colorectal Carcinoma, Breast cancer, Melanoma, Thyroid Cancer | Adult | Pediatric | Adult Group |
| “AYA type” | Acute Myeloid Leukemia, Hodgkin Lymphoma, Germ Cell Tumor | Both | Not Applicable | Inter-Group |
One such example is the newly opened NCTN study for non-rhabdomyosarcoma soft tissue sarcoma (NRSTS), ARST1321 (Table 1). This phase II/III clinical trial explores the efficacy of a novel multi-targeted tyrosine kinase inhibitor, pazopanib, which has been found to be beneficial in children and adults with NRSTS.20,21 It is the first study to be co-conceptualized, developed and conducted by a pediatric and adult cooperative group (COG and NRG Oncology) for patients of all ages (eligibility ≥2 years) with a single disease. Each medical discipline (e.g. radiation oncology, pathology, diagnostic imaging) on the study committee includes representatives from both cooperative groups, which achieved consensus on vital protocol components such as chemotherapy dosing, dose modifications for toxicity, and radiation therapy target volumes. Furthermore, embedded correlative studies may yield critical insights into the biological characteristics of NRSTS in children, adolescents and adults.
A newly designated facet of the NCTN is NCORP, the NCI Community Oncology Research Program. This sub-network of the NCTN builds upon the successful, long-standing NCI Community Clinical Oncology Program (CCOP). As of 2015, there are 34 community and 12 minority/underserved NCORP sites. The majority of these provide both medical and pediatric oncology services, while some are adult or pediatric only. Currently, seven research bases are available for affiliation with NCORP sites through independent agreements (five NCTN groups plus two university bases). The overall goals of the network are to bring a community- and population-based perspective to NCTN clinical trial design and development, and to expand access to the benefits of NCTN trials for community-based populations and others challenged by health disparities (racial/ethnic minorities, low socioeconomic status and rural location). Another important goal is to facilitate conduct of a new category of NCI-funded investigation called Cancer Care Delivery Research (CCDR). In this context, CCDR is defined as the multidisciplinary field of scientific investigation that studies how social factors, financing systems, organizational structures and processes, health technologies, provider and individual behaviors affect cancer outcomes, including access to cancer care, the quality and cost of cancer care, and ultimately the health and well-being of cancer patients.22 The NCORP initiative could be an effective mechanism for improving outcomes through the availability of NCTN trials in the communities where AYAs tend to seek care, and through CCDR that addresses issues highly relevant to AYAs, including supportive care, access to care, health care transitions, mobile health technologies, and health care delivery systems.
STRATEGIES FOR FACILITATING AYAO RESEARCH IN THE NCTN GROUPS
In addition to the federal initiatives noted above, internal cooperative group opportunities are available for enhancing AYA-focused research. In 2002, the COG became the first North American cooperative oncology group to develop a formal committee devoted to AYA oncology. The overall objective of the COG AYA Oncology Discipline Committee is to improve survival and quality of life for AYA patients through a greater understanding of differences in cancer and host biology, and their adjustment to the cancer experience. The population focus of the COG AYA Committee is three-fold: 15-21 year olds newly diagnosed with cancer, young adults with malignancies more commonly encountered in pediatrics (e.g., acute lymphoblastic leukemia and bone sarcomas), and AYA survivors of childhood and adolescent cancer. Its research approach involves highly collaborative relationships with COG scientific committees focused on specific cancers relevant to AYAs, or on survivorship/outcomes, cancer control/supportive care, nursing, behavioral science, pharmacology, developmental therapeutics, bioethics, and others.
The purpose of these relationships is to leverage the disease- or discipline-specific expertise within those committees for addressing AYA questions. Thus, the role of the COG AYA Committee is pursuit of an AYA-focused research plan in COG that is compelling, coherent, coordinated, and cross-committee. The centerpiece of its committee structure is a core of AYA Liaisons representing the various collaborating scientific steering committees. The relationship is bi-directional: Liaisons bring both specific scientific expertise to the AYA Committee and an AYA focus to their home scientific committees. To date, the research strategy has been to characterize more completely age-dependent disparities through secondary analyses of existing COG and legacy group datasets, leading to AYA-focused hypotheses and aims for incorporation in new COG clinical trials. These efforts have identified significant differences in both survival and treatment-related toxicity among AYAs compared with younger patients treated on COG trials.23 For leadership of these projects, there is a strong emphasis on identifying young investigators mentored by more senior AYA Liaisons. With emergence of the NCTN, an increasing priority of the COG AYA Committee has been to foster intergroup AYA study development, and to establish AYA-focused partnerships with adult NCTN groups.
Similar AYA committees and working groups are developing within the adult NCTN groups. In May 2013, the SWOG AYA Committee was formed. As the organizational structure and agenda for this committee are developed, productive relationships have been initiated with SWOG committees for rare tumors/early therapeutics, genitourinary cancer, breast cancer, melanoma, cancer survivorship, CCDR/NCORP, and social media. At this time, investigators from COG, SWOG and other adult groups are actively exploring potential intergroup initiatives in acute lymphoblastic leukemia, acute myeloid leukemia, Hodgkin lymphoma, malignant germ cell tumors, and others. In addition, these AYA committees have established important relationships with external AYA advocacy organizations, such as the Critical Mass Young Adult Cancer Alliance.24 Such partnerships involving the scientific and advocacy communities may be valuable for overcoming common barriers to AYAs participating in NCTN trials and receiving optimal cancer care.
Finally, the COG and SWOG AYA Committees have co-led development of the NCTN AYA Working Group, a new entity formed with the goal of leveraging the NCTN to advance AYA cancer research and care. The inaugural meeting of the NCTN AYA Working Group in November 2013 represents the first time in history that representatives of the NCI-funded cooperative groups convened to address AYAO. Membership now includes representatives from all key NCTN components, including COG and the five adult-focused NCTN groups, NCI/CTEP, the NCORP initiative, and NCTN Program leadership. Regular in-person and teleconference meetings are held. The over-arching goals of the NCTN AYA Working Group are to increase enrollment of AYAs onto NCTN trials and to facilitate development and conduct of AYA-relevant trials. Strategies for achieving this include raising awareness of existing trials, calling attention to gaps where new trials are needed, and working with CTEP to optimize the concept review process for AYAs and to adapt tracking mechanisms for AYA enrollment.
CONCLUSION
Due to the unique convergence of age, diseases, and characteristic clinical challenges, enhancing AYAO research requires deep integration of pediatric and medical oncology expertise. With creation of the NCTN, an unprecedented historical opportunity has arrived for achieving this. Individual NCTN groups have already spawned new studies, formed AYA committees, and launched other initiatives to increase AYAO research internally. The next frontier is AYA integration across the NCTN groups and internationally. Though hurdles remain, it is encouraging that as of 2015 there are important signs of progress.
Acknowledgments
Supported in part by U10 CA180886 (ARW), U10 CA98543 (DRF), U10 CA180888 (CRN), PHS/DHHS grant National Cancer Institute (NCI), National Clinical Trials Network (NCTN): 1U10CA180888-01 (C. Blanke, PI). and the Aflac Foundation (DRF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: none declared
Conflicts of interest: none declared
References
- 1.Bleyer A, Barr R. Cancer in young adults 20 to 39 years of age: overview. Seminars in oncology. 2009;36:194–206. doi: 10.1053/j.seminoncol.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Livestrong Youth Adult Alliance . Adolescent and Young Adult Oncology Progress Review Group: Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer [internet] National Institutes of Health: National Cancer Institute; 2006. [April 20 2015]. (at http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf.) [Google Scholar]
- 3.Parsons HM, Harlan LC, Seibel NL, Stevens JL, Keegan TH. Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4045–53. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canner J, Alonzo TA, Franklin J, Freyer DR, Gamis A, Gerbing RB, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children's Oncology Group. Cancer. 2013;119:4162–9. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Landier W, Hageman L, Chen Y, Kim H, Sun C, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: A children's oncology group study. JAMA Oncology. 2015 doi: 10.1001/jamaoncol.2015.0245. Epub 2015 March 26 doi:101001/jamaoncol20150245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes-Lattin B. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. Journal of the National Cancer Institute. 2011;103:628–35. doi: 10.1093/jnci/djr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veal GJ, Hartford CM, Stewart CF. Clinical pharmacology in the adolescent oncology patient. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4790–9. doi: 10.1200/JCO.2010.28.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gokbuget N, Hoelzer D. Treatment of adult acute lymphoblastic leukemia. Seminars in hematology. 2009;46:64–75. doi: 10.1053/j.seminhematol.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Mullighan CG, Evans WE, Relling MV. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood. 2012;120:1165–74. doi: 10.1182/blood-2012-05-378943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend W, Linch D. Hodgkin's lymphoma in adults. Lancet. 2012;380:836–47. doi: 10.1016/S0140-6736(12)60035-X. [DOI] [PubMed] [Google Scholar]
- 11.Freyer D, Seibel N. The Clinical Trials Gap for Adolescents and Young Adults with Cancer: Recent Progress and Conceptual Framework for Continued Research. Curr Pediatr Rep. 2015:1–9. doi: 10.1007/s40124-015-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Medical and pediatric oncology. 2002;38:1–10. doi: 10.1002/mpo.1257. [DOI] [PubMed] [Google Scholar]
- 13.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, et al., editors. SEER Cancer Statistics Review, 1975-2000. National Cancer Institute; Bethesda, MD: 2003. [Google Scholar]
- 14.Isakoff MS, Freyer DR, Bleyer A. Young adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen do not need a bone marrow transplant in first remission. Blood. 2013;121:5253–5. doi: 10.1182/blood-2013-03-484592. [DOI] [PubMed] [Google Scholar]
- 15.McNeer JL, Raetz EA. Acute lymphoblastic leukemia in young adults: which treatment? Current opinion in oncology. 2012;24:487–94. doi: 10.1097/CCO.0b013e32835538f8. [DOI] [PubMed] [Google Scholar]
- 16.Ram R, Wolach O, Vidal L, Gafter-Gvili A, Shpilberg O, Raanani P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. American journal of hematology. 2012;87:472–8. doi: 10.1002/ajh.23149. [DOI] [PubMed] [Google Scholar]
- 17.Suttorp M, Eckardt L, Tauer JT, Millot F. Management of chronic myeloid leukemia in childhood. Current hematologic malignancy reports. 2012;7:116–24. doi: 10.1007/s11899-012-0113-6. [DOI] [PubMed] [Google Scholar]
- 18.Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2010;2010:368–76. doi: 10.1182/asheducation-2010.1.368. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine Report on a National Clinical Trials System for the 21st Century [April 23 2015];NCI Perspective and Current Activities. 2011 (at http://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/2011-Mar-21-Implementation-Workshop/Doroshow%20Presentation.pdf.).
- 20.Glade Bender JL, Lee A, Reid JM, Baruchel S, Roberts T, Voss SD, et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3034–43. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–86. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 22. [April 23 2015];NCI Community Oncology Research Program Webpage. (at http://ncorp.cancer.gov/research/.)
- 23.Freyer DR, Felgenhauer J, Perentesis J, Adolescent COG. Young Adult Oncology Discipline C. Children's Oncology Group's 2013 blueprint for research: adolescent and young adult oncology. Pediatric blood & cancer. 2013;60:1055–8. doi: 10.1002/pbc.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [April 16 2015];Critical Mass Young Adult Cancer Alliance. (at http://criticalmass.org.)
- 25. [April 16 2015];National Clinical Trials Network Webpage. (at http://www.cancer.gov/clinicaltrials/nctn.)