Abstract
Background
An association between pneumococcal serotypes and mortality has been suggested. We aimed to investigate this among individuals aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa.
Methods
IPD cases were identified through national laboratory-based surveillance at 25 sites, pre-pneumococcal conjugate vaccine (PCV) introduction, from 2003–2008. We assessed the association between the 20 commonest serotypes and in-hospital mortality using logistic regression with serotype 4 (the third commonest serotype with intermediate case-fatality ratio (CFR)) as referent.
Results
Among 3953 IPD cases, CFR was 55% (641/1166) for meningitis and 23% (576/2484) for bacteremia (p<0.001). Serotype 19F had the highest CFR (48%, 100/207), followed by serotype 23F (39%, 99/252) and serotype 1 (38%, 246/651). On multivariable analysis, factors independently associated with mortality included serotype 1 (OR 1.9, 95%CI 1.1–3.5) and 19F (OR 2.9, 95%CI 1.4–6.1) vs. serotype 4; increasing age (25–44 years, OR 1.8, 95%CI 1.0–3.0; 45–64 years, OR 3.6, 95%CI 2.0–6.4; ≥65 years, OR 5.2, 95%CI 1.9–14.1; vs. 15–24 years); meningitis (OR 4.1, 95%CI 3.0–5.6) vs. bacteremic pneumonia; and HIV infection (OR1.7, 95%CI 1.0–2.8). On stratified multivariate analysis, serotype 19F was associated with increased mortality amongst bacteremic pneumococcal pneumonia cases, while no serotype was associated with increased mortality in meningitis cases.
Conclusion
Mortality was increased in HIV-infected individuals, which may be reduced by increased antiretroviral therapy availability. Serotypes associated with increased mortality are included in the 10-and-13-valent PCV and may become less common in adults due to indirect effects following routine infant immunization.
Introduction
Streptococcus pneumoniae is a common cause of pneumonia, meningitis and septicemia and is associated with substantial morbidity and mortality worldwide[1, 2]. Since the introduction of pneumococcal conjugate vaccines (PCVs), a decline in the incidence of vaccine-serotype invasive pneumococcal disease (IPD) has been observed in vaccinated children and unvaccinated adults (through herd effect) in areas where the vaccine is widely used[3]. A significant burden of IPD, however, exists worldwide due to serotypes that are not included in PCV, which has increased in children and adults since the introduction of PCV into childhood immunization programs[4].
Incidence, severity and mortality of IPD are influenced by host- and organism-related factors. The host-related factors include extremes of age, underlying chronic illness, immunosuppression and access to antibiotic treatment[5–8]. Pathogen-related factors include the polysaccharide capsule which is the serotype determinant[9, 10]. Previous studies have shown an association between pneumococcal serotypes and mortality in adults[11, 12]. These associations could differ in a middle-income country, such as South Africa, where there is a high HIV prevalence (19% in adults aged 15–49 years in 2012)[13, 14] and a greater diversity of serotypes associated with IPD[15]. In addition, few studies have examined the association between serotypes and mortality in the absence of potential effects of pneumococcal vaccination and none have examined the association between serotype and mortality separately in HIV-infected and HIV-uninfected individuals[12].
We aimed to determine the association between pneumococcal serotype and in-hospital mortality among patients aged ≥15 years with IPD in South Africa during the period 2003–2008, prior to the introduction of PCV in the routine immunization program.
Methods
Surveillance for IPD
From 2003, national, active, population-based, laboratory-based surveillance for IPD was conducted through the GERMS-SA programme [16]. Reports of laboratory-confirmed IPD, including demographic details as well as date of specimen, and source of isolate together with isolates were sent to the National Institute for Communicable Diseases (NICD) in Johannesburg from >130 laboratories nationally. We conducted annual laboratory audits of all public-sector laboratories in 8 provinces annually using Disa*Lab Laboratory Information Management System. We included cases identified by audit. At 25 sentinel enhanced surveillance hospitals in all nine provinces we collected additional information from patient interviews including HIV serological status, admission date, discharge diagnosis and outcome.
Cases were defined as patients ≥15 years of age with S. pneumoniae cultured from normally sterile site specimens (e.g., cerebrospinal fluid [CSF], blood, pleural, peritoneal or joint fluid) from January 2003 through December 2008. We excluded repeat isolates from the same individual within 21 days of the initial positive culture. We defined specimen source using a hierarchical definition, as follows: CSF specimen regardless of other specimens; blood specimen regardless of other specimens (excluding CSF); and other e.g., pleural fluid, joint fluid etc. without CSF or blood. We only obtained information on clinical syndrome for enhanced surveillance sites and we defined clinical syndrome using a hierarchical definition, as follows: meningitis if a clinical diagnosis of meningitis was noted in the medical records or the pneumococcus was isolated from CSF; bacteremic pneumonia if the clinical diagnosis of pneumonia was noted in the medical records and the pneumococcus was isolated from blood culture; other (including any diagnoses not including the preceding two, including bacteremia without any localising site and localized pneumonia with pneumococcus isolated from other sterile sites only). We defined predisposing conditions other than HIV infection as follows: asplenia, sickle cell anemia; chronic illnesses including lung, liver, renal, cardiac disease and diabetes; other immunocompromising conditions including malignancy, primary immunodeficiency, immunotherapy and organ transplant; as well as other Advisory Committee on Immunization Practices [17] risk factors including head injury with CSF leak, alcohol and smoking. Disease severity was measured using the Pitt bacteremia score, which is a composite severity score including patient temperature, presence of hypotension, receipt of mechanical ventilation, cardiac arrest and mental status[18, 19]. We defined acute severe illness as a Pitt bacteremia score of ≥4 at the time of specimen collection. Provinces were grouped into 3 categories based on poverty rates (low, intermediate and high) determined by the findings of the living conditions survey conducted by Statistics South Africa in 2008–2009[20].
Serotyping and susceptibility testing
The Quellung reaction using specific antisera (Statens Serum Institut, Copenhagen, Denmark) was used for pneumococcal serotyping. We separately distinguished serotypes 6A, B, C and D for the whole study period, although serotypes 6C and 6D were identified from <1% of cases[21]. Isolates were screened for penicillin resistance using oxacillin disc diffusion (Mast Diagnostics, Merseyside, United Kingdom) [22] and minimum inhibitory concentrations (MICs) of the potentially resistant isolates were determined using agar dilution or Etest® (AB-Biodisk, Solna, Sweden). We interpreted results as non-susceptible (intermediately resistant and resistant) or susceptible using 2008 Clinical and Laboratory Standards Institute (CLSI) definitions[22]. We considered isolates with MICs ≥0.12mg/L to be non-susceptible to penicillin at using the oral penicillin breakpoint. We defined multi-drug resistance as non-susceptibility to any three or more different antibiotic classes, according to the 2009 definitions of the CLSI. [22] For determination of appropriate antibiotic prescription, data on the antibiotics used for patient management was compared with the recommended antibiotic guidelines to determine whether the appropriate antibiotic was administered to the patient[23].
Statistical analysis
The study population included all patients with IPD aged ≥15 years from 2003 to 2008. Because our main focus was on the association between serotypes and in-hospital outcome, we included only patients with known in-hospital outcome at enhanced surveillance sites and presenting with one of the 20 most common serotypes, to allow sufficient numbers in each group for comparison. We a priori included the serotypes in PCV-13 i.e 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F. In addition we included serotypes 8, 12F, 16, 9N, 22F, 25 and 13.
Mortality was defined as death in hospital and within 30 days of the IPD episode, to avoid inclusion of deaths unrelated to IPD. To explore possible bias introduced by including only patients presenting to enhanced surveillance sites we compared the characteristics of patients presenting to enhanced and non-enhanced sites using logistic regression.
To assess differences between serotypes we evaluated the association of age (age group 15–24 years, 25–44 years, 45–64 years, ≥65 years), clinical syndrome (meningitis, bacteremic pneumonia and other IPD), HIV co-infection and in-hospital mortality for each serotype compared to serotype 4. We chose serotype 4 as the referent as it was the third most common serotype (289/3953, 7%) identified and we wanted to use a serotype with an intermediate CFR (29%) as referent to allow for identification of serotypes with particularly high or low CFR. We used univariate multinomial regression models, generating a separate estimate of effect for each predictor on each outcome relative to the base level. The effect measures are the ratios of two relative risks (relative risk ratios) with each relative risk describing the probability of the outcome in the category of interest relative to the baseline category[24].
To determine factors associated with in-hospital mortality, we used logistic regression. We assessed all variables that were significant at p<0.2 on univariate analysis, and eliminated from the model non-significant factors (p≥0.05) with stepwise backward selection. Patients with missing data for included variables were excluded from the model. Serotype was retained in all multivariate models a priori because it was the main variable of interest and clinical syndrome because it is an important potential confounder. For the analysis of factors associated with mortality in HIV-infected individuals CD4 + T cell count was not included in the multivariable model because of a large amount (>40%) of missing data, however, CD4+ T cell count was not associated with serotype. All analyses were done using Stata Version 13 (StataCorp Limited). Two-sided p values <0.05 were considered significant. We performed stratified analyses for meningitis and bacteremic pneumonia separately and HIV-infected and—uninfected individuals separately, to explore whether the association between serotype and mortality varied within these subgroups. For each analysis, we used all available case information. Variables were binary (yes/no), defined as the presence or absence of the attribute excluding missing data, or categorical variables in multiple levels. We performed a sensitivity analysis restricting the study population to individuals aged ≥18 years.
Ethics Procedures
The national surveillance programme includes national laboratory-based surveillance where demographic data and isolates are sent to NICD routinely without requiring patient consent as part of the NICD’s national public health surveillance responsibility. In addition, at sentinel enhanced surveillance sites, additional data is obtained from patient interviews, only from individuals who provide written informed consent. For participants under the age of 18, written informed consent was obtained from parents or legal guardians. The surveillance protocol (laboratory-based and enhanced surveillance) was approved by the University of the Witwatersrand Human Research Ethics Committee (Medical) (M0801117) and other relevant institutional ethics committees.
Results
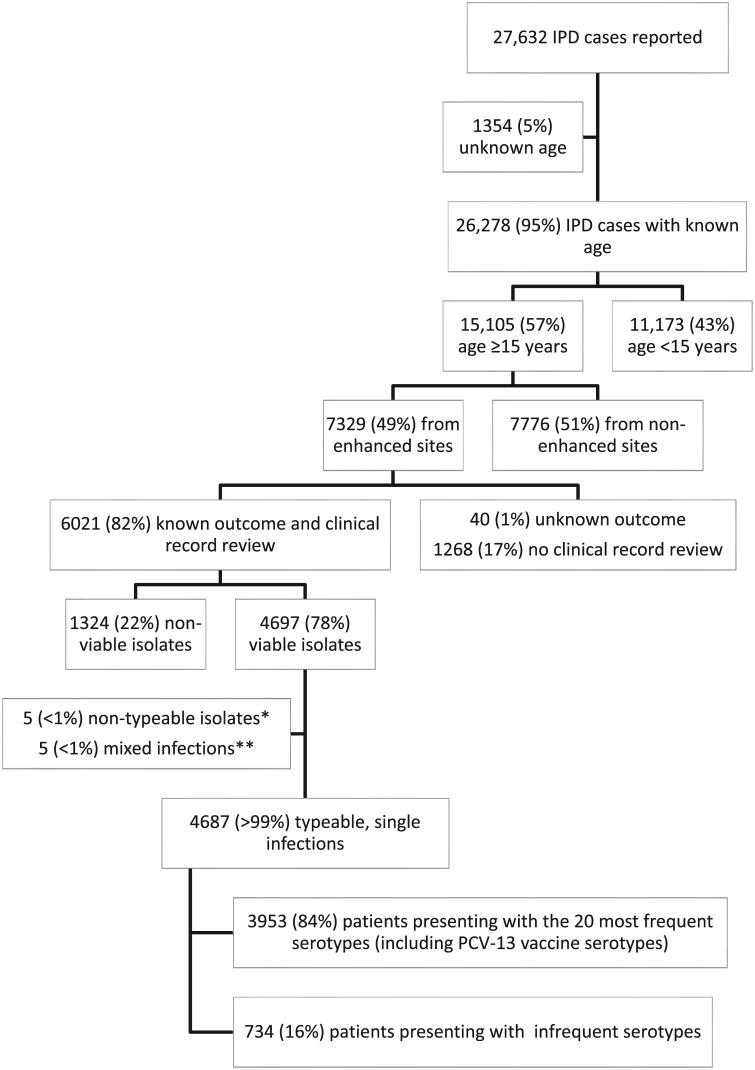
From 2003–2008, 27,632 patients with IPD were reported to the GERMS-SA surveillance program (Fig 1). Among 26,278 (95%) with known age, 15,105 (57%) were aged ≥15 years. Among these, 49% (7329) were from enhanced sites and 82% (6021) of these had known in-hospital outcome. Of patients with known outcomes, 4697 (78%) had viable, serotypeable isolates from a single IPD episode and 3953 (84%) of these were due to the 20 most frequent serotypes including PCV-13 vaccine serotypes. Data on clinical diagnosis was available for 99% (3923/3953) of individuals.
Fig 1. Flow diagram of patients with invasive pneumococcal disease (IPD) in South Africa, 2003–2008 and included in the analysis.
*Pneumococci that did not have a capsule and could not be serotyped. **Isolates from patients that were simultaneously infected with two strains of pneumococci with different serotypes.
The overall case-fatality ratio (CFR) was 34% (1333/3953): 55% (641/1166) amongst patients with meningitis, 23% (576/2484) in patients with bacteremic pneumonia and 36% (98/273) in patients with other IPD. Serotype 1 was the commonest serotype identified (651/3953, 16%), followed by serotype 19A (443/3953, 11%), serotype 4 (289/3953, 7%) and serotype 3 (284/3953, 7%). The majority of patients (64%, 2527/3953) were aged 25–44 years, 54% (2146/3952) were female and of those with known status, 89% (2309/2580) were HIV infected.
Comparing patients at enhanced to non-enhanced surveillance sites, on multivariable analysis, patients from enhanced sites were more likely to come from an intermediate poverty level province (odds ratio [OR] 1.3, 95% confidence interval [CI] 1.2-1.5) and less likely to come from a high poverty level province (OR 0.5, 95% CI 0.4-0.6)(vs low poverty province), more likely to be of non-black race (OR 3.3, 95% CI 2.5-4.2), more likely to have blood (OR 2.4, 95% CI 2.1–2.7) or other (OR 1.3, 95% CI 1.0–1.5) specimen type (vs CSF) and more likely to have disease due to serotype 9N (OR 1.6, 95% CI 1.1–2.5) or 22F (OR 22.1, 95% CI 5.3–91.6)(vs serotype 4) (Table 1).
Table 1. Comparison of demographic and clinical characteristics of patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from GERMS-SA enhanced and non-enhanced sites, 2003–2008.
| Variable | Enhanced sites | Non-enhanced sites | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | p | OR (95% CI) | p | ||
| Province* | Low poverty | 5377/7329 (73) | 4601/7776 (59) | Reference | Reference | Reference | Reference |
| Intermediate poverty | 1431/7329 (20) | 1539/7776 (20) | 0.8 (0.7–0.9) | <0.001 | 1.3 (1.2–1.5) | <0.001 | |
| High poverty | 521/7329 (7) | 1636/7776 (21) | 0.27 (0.3–0.3) | <0.001 | 0.5 (0.4–0.6) | <0.001 | |
| Race | Non-black | 539/6779 (8) | 93/4242 (2) | 3.9 (3.1–4.8) | <0.001 | 3.3 (2.5–4.2) | <0.001 |
| Specimen | CSF | 1727/7329 (24) | 3398/7776 (44) | Reference | Reference | Reference | Reference |
| Blood | 4955/7329 (67) | 3161/7776 (41) | 3.1 (2.9–3.3) | <0.001 | 2.4 (2.1–2.7) | <0.001 | |
| Other | 647/7329 (9) | 1217/7776 (16) | 1.1(0.9–1.2) | 0.021 | 1.3 (1.0–1.5) | 0.007 | |
| Serotype | 1 | 463/4528 (17) | 920/4434 (21) | 0.9 (0.8–1.1) | 0.532 | 0.9 (0.8–1.2) | 0.541 |
| 19A | 486/4528 (11) | 401/4434 (9) | 1.4 (1.1–1.7) | 0.002 | 1.06 (0.8–1.3) | 0.332 | |
| 4 | 323/4528 (7) | 368/4434 (8) | Reference | Reference | Reference | Reference | |
| 3 | 323/4528 (7) | 251/4434 (6) | 1.5 (1.2–1.8) | 0.001 | 1.1 (0.8–1.4) | 0.559 | |
| 6A | 300/4528 (7) | 321/4434 (7) | 1.1 (0.9–1.3) | 0.751 | 1.0 (0.8–1.3) | 0.515 | |
| 14 | 284/4528 (6) | 282/4434 (6) | 1.1 (0.9–1.4) | 0.374 | 1.05 (0.7–1.2) | 0.516 | |
| 23F | 301/4528 (6) | 285/4434 (7) | 1.2 (0.9–1.4) | 0.761 | 1.3 (1.0–1.7) | 0.031 | |
| 8 | 230/4528 (5) | 243/4434 (5) | 1.1 (0.9–1.4) | 0.968 | 1.0 (0.7–1.3) | 0.933 | |
| 19F | 240/4528 (5) | 200/4434 (5) | 1.4 (1.1–1.7) | 0.011 | 1.3 (0.9–1.7) | 0.079 | |
| 6B | 230/4528 (5) | 234/4434 (6) | 1.1 (0.8–1.3) | 0.571 | 1.0 (0.8–1.3) | 0.702 | |
| 12F | 215/4528 (5) | 257/4434 (6) | 1.0 (0.8–1.2) | 0.203 | 0.9 (0.7–1.3) | 0.858 | |
| 9V | 163/4528 (4) | 157/4434 (4) | 1.18 (0.9–1.5) | 0.708 | 1.3 (0.9–1.7) | 0.431 | |
| 16 | 116/4528 (3) | 108/4434 (2) | 1.2 (0.9–1.6) | 0.588 | 1.1 (0.7–1.6) | 0.583 | |
| 9N | 101/4528 (2) | 68/4434 (2) | 1.7 (1.2–2.4) | 0.003 | 1.6 (1.1–2.5) | 0.029 | |
| 7F | 80/4528 (2) | 67/4434 (2) | 1.4 (0.9–2.0) | 0.067 | 1.1 (0.7–1.6) | 0.462 | |
| 18C | 81/4528 (2) | 89/4434 (2) | 1.0 (0.7–1.4) | 0.583 | 1.1 (0.7–1.6) | 0.820 | |
| 25 | 74/4528 (2) | 52/4434 (1) | 1.6 (1.1–2.4) | 0.014 | 1.1 (0.7–1.7) | 0.401 | |
| 22F | 74/4528 (2) | 2/4434 (0.1) | 42.2 (10.3–173.1) | <0.001 | 22.1 (5.3–91.6) | <0.001 | |
| 13 | 78/4528 (2) | 65/4434 (1) | 1.4 (1.0–2.0) | 0.173 | 1.4 (0.9–2.1) | 0.151 | |
| 5 | 64/4528 (1) | 64/4434 (1) | 1.1 (0.8–1.6) | 0.944 | 0.9 (0.6–1.5) | 0.656 | |
N-number, OR—Odds ratio, CI—confidence interval, CSF- cerebrospinal fluid. Only factors statistically significant on univariate analysis are included in the table. Additional variables evaluated were age group and gender.
*The low poverty rate group consisted of Gauteng and Western Cape. The intermediate poverty rate group consisted of KwaZulu-Natal, Free State, Northern Cape and North West. The high poverty rate group consisted of Eastern Cape, Mpumalanga and Limpopo.
Univariate multinomial analysis of the 20 commonest serotypes by age group, syndrome, in-hospital outcome and HIV co-infection
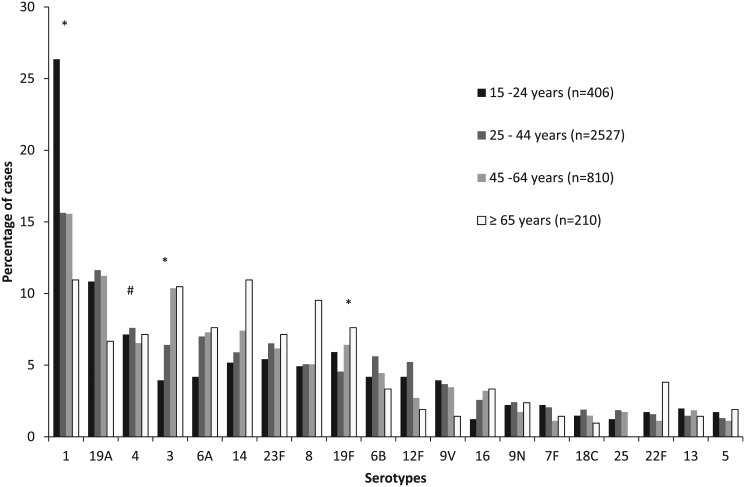
Analyzing the 20 most common serotypes, using multinomial regression, comparing the age distribution of all other serotypes to the age distribution of serotype 4 (25–44 years as the referent group), serotype 3 was significantly more likely to be identified from patients 45–64 years of age (relative risk ratio [RRR] 1.9, 95% CI 1.2–2.8), serotype 1 was significantly more likely to be isolated from patients 15–24 years of age (RRR 1.8. 95% CI 1.2–2.8) and serotype 19F was significantly more likely to be isolated from patients 45–64 years of age (RRR 1.6 95% CI 1.1–2. 6) (Fig 2).
Fig 2. Distribution of pneumococcal serotypes amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from 2003–2008, by age group.
# Reference group. *Statistically significant at p<0.05.
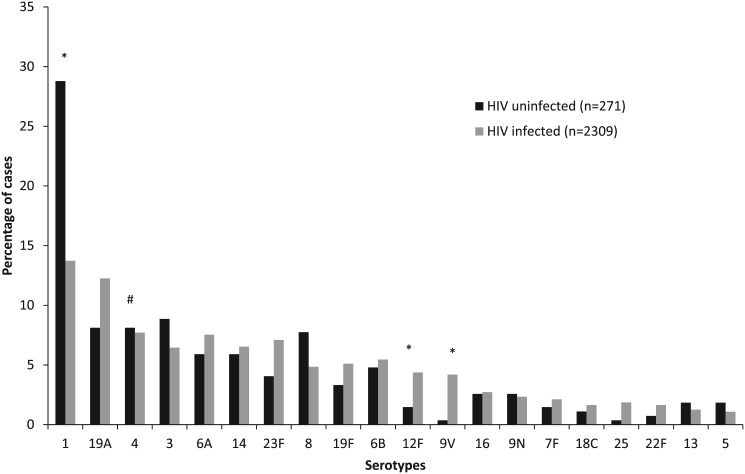
Compared to serotype 4, serotypes 12F (RRR 3.1, 95% CI 1.1–9.3) and 9V (RRR 12.0, 95% CI 1.6–90.3) caused significantly more disease in HIV-infected individuals, whereas serotype 1 (RRR 0.5, 95% CI 0.3–0.8) was significantly less likely in HIV-infected patients (Fig 3).
Fig 3. Distribution of pneumococcal serotypes amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from 2003–2008, by HIV-infection status.
# Reference group. *Statistically significant at p<0.05.
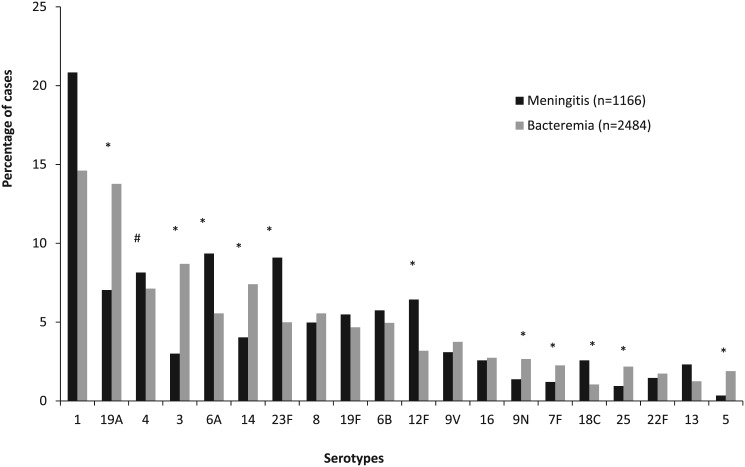
Serotypes 6A (RRR 1.5, 95% CI 1.0–2.1), 23F (RRR 1.6, 95% CI 1.1–2.3), 12F (RRR 1.8, 95% CI 1.2–2.7) and 18C (RRR 2.2, 95% CI 1.2–3.8) were more likely to present as meningitis, while serotypes 19A (RRR 2.2, 95% CI 1.6–3.2), 3 (RRR 3.3, 95% CI 2.1–5.1), 14 (RRR 2.1, 95% CI 1.4–3.2), 9N (RRR 2.2, 95% CI 1.2–4.0), 7F (RRR 2.1, 95% CI 1.1–4.1), 25 (RRR 2.6, 95% CI 1.3–5.3) and 5 (RRR 6.3, 95% CI 2.2–18.0) were more likely to present as bacteremia when compared to serotype 4 (Fig 4).
Fig 4. Distribution of pneumococcal serotypes amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from 2003–2008, by clinical syndrome.
# Reference group. *Statistically significant at p<0.05
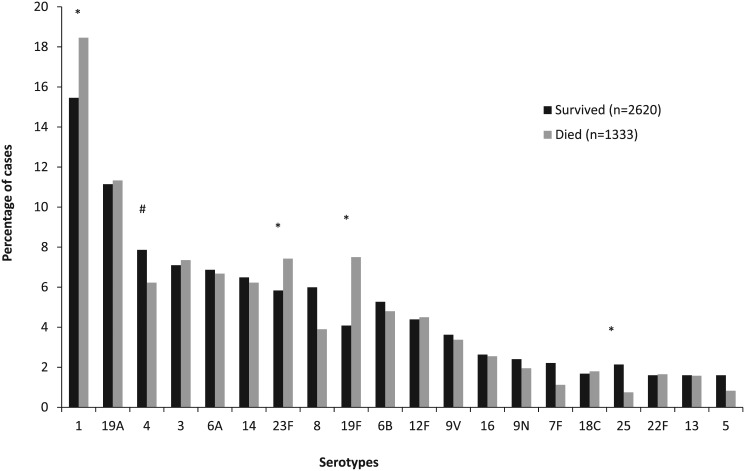
In addition, serotypes 1 (RRR 1.5, 95% CI 1.1–2.0), 23F (RRR 1.6, 95% CI 1.1–2.3) and 19F (RRR 2.3, 95% CI 1.6–3.4) were significantly more likely to cause death compared to serotype 4. Serotype 25 (RRR 0.4, 95% CI 0.2–0.9) was associated with a lower probability of death compared to serotype 4 (Fig 5).
Fig 5. Distribution of pneumococcal serotypes amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from 2003–2008, by in-hospital outcome.
# Reference group. *Statistically significant at p<0.05
Factors associated with in-hospital mortality overall
Among all patients with IPD aged ≥15 years, on multivariable analysis, compared to serotype 4, infection with serotype 1 (OR 1.9, 95% CI 1.1–3.5), 14 (OR 2.1, 95% CI 1.0–4.2) and 19F (OR 2.9, 95% CI 1.4–6.1) was associated with increased in-hospital mortality (Table 2). Additional factors associated with in-hospital mortality on multivariable analysis controlling for province were increasing age (25–44 years OR 1.8 95% CI 1.0–3.0; 45–64 years OR 3.6, 95% CI 2.0–6.4; ≥65 years OR 5.2, 95% CI 1.9–14.1 vs 15–24 years), meningitis (vs bacteremia) (OR 4.1, 95% CI 3.0–5.6), history of antibiotic use in the two months preceding the IPD episode (OR 3.9 95% CI 2.5–6.2), inappropriate antibiotic prescription (OR 2.4, 95% CI 1.7–3.2) and being HIV infected (OR 1.7, 95% CI 1.0–2.8).
Table 2. Univariate and multivariable analysis of factors associated with mortality amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa, 2003–2008.
| Risk factor | Percent of all enrolled cases | Case-fatality ratio | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|---|---|
| n/N (%) | n/N (%) | OR (95% CI) | p | OR (95% CI) | p | ||
| Age group (years) | 15–24 | 406/3953 (10) | 92/406 (23) | Reference | Reference | Reference | Reference |
| 25–44 | 2527/3953 (64) | 793/2527 (31) | 1.6 (1.2–2.0) | <0.001 | 1.8 (1.0–3.0) | 0.037 | |
| 45–64 | 810/3953 (21) | 348/810 (43) | 2.6 (2.0–3.4) | <0.001 | 3.6 (2.0–6.4) | <0.001 | |
| ≥65 | 210/3953 (5) | 100/210 (48) | 3.1 (2.2–4.4) | <0.001 | 5.2 (1. 9–14.1) | 0.001 | |
| Province poverty level* | Low | 2934/3953 (74) | 915/2934 (31) | Reference | Reference | Reference | Reference |
| Intermediate | 780/3953 (20) | 301/780 (39) | 1.4 (1.2–1.6) | <0.001 | 1.6 (1.2–2.4) | 0.005 | |
| High | 239/3953 (6) | 117/239 (49) | 2.1 (1.6–2.8) | <0.001 | 1.7 (1.0–3.0) | 0.042 | |
| Disease syndrome | Meningitis | 1166/3923 (30) | 641/1166 (55) | 4.0 (1.2–2.2) | <0.001 | 4.1 (3.0–5.6) | <0.001 |
| Bacteremic pneumonia | 2484/3923 (63) | 576/2484 (23) | Reference | Reference | Reference | Reference | |
| Other | 273/3923(7) | 98/273 (36) | 1.9 (0.3–0.5) | <0.001 | 1.6 (0.9–2.9) | 0.104 | |
| Pitt bacteremia score | <4 | 2993/3953 (76) | 864/2993 (29) | Reference | Reference | ||
| ≥4 | 960/3953 (24) | 469/960 (49) | 2.4 (2.0–2.7) | <0.001 | |||
| Prior antibiotic use (24 hours) | Yes | 96/2968 (3) | 41/96 (43) | 2.1 (1.4–3.1) | 0.001 | ||
| No | 2872/2968 (97) | 759/2872 (26) | Reference | Reference | |||
| Prior antibiotic use (2 months) | Yes | 149/2402 (6) | 59/149 (40) | 2.6 (1.8–3.6) | <0.001 | 3.9 (2.5–6.2) | <0.001 |
| No | 2253/2402 (94) | 458/2253 (20) | Reference | Reference | Reference | Reference | |
| Appropriate antibiotic prescription | Yes | 3005/3923 (77) | 900/3005 (30) | Reference | Reference | Reference | Reference |
| No | 918/3923 (23) | 415/918 (45) | 1.9 (1.7–2.3) | <0.001 | 2.4 (1.7–3.2) | <0.001 | |
| HIV status | Positive | 2309/2580 (89) | 687/2309 (30) | 1.5(1.1–2.0) | 0.013 | 1.7 (1.0–2.8) | 0.035 |
| Negative | 271/2580 (11) | 61/271 (23) | Reference | Reference | Reference | Reference | |
| Nosocomial infection | Yes | 168/3953 (4) | 73/168 (44) | 1.5 (1.1–2.1) | 0.007 | ||
| No | 3785/3953(96) | 1260/3785 (33) | Reference | Reference | |||
| Multi-drug resistant | Yes | 609/3953 (15) | 230/609 (38) | 1.2 (1.0–1.5) | 0.022 | ||
| No | 3344 (85) | 1103/3344(33) | Reference | Reference | |||
| Serotype | 1 | 651/3953 (17) | 246/651 (38) | 1.5 (1.1–2.0) | 0.007 | 1.9 (1.1–3.5) | 0.034 |
| 19A | 443/3953 (11) | 151/443 (34) | 1.3 (0.9–1.8) | 0.128 | 1.8 (0.9–3.4) | 0.075 | |
| 4 | 289/3953 (7) | 83/289 (29) | Reference | Reference | Reference | Reference | |
| 3 | 284/3953 (7) | 98/284 (35) | 1.3 (0.9–1.9) | 0.137 | 1.2 (0.5–2.5) | 0.727 | |
| 6A | 269/3953 (7) | 89/269 (33) | 1.2 (0.9–1.8) | 0.265 | 0.8 (0.4–1.6) | 0.501 | |
| 14 | 253/3953 (6) | 83/253 (33) | 1.2 (0.8–1.8) | 0.303 | 2.1 (1.0–4.2) | 0.049 | |
| 23F | 252/3953 (6) | 99/252 (39) | 1.6 (1.1–2.3) | 0.010 | 1.9 (0.9–3.8) | 0.073 | |
| 8 | 209/3953 (5) | 52/209 (25) | 0.8 (0.6–1.2) | 0.342 | 1.2 (0.5–2.7) | 0.637 | |
| 19F | 207/3953 (5) | 100/207 (48) | 2.3 (1.6–3.4) | <0.001 | 2.9 (1.4–6.1) | 0.005 | |
| 6B | 202/3953 (5) | 64/202 (32) | 1.2 (0.8–1.7) | 0.481 | 1.4 (0.7–2.8) | 0.428 | |
| 12F | 175/3953 (4) | 60/175 (34) | 1.3 (0.9–1.9) | 0.209 | 1.2 (0.5–2.9) | 0.757 | |
| 9V | 140/3953 (4) | 45/140 (32) | 1.18 (0.8–1.8) | 0.468 | 1.8 (0.8–4.4) | 0.187 | |
| 16 | 103/3953 (3) | 34/103 (33) | 1.22 (0.8–2.0) | 0.414 | 0.4 (0.1–1.5) | 0.201 | |
| 9N | 89/3953 (2) | 26/89 (29) | 1.0 (0.6–1.7) | 0.928 | 0.9 (0.3–3.0) | 0.888 | |
| 7F | 73/3953 (2) | 15/73 (21) | 0.6 (0.3–1.2) | 0.163 | 1.0 (0.3–3.0) | 0.965 | |
| 18C | 68/3953 (2) | 24/68 (35) | 1.4 (0.8–2.4) | 0.288 | 1.3 (0.4–4.1) | 0.611 | |
| 25 | 66/3953 (2) | 10/66 (15) | 0.4 (0.2–0.9) | 0.027 | 0.8 (0.2–3.1) | 0.701 | |
| 22F | 64/3953 (2) | 22/64 (34) | 1.3 (0.7–2.3) | 0.371 | 1.3 (0.4–4.7) | 0.674 | |
| 13 | 63/3953 (2) | 21/63 (33) | 1.2 (0.7–2.2) | 0.468 | 2.0 (0.7–5.8) | 0.234 | |
| 5 | 53/3953 (1) | 11/53 (21) | 0.7 (0.3–1.3) | 0.235 | 0.3 (0.0–3.0) | 0.291 | |
N-number, OR—Odds ratio, CI—confidence interval, HIV—Human immunodeficiency virus, PCV- pneumococcal conjugate vaccine. Only factors statistically significant on univariate analysis are included in the table. Additional variables evaluated were gender, race, presence of underlying medical conditions other than HIV and penicillin non-susceptibility.
*The low poverty rate group consisted of Gauteng and Western Cape. The intermediate poverty rate group consisted of KwaZulu-Natal, Free State, Northern Cape and North West. The high poverty rate group consisted of Eastern Cape, Mpumalanga and Limpopo.
Factors associated with in-hospital mortality stratified by clinical syndrome
Among patients with bacteremic pneumonia, on multivariable analysis, compared to serotype 4, infection with serotype 14 (OR 2.2, 95% CI 1.1–4.7) and 19F (OR 3.5, 95% CI 1.6–8.0) was associated with increased in-hospital mortality (Table 3). Additional factors associated with in-hospital mortality on multivariable analysis among patients with bacteremic pneumonia were increasing age group (25–44 years OR 2.7 95% CI 1.5–5.0; 45–64 years OR 6.2, 95% CI 3.1–12.7; ≥65 years OR 11.7, 95% CI 3.4–40.4 vs 15–24 years), history of antibiotic use in the 2 months preceding the IPD episode (OR 2.3 95% CI 1.4–3.9) and inappropriate antibiotic prescription (OR 2.8, 95% CI 2.0–3.8).
Table 3. Multivariable analysis of factors associated with in-hospital mortality amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from 2003–2008, with bacteremic pneumonia and meningitis separately.
| Risk factor | Bacteremic pneumonia | Meningitis | |||||
|---|---|---|---|---|---|---|---|
| Case-fatality ratio n/N (%) | Adjusted odds ratio (95% confidence interval) | p | Case-fatality ratio n/N (%) | Adjusted odds ratio (95% confidence interval) | p | ||
| Age group (years) | 15–24 | 23/225 (10) | Reference | Reference | 54/141 (38) | Reference | Reference |
| 25–44 | 320/1582 (20) | 2.7 (1.5–5.0) | 0.009 | 421/770 (55) | 1.8 (0.9–4.1) | 0.110 | |
| 45–64 | 175/527 (33) | 6.2 (3.1–12.7) | <0.001 | 145/226 (64) | 7.1 (2.8–18.2) | <0.001 | |
| ≥65 | 58/150 (39) | 11.7 (3.4–40.4) | <0.001 | 21/29 (72) | 8.3 (0.6–108.4) | 0.106 | |
| Pitt bacteremia score | <4 | 401/807 (50) | Reference | ||||
| ≥4 | 240/359 (67) | 2.4 (1.4–4.1) | <0.001 | ||||
| Prior antibiotic use (2 months) | Yes | 27/95 (28) | 2.3 (1.4–3.9) | 0.001 | 22/34 (65) | 5.0 (2.0–12.5) | <0.001 |
| No | 200/1519 (13) | Reference | Reference | 220/577 (38) | Reference | Reference | |
| Appropriate antibiotic prescription | Yes | 397/1976 (20) | Reference | Reference | |||
| No | 179/508 (35) | 2.8 (2.0–3.8) | <0.001 | ||||
| HIV status | Positive | 336/634 (53) | 6.1 (2.1–17.6) | 0.001 | |||
| Negative | 19/73 (26) | Reference | Reference | ||||
| Serotype | 1 | 60/363 (17) | 1.2 (0.6–2.4) | 0.709 | 174/243 (72) | 2.0 (0.8–4.6) | 0.149 |
| 19A | 93/342 (27) | 1.5 (0.8–3.1) | 0.251 | 52/82 (63) | 1.1 (0.4–3.2) | 0.811 | |
| 4 | 29/177 (16) | Reference | Reference | 51/95 (54) | Reference | Reference | |
| 3 | 69/216 (32) | 2.0 (1.0–4.2) | 0.061 | 13/35 (37) | 0.2 (0.02–1.5) | 0.103 | |
| 6A | 31/138 (22) | 0.7 (1.3–2.0) | 0.517 | 50/109 (46) | 0.5 (0.2–1.2) | 0.125 | |
| 14 | 55/184 (30) | 2.2 (1.1–4.7) | 0.039 | 20/47 (43) | 0.5 (0.1–1.8) | 0.267 | |
| 23F | 27/124 (22) | 1.5 (0.6–3.7) | 0.352 | 59/106 (56) | 0.9 (0.2–2.9) | 0.765 | |
| 8 | 14/138 (10) | 0.8 (0.3–2.0) | 0.649 | 32/528 (55) | 0.9 (0.3–3.1) | 0.851 | |
| 19F | 51/116 (44) | 3.5 (1.6–8.0) | 0.002 | 35/64 (55) | 0.7 (0.2–2.5) | 0.567 | |
| 6B | 22/123 (18) | 1.0 (0.4–2.5) | 0.954 | 38/67 (57) | 1.0 (0.4–3.0) | 0.975 | |
| 12F | 16/79 (20) | 1.2 (0.4–3.3) | 0.778 | 38/75 (51) | 0.7 (0.2–2.6) | 0.598 | |
| 9V | 23/93 (25) | 2.0 (0.8–5.2) | 0.153 | 19/36 (53) | 0.2 (0.01–1.4) | 0.230 | |
| 16 | 19/68 (28) | 0.6 (0.2–2.4) | 0.508 | 12/30 (40) | 0.2 (0.03–0.9) | 0.040 | |
| 9N | 17/66 (26) | 1.1 (0.4–3.5) | 0.863 | 6/16 (38) | 0.4 (0.1–4.0) | 0.444 | |
| 7F | 10/56 (18) | 0.9 (0.2–3.5) | 0.868 | 3/14 (21) | 0.4 (0.1–2.1) | 0.271 | |
| 18C | 6/26 (23) | 1.5 (0.4–85.8) | 0.583 | 10/30 (47) | 1.4 (0.3–7.0) | 0.716 | |
| 25 | 7/54 (13) | 0.5 (0.1–2.6) | 0.440 | 3/11 (27) | 0.2 (0.01–2.0) | 0.155 | |
| 22F | 11/43 (26) | 1.6 (0.4–5.6) | 0.498 | 9/17 (53) | 0.5 (0.1–3.0) | 0.409 | |
| 13 | 7/31 (23) | 1.6 (0.5–5.7) | 0.435 | 11/27 (41) | 1.1 (0.3–5.2) | 0.851 | |
| 5 | 9/47 (19) | 0.7 (0.2–3.6) | 0.703 | 2/4 (50) | 0.8 (0.02–36.0) | 0.923 | |
N-number, OR—Odds ratio, CI—confidence interval, HIV—Human immunodeficiency virus. Serotype was retained in the multivariate models a priori because it was the main variable of interest. Only factors statistically significant on multivariable analysis for the syndrome of interest are presented in the table. Additional factors evaluated for patients with bacteremic pneumonia were: (a) factors non-significant on univariate analysis: race, gender, province poverty level, appropriate antibiotic prescription (b) factors significant on univariate analysis but not on multivariable analysis: underlying medical conditions other than HIV, serotype, receipt of antibiotics in the 24 hours preceding culture, nosocomial infection, penicillin non-susceptibility, multidrug resistance. Additional factors evaluated for patients with meningitis were: (a) factors non-significant on univariate analysis: gender, province poverty level, receipt of antibiotics in the 24 hours preceding culture, appropriate antibiotic prescription, nosocomial infection, penicillin non-susceptibility, multidrug resistance (b) factors significant on univariate analysis but not on multivariable analysis: race, underlying medical conditions other than HIV, serotype.
Among patients with meningitis, on multivariable analysis, compared to serotype 4, infection with serotype 16 (OR 0.2, 95% CI 0.03–0.9) was associated with lower in-hospital mortality (Table 3). Additional factors associated with in-hospital mortality on multivariable analysis among patients with meningitis were age group 45–64 years (OR 7.1, 95% CI 2.8–18.2 vs 15–24 years), Pitt bacteremia score ≥4 (OR 2.4, 95% CI 1.4–4.1), history of antibiotic use in the 2 months preceding the IPD episode (OR 5.0 95% CI 2.0–12.5) and being HIV infected (OR 6.1, 95% CI 2.1–17.6).
Factors associated with in-hospital mortality stratified by HIV-infection status
Amongst HIV-infected individuals, on multivariable analysis, compared to serotype 4, infection with serotype 19F (OR 2.6, 95% CI 1.2–5.7) was associated with increased in-hospital mortality (Table 4). Additional factors associated with in-hospital mortality on multivariable analysis among HIV-infected individuals included increasing age group (25–44 years OR 1.9, 95% CI 1.1–3.4; 45–64 years OR 3.6, 95% CI 1.9–68; ≥65 years OR 6.9, 95% CI 1.8–25.9 vs 15–24 years), history of antibiotic use in the 2 months preceding the IPD episode (OR 4.3 95% CI 2.7–7.1), inappropriate antibiotic prescription (OR 2.5, 95% CI 1.8–3.4) and meningitis (OR 4.6 95% CI 3.3–6.0 vs bacteremia). CD4+ T cell count was available for only 1315 of 2309 (57%) HIV-infected individuals, of whom 1031 (45%) were <200 cells/mm3. CFRs were higher in patients with CD4+ T cell count <200 (297/1031, 29%) compared to those with CD4+ T cell count ≥200 cells/mm3 (38/284, 13%, p<0.001). CD4 + T cell count <200 cells/mm3 was not associated with serotype (p = 0.754). Amongst 1744 HIV-infected individuals with available data, 10% (n = 178) reported receiving anti-retroviral therapy (ART). CFRs were similar in individuals receiving (47/178, 26%) and not receiving (354/1566, 23%) ART (p = 0.254).
Table 4. Multivariable analysis of factors associated with in-hospital mortality amongst patients aged ≥15 years with invasive pneumococcal disease (IPD) in South Africa from 2003–2008, for HIV-infected and—uninfected patients separately.
| Risk factor | HIV-infected | HIV-uninfected | |||||
|---|---|---|---|---|---|---|---|
| Case-fatality ratio n/N (%) | Adjusted odds ratio (95% confidence interval) | p | Case-fatality ratio n/N (%) | Adjusted odds ratio (95% confidence interval) | p | ||
| Age group (years) | 15–24 | 48/210 (23) | Reference | 4/44 (9) | Reference | ||
| 25–44 | 482/1688 (29) | 1.9 (1.1–3.4) | 0.025 | 25/118 (21) | 4.7 (1.4–16.4) | 0.015 | |
| 45–64 | 145/384 (38) | 3.6 (1.9–6.8) | <0.001 | 24/84 (29) | 11.8 (3.1–44.6) | <0.001 | |
| ≥65 | 11/27 (41) | 6.9 (1.8–25.9) | 0.004 | 8/25 (32) | 12.9 (2.6–64.6) | 0.002 | |
| Race | Black | 55/214 (26) | Reference | ||||
| Other | 6/56 (11) | 0.2 (0.1–0.5) | 0.002 | ||||
| Pitt bacteremia score | <4 | 37/211 (18) | Reference | ||||
| ≥4 | 24/60 (40) | 3.9 (1.8–8.6) | 0.001 | ||||
| Province poverty level* | Low | 517/1855 (28) | Reference | ||||
| Intermediate | 129/357 (36) | 1.9 (1.3–2.7) | 0.001 | ||||
| High | 41/97 (42) | 1.9 (1.1–3.3) | 0.025 | ||||
| Prior antibiotic use (2 months) | Yes | 43/95 (45) | 4.3 (2.7–7.1) | <0.001 | |||
| No | 232/1383 (17) | Reference | |||||
| Appropriate antibiotic prescription | Yes | 469/1782 (26) | 2.5 (1.8–3.4) | <0.001 | |||
| No | 213/515 (41) | Reference | |||||
| Disease syndrome | Meningitis | 336/634 (53) | 4.6 (3.3–6.0) | <0.001 | 19/73 (26) | 1.6 (0.7–3.7) | 0.226 |
| Bacteremic pneumonia | 317/1550 (20) | Reference | 34/170 (20) | Reference | |||
| Other | 29/113 (26) | 1.6 (0.8–3.0) | 0.001 | 7/27 (26) | 1.7 (0.6–5.3) | 0.349 | |
| Serotype | 1 | 104/317 (33) | 1.7 (0.9–3.2) | 0.104 | 20/78 (26) | 5.0 (0.6–44.6) | 0.151 |
| 19A | 91/283 (32) | 1.7 (0.9–3.4) | 0.098 | 5/22 (23) | 6.2 (0.6–65.5) | 0.132 | |
| 4 | 45/178 (25) | Reference | 2/22 (9) | Reference | |||
| 3 | 30/149 (20) | 0.8 (0.3–1.9) | 0.480 | 7/24 (29) | 7.2 (0.7–73.7) | 0.095 | |
| 6A | 51/174 (29) | 0.6 (0.3–1.3) | 0.200 | 5/16 (32) | 6.5 (0.6–72.1) | 0.128 | |
| 14 | 44/151 (29) | 2.1 (1.0–4.4) | 0.052 | 2/16 (13) | 2.5 (0.2–34.4) | 0.483 | |
| 23F | 69/164 (42) | 1.6 (0.8–3.2) | 0.898 | 3/11 (27) | 7.9 (0.6–103.2) | 0.116 | |
| 8 | 23/112 (21) | 1.1 (0.5–2.6) | 0.726 | 4/21 (19) | 3.5 (0.3–40.1) | 0.317 | |
| 19F | 53/118 (45) | 2.6 (1.2–5.7) | 0.014 | 3/9 (33) | 12.3 (0.9–162.2) | 0.056 | |
| 6B | 36/126 (29) | 1.2 (0.6–2.6) | 0.994 | 1/13 (8) | 2.4 (0.1–45.9) | 0.561 | |
| 12F | 30/101 (30) | 1.0 (0.5–1.9) | 0.907 | 2/4 (50) | 23.9 (1.2–476.5) | 0.037 | |
| 9V | 30/97 (31) | 1.7 (0.7–4.1) | 0.257 | 0/1 (0) | Omitted | ||
| 16 | 16/63 (25) | 0.3 (0.1–1.1) | 0.060 | 3/7 (43) | 28.3 (1.9–414.9) | 0.015 | |
| 9N | 13/54 (24) | 0.7 (0.2–2.7) | 0.716 | 2/7 (29) | 16.3 (1.1–255.8) | 0.047 | |
| 7F | 12/49 (24) | 0.9 (0.3–2.9) | 0.300 | 0/4 (0) | Omitted | ||
| 18C | 12/38 (32) | 1.2 (0.4–3.7) | 0.828 | 1/3 (33) | 7.5 (0.3–198.6) | 0.226 | |
| 25 | 6/43 (14) | 0.7 (0.2–2.9) | 0.645 | 0/1 (0) | Omitted | ||
| 22F | 10/38 (26) | 1.3 (0.3–4.6) | 0.736 | 0/2 (0) | Omitted | ||
| 13 | 9/29 (31) | 2.2 (0.7–6.8) | 0.785 | 1/5 (20) | 2.6 (0.1–78.6) | 0.581 | |
| 5 | 3/25 (12) | 0.2 (0.1–2.9) | 0.267 | 0/5 (0) | Omitted | ||
N-number, OR—Odds ratio, CI—confidence interval, HIV—Human immunodeficiency virus. Only factors statistically significant on multivariable analysis for the syndrome of interest are presented in the table. Serotype was retained in the multivariate models a priori because it was the main variable of interest, and clinical diagnosis because it is an important potential confounder of the association between serotype and mortality.
Amongst HIV-uninfected individuals, on multivariable analysis, compared to serotype 4, infection with serotype 12F (OR 23.9, 95% CI 1.2–176.5), serotype 16 (OR 28.3 95% CI 1.9–414.9) and serotype 9N (OR 16.3 95% CI 1.1–255.8) was associated with increased in-hospital mortality. Additional factors associated with in-hospital mortality among HIV-uninfected individuals included increasing age group (25–44 years OR 4.7, 95% CI 1.4–16.4; 45–64 years OR 11.8, 95% CI 3.1–44.6; ≥65 years OR 12.9, 95% CI 2.6–64.6 vs 15–24 years) and elevated Pitt bacteraemia score (OR 3.9, 95% CI 1.8–8.6).
Individuals aged 15–17 years made up only 1% (51/3953) of included individuals. Sensitivity analysis excluding individuals aged 15–17 years did not change any of the study conclusions (data not shown).
Discussion
We have described extremely high in-hospital mortality in individuals aged ≥15 years with IPD in South Africa, particularly for meningitis, where CFRs exceed 50%. Individual serotypes (1, 14 and 19F) were associated with increased mortality, even after controlling for confounders, suggesting pneumococcal capsule characteristics may be related to virulence. Other important factors associated with increased in-hospital mortality were increasing age and underlying HIV infection. All of the serotypes associated with increased mortality are included in PCV-10 and -13 and use of these vaccines in infants has reduced the burden of these serotypes in children as well as among adults through a herd effect [25, 26].
As has been described in other studies, serotypes causing IPD varied by age group, HIV status and clinical syndrome, all important factors potentially associated with mortality[27, 28]. For this reason, it is important to control for potential confounders when assessing the association between serotype and mortality. We found serotypes 1, 14 and 19F to be significantly associated with mortality, and particularly when presenting with bacteremia. Previous studies have found serotype 19F to be associated with increased CFR [11, 12, 29]. Serotype 14 was also associated with increased mortality in the current study, which has not been previously described. Serotype 1 was the commonest serotype in our study population and we found it to be associated with increased CFR. Some studies have found serotype 1 to be associated with decreased risk of death [11, 12], although increased CFR as found in our study has been observed previously [30]. Previous studies have suggested that serotypes with increased relative risks of death had a higher carriage prevalence and low invasiveness[11]. Serotype 1 has been described as among the more invasive serotypes and serotype 19F among the less invasive serotypes [12, 29, 31, 32].
Differences in the association between serotype and mortality in our study compared to previous studies could be partly as a result of the high HIV-prevalence (89% HIV prevalence amongst included IPD cases) in the population studied. Interestingly, when stratified by HIV-infection status, different serotypes were found to be associated with increased mortality in HIV-infected (serotype 19F) and HIV-uninfected (serotypes 12F, 16 and 9N) individuals. Serotype 1 was not found to be independently associated with increased mortality in either the HIV-infected or -uninfected group, although CFRs were elevated for this serotype in both HIV-infected and—uninfected individuals. Other factors associated with mortality also differed between the two groups. In the HIV-infected subgroup, meningitis was associated with almost five times increased odds of death, but mortality was not significantly elevated in patients with meningitis in the HIV-uninfected group. The overall CFR was extremely high (55%) in patients with meningitis and elevated compared to individuals with bacteremic pneumonia. A study from Malawi, a country with a high population HIV prevalence, found 65% mortality in patients with meningitis compared to 20% for bacteremia[33]. In HIV-infected individuals consumption of antibiotics in the two months preceding hospitalization was predictive of elevated mortality; this likely reflected the severity of underlying HIV-related illness, rather than infection with antibiotic non-susceptible organisms, because strain susceptibility was not related to mortality on multivariable analysis. Residence in intermediate and high poverty level provinces was also associated with increased mortality in HIV infected individuals. This is possibly related to different specimen-taking practices[34], where in less-resourced provinces only the sickest individuals have specimens collected for culture; or due to worse access to care or quality of care, although we do not have data to support this hypothesis.
We found HIV-infected individuals to have an overall two times greater odds ratio of in-hospital death compared to HIV-uninfected individuals. When stratified by syndrome, this association was only seen in patients with meningitis (who experience six times increased odds of death) but not in patients with bacteremic pneumonia. This difference in mortality association by clinical syndrome is similar to what was found in a study of IPD in patients aged <15 years from South Africa in the pre-PCV era[35]. Published data on the association between HIV infection and mortality vary, with some studies describing increased mortality in HIV-infected individuals and others not finding this association[36–39]. These varying findings could result from different populations studied including differing clinical syndromes included, different proportions of patients receiving ART as well as limited statistical power to detect an association in some studies.
Studies of the association between serotype and mortality have varied in the patient populations included, with some studies including only patients with bacteremic pneumonia and others including all IPD[11, 29, 40]. When stratified by clinical syndrome, amongst patients with bacteremic pneumococcal pneumonia, serotype 19F remained associated with increased mortality, while no serotype was associated with increased mortality in patients with meningitis. This suggests that the association between serotype and mortality may vary depending on the clinical syndrome evaluated.
Other factors associated with increased mortality included increasing age group, which has been described in several studies, most likely as a result of immunosenescence[41–44]. Notably, the numbers of cases aged ≥65 years was low, possibly as a result of reduced health seeking in this age group as well as the relatively younger population than many high income countries and the relatively low life expectancy in South Africa[45, 46]. In contrast to previous studies, we did not find underlying illnesses (other than HIV) to be associated with increased in-hospital mortality. This could reflect poor diagnosis and documentation of underlying illness data in our setting or reflect the relatively smaller contribution of underlying illness to in-hospital outcome in this predominantly HIV-infected population[5–7].
Our study had some limitations. Only patients admitted to enhanced surveillance sites with viable isolates were included. Patients at enhanced sites differed from those presenting to non-enhanced sites with regard to a number of characteristics and this could potentially have introduced bias. Because of the nature of laboratory-based surveillance, only patients who presented to a healthcare facility and who had specimens taken could be included; these individuals may not be fully representative, especially in the poorer provinces. We examined a large number of serotypes individually, which may have reduced our power to detect significant associations, however we did have data on almost 4000 individuals and >1000 deaths. The ability to detect a significant association with mortality for different serotypes could have been affected by available numbers of cases allowing insufficient power to detect statistically significant associations in some cases. Some previous studies did not assess severity association for individual serotypes but rather grouped them, limiting the ability to compare with our findings [40]. We elected not to group serotypes for the mortality analysis as several different grouping have been used in the literature, and all groupings are somewhat arbitrary and could introduce spurious associations[11, 29, 40]. We included individuals aged ≥15 years in our study, although a cut-off of ≥18 years is more standard for adults. We did this because in South Africa 15 years is the age at which patients are generally admitted to adult rather than pediatric wards in South Africa. Importantly, individuals aged 15–17 years only made up 1% of the study population and study conclusions remained unchanged on sensitivity analysis excluding this group of individuals. CD4+ T cell count data was only available for 56% of HIV-infected patients and we therefore did not include it in the analysis of factors associated with mortality; CD4+ T cell count, however, was not associated with serotype and it is therefore unlikely to be a confounder of the association between serotype and mortality.
In conclusion, we have found that individual serotypes, included in PCV-13, were associated with increasing mortality in the pre-PCV era in South Africa, even controlling for potential confounding. HIV infection was also an important risk factor for death in our study, especially among patients with meningitis and older individuals. Studies of the association of pneumococcal serotype and mortality, following widespread PCV introduction and possible serotype replacement, may be informative.
Acknowledgments
Members of the GERMS-SA surveillance network are: Sandeep Vasaikar, Vivek Bhat (Eastern Cape); Peter Smith, Anne-Marie Pretorius (Free State); Pyu-Pyu Sein, Anwar Hoosen, Ruth Lekalakala, Olga Perovic, Charles Feldman, Alan Karstaedt, Kathy Lindeque, Jeannette Wadula (Gauteng); Halima Dawood, Sindisiwe Sithole, Sumayya Haffajee, Yacoob Coovadia (KwaZulu Natal); Greta Hoyland, Jacob Lebudi (Mpumalanga) Stan Harvey, Pieter Jooste (Northern Cape) Andrew Rampe (North West) Elizabeth Wasserman, Andrew Whitelaw, Siseko Martin (Western Cape); Ken Hamese (Limpopo)Adrian Brink, Inge Zietsman, Suzy Budavari, Xoliswa Poswa (Ampath laboratories), Keshree Pillay, Jaunita Smit (Lancet laboratories), Marthinus Senekal (PathCare); Anne Schuchat, Stephanie Schrag (CDC); Keith Klugman, Anne von Gottberg, Linda de Gouveia, Karen Keddy, Arvinda Sooka, John Frean, Leigh Dini, Nelesh Govender, Jay Patel, Vanessa Quan, Cheryl Cohen, Susan Meiring, Penny Crowther, Desiree du Plessis (NICD)
Institution where work was done: Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases, Private Bag X4, Sandringham, 2131, Gauteng, South Africa.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Requests for reprints may be addressed to: Cheryl Cohen, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases, Private Bag X4, Sandringham, 2131, Gauteng, South Africa, Telephone: 27 11 386 6593. Fax: 27 11 882 9979. E-mail: cherylc@nicd.ac.za
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by funding from the National Institute for Communicable Diseases, a division of the National health Laboratory Service, and was supported in part by funds from the United States Agency for International Development’s Antimicrobial Resistance Initiative, transferred via a cooperative agreement (number U60/CCU022088) from the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; and cooperative agreement U62/CCU022901 from the CDC. (The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.)
References
- 1. Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet. 2006;368(9541):1048–50. [DOI] [PubMed] [Google Scholar]
- 2. Ostergaard C, Konradsen HB, Samuelsson S. Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according to the focus of infection. BMC Infect Dis. 2005;5:93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Black S. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. J Pediatr. 2003;143(5):688–9. . [PubMed] [Google Scholar]
- 4. Rodgers GL, Klugman KP. The future of pneumococcal disease prevention. Vaccine. 2011;29 Suppl 3:C43–8. 10.1016/j.vaccine.2011.07.047 . [DOI] [PubMed] [Google Scholar]
- 5. Feikin DR, Schuchat A, Kolczak M, Barrett NL, Harrison LH, Lefkowitz L, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90(2):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moroney JF, Fiore AE, Harrison LH, Patterson JE, Farley MM, Jorgensen JH, et al. Clinical outcomes of bacteremic pneumococcal pneumonia in the era of antibiotic resistance. Clin Infect Dis. 2001;33(6):797–805. [DOI] [PubMed] [Google Scholar]
- 7. Yu VL, Chiou CC, Feldman C, Ortqvist A, Rello J, Morris AJ, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37(2):230–7. [DOI] [PubMed] [Google Scholar]
- 8. Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–54. [DOI] [PubMed] [Google Scholar]
- 9. Gilbert K, Fine MJ. Assessing prognosis and predicting patient outcomes in community-acquired pneumonia. Semin Respir Infect. 1994;9(3):140–52. . [PubMed] [Google Scholar]
- 10. Henriques B, Kalin M, Ortqvist A, Olsson Liljequist B, Almela M, Marrie TJ, et al. Molecular epidemiology of Streptococcus pneumoniae causing invasive disease in 5 countries. J Infect Dis. 2000;182(3):833–9. 10.1086/315761 . [DOI] [PubMed] [Google Scholar]
- 11. Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, et al. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis. 2010;51(6):692–9. 10.1086/655828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen AG, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, et al. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49(2):e23–9. 10.1086/600045 . [DOI] [PubMed] [Google Scholar]
- 13. Shisana O, Rehle T, Simbayi L, Zuma K, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: HSRC Press; 2014. [DOI] [PubMed] [Google Scholar]
- 14.National Department of Health. The National Antenatal Sentinel HIV and Syphillis Prevalence Survey, South Africa, 2011. National Department of Health. 2012.
- 15. Nunes MC, Madhi SA. Safety, immunogenicity and efficacy of pneumococcal conjugate vaccine in HIV-infected individuals. Hum Vaccin Immunother. 2012;8(2):161–73. 10.4161/hv.18432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huebner RE, Klugman KP, Matai U, Eggers R, Hussey G. Laboratory surveillance for Haemophilus influenzae type B meningococcal, and pneumococcal disease. Haemophilus Surveillance Working Group. S Afr Med J. 1999;89(9):924–5. . [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816–9. . [PubMed] [Google Scholar]
- 18. Baddour LM, Yu VL, Klugman KP, Feldman C, Ortqvist A, Rello J, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440–4. [DOI] [PubMed] [Google Scholar]
- 19. Paterson DL, Ko WC, Von GA, Mohapatra S, Casellas JM, Goossens H, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39(1):31–7. [DOI] [PubMed] [Google Scholar]
- 20.Satistics South Africa. Subjective Poverty in South Africa. Findings of the living conditions survey; 2008–2009. 2009.
- 21. du Plessis M, von Gottberg A, Madhi SA, Hattingh O, de Gouveia L, Klugman KP. Serotype 6C is associated with penicillin-susceptible meningeal infections in human immunodeficiency virus (HIV)-infected adults among invasive pneumococcal isolates previously identified as serotype 6A in South Africa. Int J Antimicrob Agents. 2008;32 Suppl 1:S66–70. Epub;%2008 Aug 23.:S66-S70. [DOI] [PubMed] [Google Scholar]
- 22. CLSI. Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement Clinical and Laboratory Standards Institute (CLSI)(Formerly NCCLS). 2012;Wayne, Pennsylvania:CLSI. [Google Scholar]
- 23. National Department of Health. Standard Treatment Guidelines and Essential Drugs List for South Africa Hospital Level Adults. National Department of Health, Pretoria, South Africa: 2006. [Google Scholar]
- 24. Modelling Multinomial Data In: Dohoo I, Martin WJ, Stryhn H, editors. Veterinary Epidemiologic Research. 1 ed Charlottetown, PE, Canada: Atlantic Veterinary College,; 2003. p. 378–9. [Google Scholar]
- 25. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. [DOI] [PubMed] [Google Scholar]
- 26. von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371(20):1889–99. 10.1056/NEJMoa1401914 . [DOI] [PubMed] [Google Scholar]
- 27. Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30(1):122–40. [DOI] [PubMed] [Google Scholar]
- 28. Buie KA, Klugman KP, von Gottberg A, Perovic O, Karstaedt A, Crewe-Brown HH, et al. Gender as a risk factor for both antibiotic resistance and infection with pediatric serogroups/serotypes, in HIV-infected and -uninfected adults with pneumococcal bacteremia. J Infect Dis. 2004;189(11):1996–2000. [DOI] [PubMed] [Google Scholar]
- 29. Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6(5):e1000081 10.1371/journal.pmed.1000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martens P, Worm SW, Lundgren B, Konradsen HB, Benfield T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect Dis. 2004;4:21 10.1186/1471-2334-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190(7):1203–11. 10.1086/423820 . [DOI] [PubMed] [Google Scholar]
- 32. Sandgren A, Sjostrom K, Olsson-Liljequist B, Christensson B, Samuelsson A, Kronvall G, et al. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis. 2004;189(5):785–96. 10.1086/381686 . [DOI] [PubMed] [Google Scholar]
- 33. Gordon SB, Chaponda M, Walsh AL, Whitty CJ, Gordon MA, Machili CE, et al. Pneumococcal disease in HIV-infected Malawian adults: acute mortality and long-term survival. AIDS. 2002;16(10):1409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheyip M, Cohen C, Von Gottberg A, Govender N, Keddy K, GERMS. Collection rates for blood culture and cerebrospinal fluid specimens and estimated burden of disease due to invasive respiratory, meningeal and enteric bacterial pathogens in South Africa. University of the Witwatersrand School of Public Health Annual Research Day, Johannesburg, South Africa: 2007. [Google Scholar]
- 35. Nyasulu P, Cohen C, de GL, Feldman C, Klugman KP, von GA. Increased risk of death in human immunodeficiency virus-infected children with pneumococcal meningitis in South Africa, 2003–2005. PediatrInfectDisJ. 2011;30(12):1075–80. [DOI] [PubMed] [Google Scholar]
- 36. Feldman C, Klugman KP, Yu VL, Ortqvist A, Choiu CC, Chedid MB, et al. Bacteraemic pneumococcal pneumonia: impact of HIV on clinical presentation and outcome. J Infect. 2007;55(2):125–35. [DOI] [PubMed] [Google Scholar]
- 37. Karstaedt AS, Khoosal M, Crewe-Brown HH. Pneumococcal bacteremia in adults in Soweto, South Africa, during the course of a decade. Clin Infect Dis. 2001;33(5):610–4. [DOI] [PubMed] [Google Scholar]
- 38. Frankel RE, Virata M, Hardalo C, Altice FL, Friedland G. Invasive pneumococcal disease: clinical features, serotypes, and antimicrobial resistance patterns in cases involving patients with and without human immunodeficiency virus infection. Clin Infect Dis. 1996;23(3):577–84. . [DOI] [PubMed] [Google Scholar]
- 39. Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132(3):182–90. . [DOI] [PubMed] [Google Scholar]
- 40. van Hoek AJ, Andrews N, Waight PA, George R, Miller E. Effect of serotype on focus and mortality of invasive pneumococcal disease: coverage of different vaccines and insight into non-vaccine serotypes. PLoS One. 2012;7(7):e39150 10.1371/journal.pone.0039150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–208. 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ewig S, Birkner N, Strauss R, Schaefer E, Pauletzki J, Bischoff H, et al. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64(12):1062–9. 10.1136/thx.2008.109785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kothe H, Bauer T, Marre R, Suttorp N, Welte T, Dalhoff K, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J. 2008;32(1):139–46. 10.1183/09031936.00092507 . [DOI] [PubMed] [Google Scholar]
- 44. Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(8):910–4. 10.1164/rccm.200310-1448OC . [DOI] [PubMed] [Google Scholar]
- 45. Cohen C, Walaza S, Moyes J, Groome M, Tempia S, Pretorius M, et al. Epidemiology of Viral-Associated Acute Lower Respiratory Tract Infection among Children <5 Years of Age in a High HIV Prevalence Setting, South Africa, 2009–2012. Pediatr Infect Dis J. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Statistics South Africa. Census 2011 Statistical release- P0301.4. Statistics South Africa. 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.