Abstract
The brain has a limited capacity and therefore needs mechanisms to selectively enhance the information most relevant to one’s current behavior. We refer to these mechanisms as ‘attention’. Attention acts by increasing the strength of selected neural representations and preferentially routing them through the brain’s large-scale network. This is a critical component of cognition and therefore has been a central topic in cognitive neuroscience. Here we review a diverse literature that has studied attention at the level of behavior, networks, circuits and neurons. We then integrate these disparate results into a unified theory of attention.
Introduction
Over 125 years ago, William James defined attention as the “taking possession by the mind … of one out of what seem simultaneously possible objects or trains of thought” (James, 1890). James’ intuitive understanding of attention is remarkably close to our modern definition: attention is the selective prioritization of the neural representations that are most relevant to one’s current behavioral goals. Such prioritization is necessary because the brain is a limited capacity information system. Representations of external stimuli and internal thoughts compete for access to these limited processing resources, and attention helps to resolve that competition in favor of the information that is currently task-relevant.
Attention research has been central to the fields of cognitive neuroscience, psychology and systems neurophysiology. This has led to the discovery of a large number of attention effects at each of these levels of observation. In the first three sections, we briefly review this literature, highlighting key insights at the behavioral, network, and neuronal levels. Our goal for this review is to integrate these disparate findings into a single unified framework, which we outline in the fourth section.
We should note that we will largely constrain our review to visual attention, as it has been the best studied. We acknowledge the importance of extending our understanding to other sensory modalities and to interactions between modalities and we hope the knowledge gained from understanding visual attention will reveal principles of neural processing that may be fundamental to cognition more generally.
Furthermore, even though attention is often studied in isolation, a mechanism that prioritizes task-relevant information will likely interface with many cognitive domains such as action control and decision making, motivation and emotions, memories at different time scales, and awareness. We will review our current knowledge of some of these interactions in the last section. Understanding the interaction of selective attention with other cognitive domains will ultimately lay the foundation for reaching a cohesive understanding of the general principles of cognition and their associated neural mechanisms (2014).
Behavioral effects - Building blocks and shifting concepts
Classical attention paradigms
The two most commonly used paradigms to study visual attention are visual spatial orienting (Posner et al., 1980) and visual search (Treisman and Gelade, 1980).
In spatial orienting tasks, subjects are instructed by a predictive cue to direct attention to a particular spatial location where they must detect or discriminate a target stimulus. The classic finding is that subjects benefit from the cue as they respond faster and more accurately to stimuli occurring at the cued location than to stimuli occurring at other locations. This facilitation comes at the expense of other objects in the visual environment, reflecting the competitive nature of attention.
While orienting tasks typically involve only a single target stimulus, visual search tasks more closely relate to our everyday experience, where we typically face cluttered scenes. In search tasks, subjects are given an array of stimuli and asked to find a particular target stimulus defined by one or more features in the array (e.g. find the green ‘T’ in an array of green and blue ‘T’s and ‘L’s; see Figure 1A). Hence, in visual search, the selection process is informed by features of the target (i.e. feature-based attention), which then guides spatial attention.
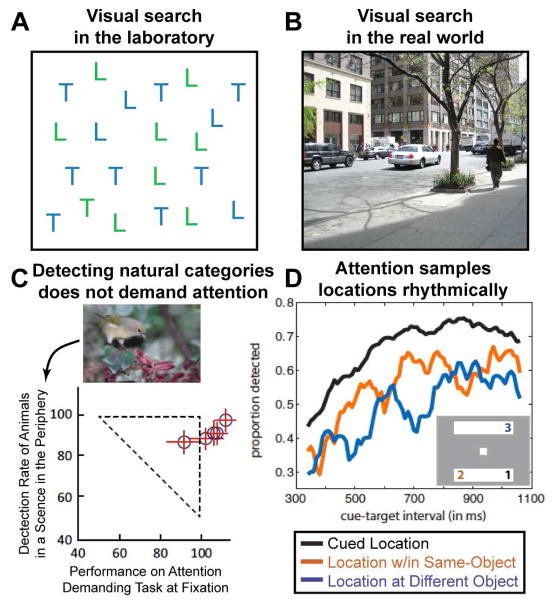
Figure 1. Behavioral studies.
(A) Visual search in artificial displays versus in real-world scenes. Detecting the presence of a green T (conjunction search) is effortful and time-consuming, such that reaction times increase as a function of display items. (B) In contrast, detecting the presence of categorical object information such as ‘people’ or ‘cars’ in real-world scenes requires only a single glance, despite the large number and variety of distracter objects. (C) The detection of animals or vehicles in natural scenes does not require focused spatial attention. In a dual-task paradigm, subjects performed a central discrimination task, while detecting animals in scenes presented in the periphery. Performance is normalized to a condition when only a single task was performed. Performance on the peripheral animal detection task was only mildly impaired by simultaneously performing the central discrimination task. Adapted from (Li et al., 2002). (D) Selective attention has rhythmic properties. Subjects detected the dimming of a part of a rectangular object at a spatially cued (black line; location #1 in the two object display depicted as an example), or at an uncued location of the same object than the cued location (orange line; location #2), or a different object (blue line; location #3). Accuracy is plotted as a function of the cue-target interval revealing the following rhythmic properties: at the cued location, detection performance fluctuated at 8Hz, whereas at the same- and different-object locations a characteristic anti-phase relationship of a 4Hz rhythm was observed. Adapted from (Fiebelkorn et al., 2013).
Performance on visual search tasks is affected by how many features the target shares with other stimuli in the array. If the target has a unique feature, such as being of different color from the distracters, the search is completed quickly and effortlessly, regardless of the number of elements in the array. This phenomenon is known as ‘pop-out’ or efficient (parallel) search. However, just by changing the distractors in the search array, the search for the same target can be made much more difficult. For example, if the target is defined by a conjunction of features that each are shared by distracters (as in Figure 1A), search time increases as a function of the number of elements in the array. This is known as inefficient search and the increase in search times is thought to reflect a serial target search, which is mediated by a spatial ‘spotlight’ mechanism that can shift from location-to-location about every 50 ms (Buschman and Miller, 2009; Wolfe et al., 2011). However, under some circumstances, only a subset of the array needs to be searched. Simple features, such as color, can be used to guide search to just those elements that share a particular target feature (Wolfe et al., 1989). Search difficulty also depends on the similarity of the target to the distracters and to the dissimilarity of the distracters to each other (Duncan and Humphreys, 1989).
The results of studies using classical attention paradigms have shaped our current theoretical concepts and have been foundational for investigations at the neural level that we will review below. However, attention mechanisms have evolved to function in real-world scenarios. Recently, there has been a growing number of studies that have asked whether the knowledge and concepts gained from simplified laboratory conditions translate to more ecologically relevant situations.
Real-world visual search
An important first step to investigate attentional prioritization under more naturalistic conditions has been to study the selection of categorical object information from natural scenes (for an in depth review, see Peelen and Kastner, 2014). In daily life, we select meaningful objects from meaningful scenes such as looking for cars when crossing a street. What would be the behavioral prediction for detecting a car in the scene of Figure 1B based on classic search paradigms? Typical scenes contain dozens of distracter objects with highly variable appearance, and there is not one feature that uniquely defines a target. Based on classical attention theories one would predict a long response time reflecting a particularly inefficient search. However, the opposite is the case. The detection of familiar object categories in scenes is extremely rapid (Thorpe et al., 1996) and search is highly efficient – adding additional items to a scene has little cost (Wolfe et al., 2011). Furthermore, one can accurately perform such real-world search tasks while simultaneously performing a second, demanding attention task at fixation (Figure 1C; Li et al., 2002). This suggests that real-world search of object categories does not require focused spatial attention.
Neuroimaging studies in humans have begun to investigate the neural basis of real-world search by having subjects detect the presence of objects from a target category in briefly presented photographs (Peelen and Kastner, 2011; Peelen et al., 2009), or short movie segments (Çukur et al., 2013). It was found that the pattern of neural activity in object-selective cortex evoked by the scenes fully depended on task-relevance: target objects embedded in natural scenes were only represented when one was actively searching for them. Responses in many parts of the brain increased with the appearance of a stimulus in the target category, or a semantically similar category, suggesting that category-based attention may have widespread influences on brain activity. Together, these results provide neural evidence that the attentional selection mechanism that biases the processing of scenes acts at the level of natural categories. Future work is needed to extend our traditional concepts of attention to incorporate mechanisms that are optimized for naturalistic conditions. Key to this will be the development of appropriate paradigms in animal models in order to study the underlying neural mechanisms in greater detail.
Rhythmic properties of selective attention
Classic attention theories (Posner et al., 1980; Treisman and Gelade, 1980) propose a unique and indivisible ‘spotlight’ of attention that highlights a selected item. To process an entire scene, this spotlight was thought to be continuously moving from location-to-location, shifting at a rate of approximately 20 Hz (Wolfe et al., 2011). Previous studies suggested that this shifting may be regular, moving the spotlight of attention in a rhythmic fashion around a visual scene (Buschman and Miller, 2009). Surprisingly, recent evidence shows that even when this spotlight is sustained at one location, it is not static, but rather appears to flash rhythmically. Using EEG, Busch and VanRullen (2010) demonstrated that the detection of a visual target at threshold was systematically related to the phase of an ongoing theta oscillation (~7 Hz). This phase-behavior relationship was contingent on the allocation of attentional resources following a cue and was absent at other locations in the visual field. The cue served not only to guide the deployment of attention, but caused the timing of the high- and low-excitability states of the oscillation to align across trials (see also Lakatos et al., 2009). Thus, it appears that the selection mechanism periodically samples the attended location, with the degree of selection fluctuating with the phase of the neural rhythm. Intriguingly, recent behavioral studies suggest that there may be at least two concurrent spatial mechanisms: the first is the ‘classic’ focusing of attention at a selected location, while the second mechanism rhythmically monitors other locations outside this focus (Figure 1C; Fiebelkorn et al., 2013; Landau and Fries, 2012). Such rhythmic monitoring of other locations and objects may be an important mechanism for flexibly gating the reallocation of attentional resources. It is important to note that the rhythmic monitoring appears to be an automatic process that is distinct from voluntarily splitting or dividing attentional resources across multiple locations. Together, these findings suggest that selective attention falls into the class of rhythmic behaviors and is a highly dynamic and flexible resource. The neural basis of the rhythmic properties of selective attention is unclear and awaits future investigation.
Studies based on careful observations of behavior have provided the foundation not only for theoretical accounts of selective visual processing, but also for the investigations that are aimed at revealing its underlying neural mechanisms, as we will discuss next.
Network effects – from functional anatomy to dynamic connectivity
In the primate brain, attentional selection is mediated by a large-scale network of regions, including the frontal, parietal, temporal and occipital cortex as well as thalamic and midbrain regions (Corbetta and Shulman, 2002; Ungerleider and Kastner, 2000). In this section, we will review the functional anatomy of the primate attention network, and its major dissociations of function. We will particularly focus on dynamic network interactions that ultimately drive the selection process and its associated specific behavior. This is not a perceptual deficit as subjects will respond if competing stimuli from the unaffected hemifield are removed.
Defining the visual attention network
Early evidence that attentional selection involves a distributed large-scale network comes from neuropsychological studies of human patients showing that unilateral brain lesions, especially of higher-order cortex, may cause impairment in spatially directing attention to the contralateral hemifield. This syndrome is known as visuospatial hemineglect. In severe cases, patients suffering from neglect will completely disregard the visual hemifield contralateral to the side of the lesion (e.g. Bisiach and Vallar, 1988). This leads to deficits in everyday behaviors; patients will read from only one side of a book, apply make-up to only one half of their face, or eat from only one side of a plate.
Visuospatial neglect may follow unilateral lesions at different sites, including most frequently the temporo-parietal junction (Mort et al., 2003) and superior temporal cortex (e.g. Karnath et al., 2001). Neglect is also, but less frequently observed following damage of the frontal lobe (e.g. Damasio et al., 1980), the anterior cingulate cortex (e.g. Janer and Pardo, 1991), other sites in parietal cortex such as the superior parietal lobule (Kenzie et al., 2015), the basal ganglia (e.g. Damasio et al., 1980), and the thalamus, in particular the pulvinar (e.g. Karnath et al., 2002). The syndrome is not confined to cortical lesions, but can also result from white matter lesions that affect structural connections between nodes of the attention network (Lunven et al., 2015). Importantly, neglect occurs more often with right-sided lesions than with left-sided lesions, which has been taken as evidence for a specialized role of the right hemisphere in attentional selection. This observed hemispheric asymmetry led to the ‘hemispatial’ theory which proposes that the right hemisphere directs attention to both visual hemifields, whereas the left hemisphere directs attention to the right visual field only (Heilman and Van Den Abell, 1980). Thus, while left hemispheric damage can be compensated for by the right hemisphere, such compensation will not be possible with right hemispheric damage, thereby resulting in neglect of the left visual field.
Human neuroimaging studies of the intact brain have provided a more detailed account of the neuroanatomy of the attention network. When subjects attend to a location in space in anticipation of the appearance of a stimulus, neural signals increase in a fronto-parietal network consisting of regions within the superior parietal lobule (SPL), the intraparietal sulcus (IPS), the frontal eye field (FEF), and the supplementary eye field (SEF; see Figure 2A for full map). This dorsal fronto-parietal attention network has been implicated in many visuospatial tasks, regardless of whether target stimuli were detected, discriminated or tracked in visual space (Ungerleider and Kastner, 2000) and regardless of whether the task required spatial attention, spatial working memory, or planning saccades (Jerde et al., 2012).
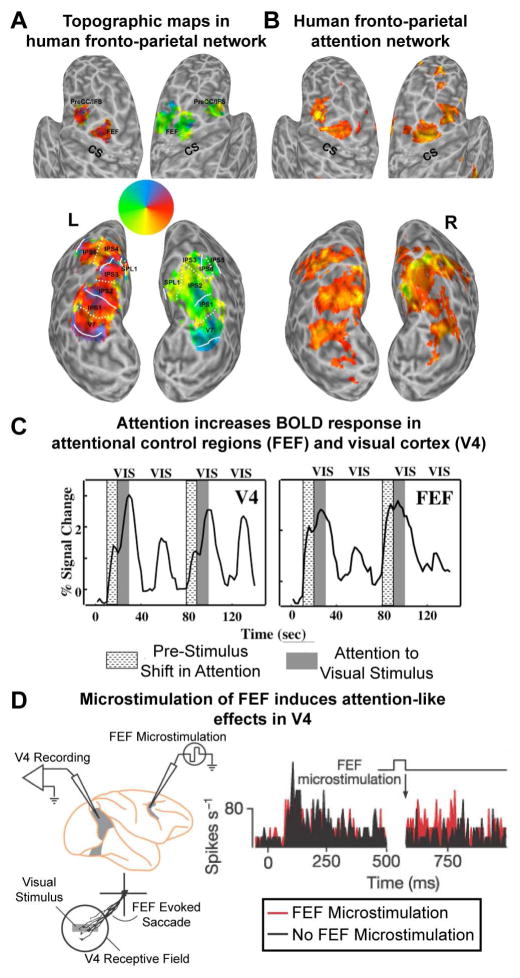
Figure 2. Fronto-parietal control of attentional selection.
(A) Topographic organization of areas in human frontal and parietal cortex. Using a memory-guided saccade task several areas with a systematic representation of the contralateral visual field were identified along the intraparietal sulcus (IPS0-5), adjacent superior parietal cortex (SPL1), and in superior (FEF) and inferior aspects of precentral cortex. Adapted from (Silver and Kastner, 2009). (B) Attention-related activations within parietal and frontal cortex in a spatial attention task. There is significant overlap between attention-related activations and topographic representations in higher-order cortex. Adapted from (Szczepanski et al., 2010). (C) Time series of fMRI signals in V4 and FEF. Directing attention to a peripheral target location in the absence of visual stimulation led to an increase of baseline activity (textured blocks), which was followed by a further increase after the onset of the stimuli (gray shaded blocks) in V4, but not in FEF, where the initially stronger baseline increase was sustained, thus reflecting the attentional operations of the task and not sensory processing. Adapted from (Kastner et al., 1999) (D) Microstimulation of FEF (below the threshold that evokes an eye movement) induces attention-like increases in the spikes/s of V4 neurons with receptive fields that overlap the FEF site (black is baseline; red with microstimulation). Adapted from (Moore and Armstrong, 2003).
The fronto-parietal attention network is also activated when subjects select non-spatial information. In studies of feature-based attention, similar activations have been found when subjects shift attention from one feature to another (e.g. from color to direction of motion in a display of colored, moving dots; (Greenberg et al., 2010), or when subjects shift attention between two spatially overlapping objects and perform object-based selections (Serences et al., 2004). Together, these studies suggest that the fronto-parietal network is a ‘domain-general’ controller without much functional specialization. However, it is not clear whether distributed subpopulations within this network subserve specific functions needed for space-, feature-, or object-based attentional control. The different neural mechanisms associated with the different selection modes (as described below in our theory section) may suggest such a functional organization.
It is important to note that the fronto-parietal network consists of a large number of topographically organized areas that coordinate their functional operations (Figure 2A). Thus far, nine topographically organized areas have been found in posterior parietal and frontal cortex, each containing a continuous representation of the contralateral visual field (for review, Silver and Kastner, 2009). The delineation of topographic organization in higher-order cortex in individual subjects has permitted a more systematic study of the dorsal attention network in the human brain.
In line with the topographic organization, spatial attention increased responses more strongly when directed contra- rather than ipsilaterally (Szczepanski et al., 2010), thus generating a contralateral spatial biasing signal in each topographic region. The sum of the biasing signals across areas was of similar magnitude across the two hemispheres, suggesting a balanced spatial control system in the intact brain. Thus, visual space within a hemifield appears to be largely controlled by the contralateral hemisphere, contradicting the hemspatial theory of attention control. Instead, these studies have provided evidence for an alternative model of neglect, first proposed by Kinsbourne (1977). According to this ‘interhemispheric competition account’, spatial attention uses an opponent processor control system, in which each hemisphere directs attention towards the contralateral visual field. In an intact system, the two hemispheric processors are balanced through mutual reciprocal inhibition, possibly through direct callosal connections, or through cortico-subcortical interactions of parietal cortex and superior colliculus. The interhemispheric competition account of attention control has received further empirical support in transcranial magnetic stimulation studies showing that attentional biasing signals can be altered in predictable ways by perturbing the frontoparietal control system (Szczepanski and Kastner, 2013).
While Kinsbourne’s original model was not able to account for the right hemispheric dominance observed with the neglect syndrome, the functional brain imaging studies in the intact brain have shown several asymmetries in the strengths of attentional biasing signals across the nodes of the dorsal attention network (Sczcepanski et al., 2010). These asymmetries can theoretically account for the observed right hemispheric dominance. Further support for the Kinsbourne model comes from clinical studies in patients suffering from hemi-neglect following a stroke to the right superior temporal cortex, who show reduced activity in the right relative to the left dorsal parietal attention network, even though these brain regions are structurally intact (Corbetta et al., 2005). Thus, the attentional deficits observed in these patients may be explained by a distal impact of the lesion. This results in an imbalance of attentional biasing signals generated by each hemisphere and, thus, an imbalance in the ability to control contralateral space. This imbalance is also accompanied by a breakdown of functional connectivity within the dorsal network between the two hemispheres (He et al., 2007).
Functional dissociations of the network
Thus far we have highlighted the distributed nature of attentional processing, which is mediated by the strongly interconnected anatomy of the brain, thereby ensuring that any information is quickly shared between regions. In this framework, computations and behavior do not arise from a single brain region but rather emerge through interactions between regions. However, this does not imply that each brain region does exactly the same computation. There are important functional dissociations that can be drawn between regions.
One broad functional dissociation that has been made is that higher-order fronto-parietal cortex acts as the ‘source’ of modulatory attention-related signals that are fed back to sensory cortex. This dissociation was observed in early human neuroimaging studies showing that when attention was directed to the location of an upcoming stimulus, activity in frontal and parietal cortex was sustained relative to activity in visual cortex, reflecting the attentional operations of the task and not sensory processing (Figure 2C; Kastner et al., 1999). To understand the different contributions of frontal and parietal cortex in controlling attention, we will now turn to electrophysiological studies in non-human primates.
The large-scale fronto-parietal attention network seen in humans is generally conserved in non-human primates. For spatial selection, important parts of the network include frontal cortex (lateral prefrontal, lPFC, and the frontal eye fields, FEF) as well as a region within the intraparietal sulcus (lateral intraparietal area, LIP). In addition, a recent neuroimaging study has shown evidence for a role of medial posterior parietal cortex including areas V6 and V6A in mediating dynamic shifts of attention across the visual field (Premereur et al., 2015). Shifts in attention are reflected in single neuron responses in all of these regions (e.g. FEF, Bichot and Schall, 1999; LIP, Bisley and Goldberg, 2003).
What then distinguishes these regions? To answer this question, Buschman and Miller (2007) used large-scale, multiple electrode recording techniques to simultaneously record the activity of neurons in lPFC, FEF, and LIP. They found that, when a monkey’s attention was externally captured by a salient stimulus (i.e. by a ‘pop-out’ stimulus, see above), this was reflected first in LIP neurons and then in FEF neurons, suggesting a flow of information from parietal to frontal cortex. In contrast, when attention was internally directed by the memory of the target stimulus (i.e. during a conjunction search), such voluntary control of attention originated in frontal cortex, and information flowed back to parietal cortex. Similar results have recently been found in humans (Li et al., 2010).
These results suggest that frontal and parietal cortex play different roles in guiding attention. First, parietal cortex (LIP) encodes a ‘saliency’ map of the visual scene, encoding which locations in space are of potentially high significance. Such saliency is largely defined by the properties of the stimuli. Consistent with this model, LIP neurons will respond to a highly salient, transiently flashed stimulus (Bisley and Goldberg, 2006) and encode the saliency of stimuli in a visual scene (Arcizet et al., 2011). In contrast, neurons in frontal cortex carry information about task-relevant stimuli, not necessarily the most salient stimulus (Hasegawa et al., 2000). Furthermore, inactivating lateral PFC disrupts tasks requiring top-down, internal direction of attention (Iba and Sawaguchi, 2003).
Further evidence that prefrontal cortex is the source of top-down signals comes from the work of Moore and colleagues, who found that electrical stimulation of the frontal eye fields (FEF) can induce attention-like effects. Stimulation of FEF increases the animal’s behavioral discriminability at the location of the FEF receptive fields, as if attentional resources had been directed there (Moore and Fallah, 2004). Furthermore, attention-like effects were observed in V4 neurons whose receptive fields overlapped with the stimulated FEF neurons (Figure 2D; Moore and Armstrong, 2003). Causal manipulations in humans using TMS have corroborated these findings by showing qualitatively similar effects (Ruff et al., 2006).
Dynamic functional connectivity
Despite these functional dissociations, it is clear that the fronto-parietal network works as a cohesive unit to direct attention based on a multitude of factors. This then raises the question – how can one network dynamically adapt to changing requirements as the situation or goals change? More globally, how might the fronto-parietal network induce attention by biasing connections throughout the brain? This isn’t likely due to anatomical changes; changes in behavior simply happen too quickly. Instead, changes in the effective connectivity between interconnected regions allows for the large-scale network to adapt as needed.
Changing the synchrony of neurons is one mechanism that may modulate effective connectivity. Theoretical and experimental work has shown that increasing the synchrony of inputs into a single neuron has a super-additive effect (Azouz and Gray, 2000; Salinas and Sejnowski, 2001). Therefore, modulating the synchrony of a population of neurons will dynamically change their downstream impact. Therefore, one way to increase the strength of an attended stimulus would be to increase the synchrony of neurons representing that stimulus. Early experimental support for such a model came from the somatosensory system, where Steinmetz and colleagues (2000) found that attending to tactile stimuli increased the synchrony of neurons. Studies on visual attention showed that neural synchrony increased in a highly specific way, that is, attention increased the high-frequency (40–80 Hz) synchronous oscillations and decreased the low-frequency (<10 Hz) oscillations in populations of neurons representing the attended location (Fries et al., 2001; Womelsdorf et al., 2006a).
In addition to boosting the effectiveness of local neuronal populations, increasing synchrony between brain regions may also change inter-areal effective connectivity. As we detail below in the section on an integrated theory of attention, oscillations in population activity likely reflect the ebb-and-flow of inhibition in a local network. Therefore, aligning such oscillations across regions could ensure that populations of neurons in inter-connected regions will be in a co-excitable state, which is one possible way to boost effective connectivity (Figure 3A; Bressler, 1996; Fries, 2005). There is growing evidence for such a model (Buschman and Miller, 2007; Gregoriou et al., 2009a; Saalmann et al., 2007; Siegel et al., 2008). In particular, a recent study by Fries and colleagues demonstrated that synchrony between regions can be highly selective, acting on a single visual object (Bosman et al., 2012). By recording simultaneously from populations of V1 neurons with receptive fields encompassing one of two stimuli as well as from V4 neurons whose receptive field overlapped both stimuli (Figure 3B, middle), they showed that, when attention was directed to a single stimulus, gamma-band oscillations were selectively synchronized between V4 and only those V1 neurons that encoded the attended stimulus location (Figure 3B, left and right).
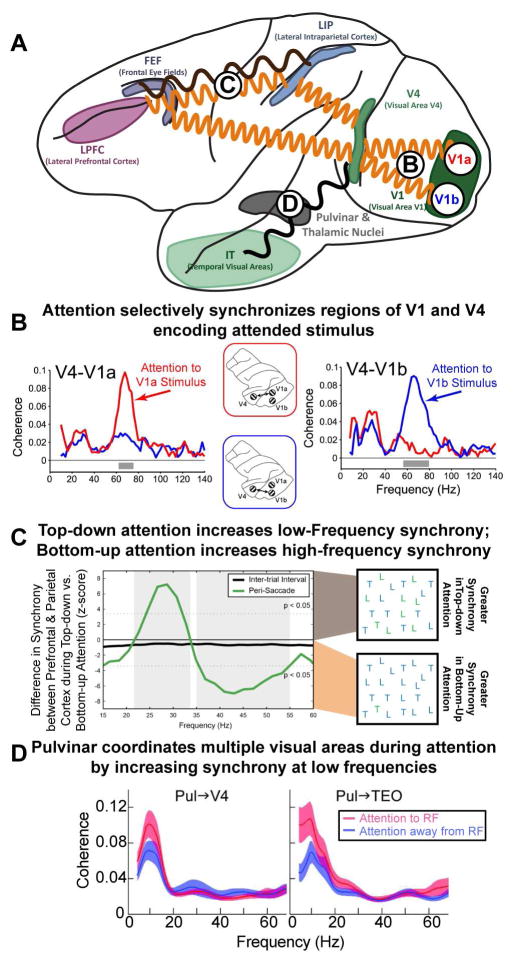
Figure 3. Attention dynamically changes the effective connectivity between brain regions.
(A) Attention modulates the synchrony between different brain regions. A minor subset of the effects of attention is outlined. Circles with letters refer to other parts of the figure. (B) Attentional modulation is specific to selected objects: synchrony between V4 and V1 is specific to those regions that encode the currently attended stimulus (red for a stimulus encoded by V1a; blue for V1b). Note that these changes in the pattern of synchrony overlay the underlying anatomy and can occur rapidly, with each shift in attention. Adapted from (Bosman et al., 2012). (C) There is also flexibility in the frequency of oscillations between brain regions. Internal (top-down) direction of attention and external (bottom-up) capture of attention emphasize different frequency bands between the same brain regions. Synchrony between prefrontal cortex (PFC) and parietal cortex (PPC) changed frequency depending on the type of attention. When attention was externally captured by a salient stimulus, this was reflected in neural activity in PPC first, followed by PFC. In addition, synchrony between PFC and PPC was observed at high-frequencies (~45 Hz; shown as negative deflection). In contrast, when attention was internally directed by the memory of a target stimulus (as in visual search) neural activity was found first in PFC and then PPC and PFC-PPC synchrony was observed at low frequencies (~25 Hz; shown as positive deflection). Adapted from (Buschman and Miller, 2007) (D) Sub-cortical regions, such as the pulvinar, play an important role in attention. In addition, they may act to coordinate activity across cortical regions: attention increased low-frequency synchrony between the pulvinar and V4/TEO (left/right, respectively), when allocated at the receptive field (RF) represented by the recorded neurons (ATT to RF) relative to a different RF location (ATT away from RF). This may organize higher-frequency oscillations, facilitating the establishment of synchrony observed between cortical regions (as in B and C). Adapted from (Saalmann et al., 2012).
These effects of synchronization are not limited to visual cortex or specifically to the gamma frequency band. Buschman and Miller (2007) found that synchrony between prefrontal and parietal cortex differed depending on whether attention was being externally captured by a salient stimulus or internally directed based on a remembered target (Figure 3C). When attention was externally captured and information flowed in a bottom-up manner (from parietal cortex to prefrontal cortex), synchrony was observed at gamma-band frequencies (the same frequency found in visual cortex). In contrast, when attention was internally controlled, and information flowed ‘top-down’ from prefrontal to parietal cortex, synchrony between prefrontal and parietal cortex was at a lower-frequency ‘beta’ band. These results suggest that attention modulates synchrony between brain regions in order to guide information flow between regions in a task-specific manner. Furthermore, these results provided the first evidence that specific frequency bands might serve specific purposes: beta-band oscillations may increase ‘top-down’ signaling while gamma-band oscillations increase ‘bottom-up signals.
Importantly, communication in cortical large-scale networks is not only mediated through cortico-cortical interactions, but also through thalamo-cortical interactions. In particular, there is growing evidence that the pulvinar, the largest nucleus in the primate thalamus, plays a key role in attention. The pulvinar is considered a higher-order thalamic nucleus, because it forms input-output loops almost exclusively with the cortex, thereby forming cortico-thalamo-cortical pathways. As a general principle, directly connected cortical areas will be indirectly connected via the pulvinar (Jones, 2001; Shipp, 2003). This indirect connectivity may be used to facilitate information transfer between cortical areas. During spatial selection, increased synchrony of slow frequency oscillations in the alpha band between two interconnected visual cortical areas (V4 and TEO) resulted from pulvino-cortical rather than cortico-cortical communication (Figure 3D; Saalmann et al., 2012). In addition, these slow oscillations were coupled to higher frequency oscillations in the gamma band in each cortical region. Such cross-frequency coupling may be an effective mechanism for coordinating long-range communication across a network, with lower frequency oscillations controlling the excitability of local neural populations in order to facilitate the coupling of higher frequency oscillations (Canolty et al., 2006; Lakatos et al., 2008). This mechanism may provide a bridge between cortico-cortical and thalamo-cortical mechanisms for large-scale communication. These studies have begun to provide a mechanistic framework for behavioral observations showing that pulvinar lesions or inactivations impair orienting responses and the exploration of visual space (Ward et al., 2002; Wilke et al., 2010).
Thus far, we have highlighted the dynamic nature of attention. Behaviorally, attention can be internally or externally controlled and even when ‘statically’ maintained, it vacillates between locations. This is reflected in the dynamic nature of attentional signals across the fronto-parietal network, including recent evidence that synchrony within and between brain regions may sculpt information flow. Next, we discuss how attention acts on the sensory representations themselves.
Neuronal effects – from single neurons to populations
When attention is allocated to a spatial location, feature, or object, its neural representation is enhanced relative to when attending elsewhere. This enhancement occurs in many different ways, ranging from changes in the responses of single neurons to changes in the dynamics of populations of neurons.
Spatial attention enhances neural responses
In one of the first studies probing attention effects in the primate brain, it was reported that directing spatial attention into the receptive field of a single parietal cortex neuron increased its response to a stimulus (Bushnell et al., 1981). Since then, studies in monkeys and humans have shown that spatial attention increases neural responses to a selected stimulus across many levels of processing. This includes cortical visual areas, such as V1, V2, V4, MT, MST, and IT (e.g. Chelazzi et al., 1993; Luck et al., 1997; Spitzer et al., 1988; Treue and Maunsell, 1999), as well as subcortical regions such as the lateral geniculate nucleus, pulvinar, reticular nucleus of the thalamus, and superior colliculus (McAlonan et al., 2006; O’Connor et al., 2002; Zénon and Krauzlis, 2012). The magnitude of the spatial attention effect increases along the cortical hierarchy, reaching its strongest effect in associative regions, such as prefrontal and parietal cortex (Rainer et al., 1998). Similarly, spatial attention effects seem to occur first in higher cortical regions and then cascade backwards (Buffalo et al., 2010). However, spatial attention does not simply increase the response rate of neurons but also increases a neuron’s sensitivity to stimuli. For example, spatial attention shifts the contrast-response function of single neurons in V4 and MT such that a neuron is more sensitive to low contrast stimuli (Figure 4A, Martínez-Trujillo and Treue, 2002; Reynolds et al., 2000). By increasing the neuronal sensitivity the perceived contrast of a stimulus can be increased due to attentional allocation, thus improving behavioral performance (Carrasco et al., 2004).
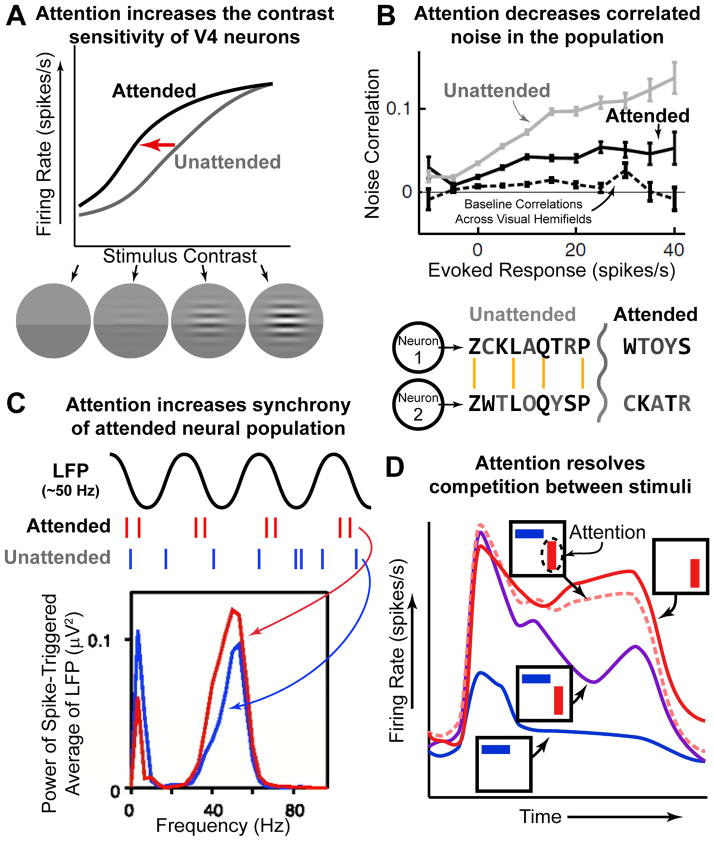
Figure 4. Attention changes neural responses.
(A) Attention increases the sensitivity of V4 neurons. Neurons increase their firing rate response to stimuli of increasing contrast; even without attention (gray line). Attending to the stimulus increases the response to stimuli at lower contrasts (black line; note leftward shift). Data is schematized from (Reynolds et al., 2000). (B) Attention reduces noise correlations in neural activity. Simultaneously recorded neurons often share uninformative ‘noise’ signals. This is schematized in the two model neurons below. Noise is schematized as black letters in the encode ‘stream’, gray letters are ‘signal’. The two neurons share much of the same ‘noise’; reducing such noise makes the message encoded by both neurons clearer (‘toy car’). Attention has such an effect in the brain (top figure; black line is below gray line). Adapted from (Cohen and Maunsell, 2009). (C) Attention increases the synchrony of selected neurons. The synchrony between individual neurons and the population (as measured by the local field potential, LFP) increases with attention allocated at RF (‘attended’, red) relative to away from RF (‘unattended’, blue). This effect is specific to a ‘gamma’ oscillation at ~50 Hz (note that synchrony is reduced at low-frequency, ~10 Hz, oscillations). Adapted from (Fries et al, 2001) (D) Attention resolves competition between stimuli. Stimuli compete for representation in V4 neurons: the response to two stimuli (purple) is approximately the average of the response to either stimuli when presented alone (red, preferred, and blue, non-preferred). Attending to a single stimulus ‘rescues’ this competition, causing the neuron to respond as if only the attended stimulus was presented (pink dashed line). Data is schematized from (Reynolds et al., 1999).
Attending to features
As noted above, attention can not only be directed to a specific location, but also to a stimulus feature. Such featural attention influences single neuron responses in much the same way as spatial attention, increasing the sensitivity of neurons that respond preferentially to stimuli matching the sought-after feature (in V1 and V4, Haenny and Schiller, 1988; in MT, Treue and Trujillo, 1999). Similar results have been reported in human neuroimaging studies (Saenz et al., 2002). Selection of a desired feature also suppresses neurons with response properties of differing selectivity (Martinez-Trujillo and Treue, 2004) and increases baseline activity in feature-specific ways even when no stimulus is present (Serences and Boynton, 2007).
Attention changes population codes
Although many of the effects of attention are observed at the level of single neurons, they also impact representations at the population level. For example, increasing the sensitivity of selected neurons will lead to an increase in the selectivity of the entire population. In addition, attention also acts to directly change the way information is represented in populations of neurons. One way that attention improves the encoding of information in a neuronal population is by decreasing noise correlations (Cohen and Maunsell, 2009; Mitchell et al., 2009). Noise correlations measure the degree to which neurons share uninformative signals that vary from trial-to-trial. Since each neuron has a limited bandwidth, correlations in their signal reduces the information carrying capacity of the population as a whole. This is perhaps most easily seen in the extreme: if each neuron carried the same signal, then the amount of information carried by the entire population would be the same as by any one neuron. Therefore, by reducing noise correlations, attention can significantly increase the information capacity of the population (Figure 4B).
However, not all correlations have a negative impact. As noted above, attention increases the synchrony of selected populations of neurons, particularly at high frequencies (~40–50 Hz, Fries et al., 2001; Figure 4C). This is thought to boost the transmission of information from the selected population (see above). It is important to note that such temporal synchrony is orthogonal to noise correlations: information is carried in the pattern of firing across a population of neurons, and redundancy in that population (such as in the case of noise correlations) reduces the information capacity of a network; synchrony is local coincidence in time and ensures the temporal precision of the firing pattern in order to drive downstream neurons. Exactly how such synchrony arises remains unknown (although we propose one theory in the Model section below). However, it may be under top-down control. For example, microstimulation of FEF induces high-frequency oscillations in parietal cortex in a topographic manner (Premereur et al., 2012).
Attention resolves competition
Thus far we have largely discussed how attention impacts the representation of isolated stimuli. However, as emphasized in the Introduction, the need for attention is greatest when multiple stimuli are present and thus there is competition among stimuli for neural representation. In a now classic experiment, Moran and Desimone demonstrated how competition is resolved within single V4 neurons (Moran and Desimone, 1985). When two stimuli were simultaneously presented in the receptive field of a V4 neuron they competed with one another, reducing the overall response of the neuron (Figure 4D, purple line). However, this effect was counteracted by attention: when attention was directed to one of the two stimuli in the receptive field, the neuron responded as if only the attended stimulus was presented (Figure 4D, pink dashed line). Similar results have been found in MT and MST (Treue and Maunsell, 1999), and corroborating evidence has been obtained in human neuroimaging studies (reviewed in Beck and Kastner, 2009). Biasing the competition between stimuli can also be conceived as a shift in a neuron’s selectivity: spatial attention collapses the receptive field of neurons towards the attended location (Connor et al., 1997; Womelsdorf et al., 2006b) while featural attention shifts the tuning curve of neurons toward an attended feature (David et al., 2008; Martinez-Trujillo and Treue, 2004).
As reviewed here, there is strong evidence that attention impacts neural representations in several different ways. Models of attention typically focus on an individual aspect of these effects. However, an integrated understanding of attention will require a unified theoretical framework that captures these diverse effects. Next, we outline a theory that attempts to build such an integrated understanding.
A unified framework for selective attention
As we have reviewed thus far, attention is a complex, multi-faceted, phenomenon with a large and diverse number of associated effects, both in the way attention impacts sensory representations as well as how attentional resources are allocated in space and time. Many different theories have been proposed that capture specific components of these attention effects. However, an integrated model of attention has yet to be developed. Here we outline a theoretical framework that builds upon several existing models of attention with a focus on integrating the disparate physiological findings reviewed above.
Basic assertions
Before we detail our theory of attention we will briefly outline three basic assertions upon which our theory is built:
Sensory cortex learns to represent visual objects; these embedded representations are then used during perception.
Normalization of responses is a fundamental aspect of neural processing in the cortex.
Oscillations largely reflect rhythmic fluctuations in inhibitory tone in a neural network.
Here, we will first explain the evidence for each assertion and, where possible, propose underlying neural circuit mechanisms. Then, we describe how these three broad observations can be combined with top-down attention signals to explain the large body of neurophysiological findings associated with attention.
Our first assertion states that sensory cortex encodes and represents visual objects (Figure 5A). Although this review is focused on visual attention, it is important to consider the computations used by sensory cortex to support perception given that visual attention affects sensory processing. Although classical models assumed that these representations were the result of fine-tuned wiring (Hubel and Wiesel, 1959), more recent theoretical and experimental work suggests that these representations are learned through experience. With the help of simple unsupervised learning rules a ‘dictionary’ can be learnt that captures the statistical regularities in the world (Simoncelli and Olshausen, 2001). At the level of primary visual cortex, such learning results in gabor-like representations (Olshausen and Field, 1996); in higher-order cortex, it likely generalizes to ‘object’-like properties (e.g. co-linearity of line segments, correlation of movement, parts of complex objects, etc). In support of this model, experiments that ‘re-wired’ auditory cortex to receive visual inputs led to neurons in auditory cortex with tuning properties that matched visual cortex (Sharma et al., 2000). In other words, the selectivity of the neurons were not defined by a developmental plan, but rather neurons learned the representations that best captured the variability in their inputs.
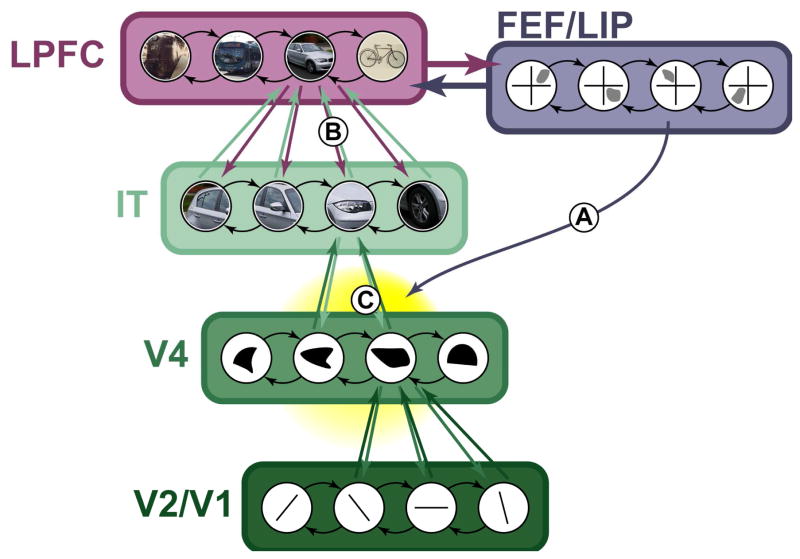
Figure 5. Theory of local attention effects.
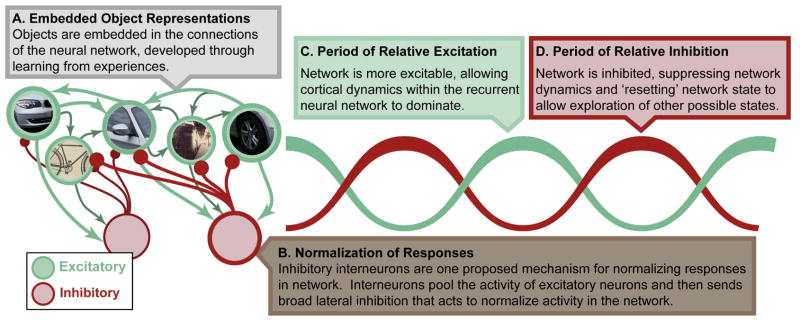
We propose many of the effects of attention to be due to local interactions within a cortical region. Attentional selection interacts with bottom-up sensory drive (not shown) as well as object representations that have been embedded within the neural network through learning (A). Attention acts on these representations by changing interactions between excitatory neurons (green) and local inhibitory interneurons (red). In particular, pooling of responses by inhibitory interneurons could form the basis for normalization of excitatory responses (B). As noted in the main text, normalization likely plays a key role in perception and attention. Furthermore, rhythmic interactions between excitatory and inhibitory neurons is proposed to underlie high-frequency oscillations (C), which are increased with attention. These oscillations may play a fundamental role to temporally organize neural activity. For example, periods of inhibition may ‘reset’ the network, allowing it to explore more than one attractor state (details in main text).
Importantly, embedding object-based representations will ensure that the system is tolerant to noise as any input will be transformed by the learned object dictionary: signals that match an expected pattern will be boosted while signals that are orthogonal to representations in the dictionary will be ignored. As the dictionary has been trained to optimally represent the world, this means the system will, in effect, perform pattern completion, settling on nearby ‘known’ representations, even when provided with a noisy input. As we detail below, this will be crucial for allowing broad, unspecific attention signals to become selective based on the combination of a learned dictionary and the current sensory drive.
Our second assertion is that response normalization is fundamental to cortical function (Figure 5B). Responses in cortex are normalized such that a constant level of overall activity in a region is maintained. For example, the response of V1 neurons to multiple stimuli of varying constrasts closely fits the predictions of a normalization model (Busse et al., 2009). The exact neural mechanisms responsible for normalizing responses remain unknown, although several possibilities have been discussed (for review, see Carandini and Heeger, 2012). In particular, one account that fits well with other observed effects of attention is that divisive normalization is the result of lateral inhibition within a cortical region (Figure 5B; Lee et al., 2012; Wilson et al., 2012; but also see Nassi et al., 2014 for a role for feedback). Normalization is thought to be important for cortical functioning for several reasons. First, by bounding the overall activity level, normalization may reduce the risk of pathologically high levels of excitation. Second, normalization acts to sparsify responses in a cortical region, possibly contributing to the formation of the optimal, sparse, responses described above (Schwartz and Simoncelli, 2001). Finally, as we detail below, a wide range of the attention effects reviewed above have been modeled as the result of attention modulating the gain of normalization (Reynolds and Heeger, 2009; Reynolds et al., 1999).
Our third assertion is that oscillations reflect rhythmic fluctuations in inhibition (Figure 5C and 5D). Rhythmic fluctuations in neural activity are observed throughout the brain across a wide variety of frequency bands (for review, see Buzsaki, 2006). The exact neural mechanisms that produce oscillatory activity in the brain remain unknown; however, there is a general consensus that inhibitory interneurons play a key role in the generation of rhythms. For example, blockade of GABA receptors reduces the high-frequency oscillations commonly modulated by attention in the cortex (Hasenstaub et al., 2005). Furthermore, optogenetic stimulation of parvalbumin-positive inhibitory interneurons preferentially generates high-frequency gamma oscillations (Cardin et al., 2009). These results suggest that oscillations in the brain reflect the ebb-and-flow of cortical excitability as inhibition is rhythmically modulated.
Outline of the theory
We propose that many of the diverse neurophysiological findings associated with attention can be explained by combining our three basic assertions with the mechanisms of top-down attentional selection reviewed in the section on network level effects. In particular, we propose interactions between excitatory pyramidal neurons and inhibitory interneurons are central to the mechanism supporting normalization and in the generation of synchronous oscillations. In brief, we propose that attention works through a cascade of effects:
Attention can either be a) automatically grabbed by salient stimuli or b) guided by task representations in frontal and parietal regions to specific spatial locations or features.
The pattern-completion nature of sensory cortex sharpens the broad top-down attentional bias, restricting it to perceptually relevant representations. Interactions with bottom-up sensory drive will emphasize specific objects.
Interneuron-mediated lateral inhibition normalizes activity and, thus, suppresses competing stimuli. This results in increased sensitivity and decreased noise correlations.
Lateral inhibition also leads to the generation of high-frequency synchronous oscillations within a cortical region. Inter-areal synchronization follows as these local oscillations synchronize along with the propagation of a bottom-up sensory drive. Both forms of synchrony act to further boost selected representations.
Further build-up of inhibition acts to ‘reset’ the network, thereby restarting the process. This reset allows the network to avoid being captured by a single stimulus and allows a positive-only selection mechanism to move over time.
As we detail next, many of the effects observed at the neural level can be explained through this cascade. In addition to noting neurophysiological observations, we will also highlight some of the theoretical models on which our unified framework is built.
Step #1: Direction of attention
Attention is controlled in one of two ways. First, attention can be captured by stimuli that are inherently salient based on their physical properties (such as their brightness, contrast, speed, etc) or other factors such as their associated valence. As noted above, saliency maps capture the saliency of all objects in a visual scene, allowing attention to be directed to stimuli in their rank order of salience (Itti and Koch, 2001).
Second, attention can be guided towards stimuli that are relevant to one’s current task. Our ability to focus our attention in such a manner is remarkably flexible: we can attend to different spatial locations, with seemingly different ‘zoom’ levels, as well as to both simple and complex features (i.e. ‘red things’ and ‘cars’). As reviewed above, such attention templates are ‘top-down’; originating in prefrontal and parietal cortex and influencing sensory cortex both through direct descending projections (e.g. from FEF to V4; Figure 6A) as well as through a backwards cascade (e.g. from PFC to IT to V4, etc; Figure 6B).
Figure 6. Cascades of spatial and featural attention across the visual hierarchy.
The brain is a densely interconnected network and so attentional selections, whether (A) spatial or (B) featural, propagate up and down the visual hierarchy. In this way, they will interact (C), allowing spatial attention to lead to selection of objects with similar features or featural selection to drive spatial attention.
Step #2: From broad to precise top-down modulation
This then presents a conundrum: what neural mechanisms would allow for both the flexibility and specificity of attentional modulation? One hypothesis is that there are specific anatomical connections that support all possible selections that could be desired. Under this model, every form of attentional selection would need a physiological correlate. For example, top-down spatial attention projections would have to be distributed across different spatial locations as well as across different spatial scales. Although this remains a possibility, current anatomical and physiological support for this model is limited (Anderson et al., 2011). Instead, we argue that broad, non-specific top-down signals are shaped by the local circuitry and activity in sensory cortex (Duncan et al., 1997).
The pattern-completion nature of sensory cortex (Assertion #1) means that non-specific inputs will be transformed into something that ‘makes sense’ to the network. In other words, any energy input to the circuit that is orthogonal to its inherent representations will be discarded while energy along its learned representations will be maintained. This effect will be further amplified when the circuit is already receiving (and processing) bottom-up inputs. In this case, sensory drive has activated a sub-set of possible activity states for the network – moving away from these would require a strong overriding input. Instead, attention is modulatory and therefore has the greatest impact on those representations that are already active in the network.
To demonstrate this effect, we can imagine a simplified case where attention is being directed to a spatial location with two competing neurons that respond to either circular or square stimuli. Attending to this location without visual stimulation will broadly boost both representations (note that representations not embedded in our network, such as triangular stimuli, will not be boosted as our simplified network does not encode them). However, if our spatial location begins receiving circular visual input this same attention signal will only be effective in increasing the activity of the ‘circle’ neuron as the ‘square’ neuron will be suppressed (either in a bottom-up manner or through competition with the circle neuron). Featural selection would work in a similar way: attending to a ‘car’ will emphasize car-components, such as circular wheels. These would be automatically selected in a top-down cascade (as seen in Figure 6B). Again, interactions with bottom-up sensory drive would collapse that selection to a particular exemplar of a category (i.e. a BMW vs. a Ford).
Generalizing from this simplified example, our theory will make a prediction how attention selects objects. As noted above in our first assertion, the visual system has learned the statistical regularities of objects and has embedded this knowledge in the connections of a distributed network (Figure 5A). Therefore, applying attention to any part of the object representation will cause the boosting signal to spread throughout the object. This prediction has extensive experimental support. First, attention to an object automatically selects all components of an object (Duncan, 1984; Egly et al., 1994; Siegel et al., 2015). In addition, recent work suggests that attention will automatically extend to other stimuli that follow Gestalt object rules (e.g. collinearity, Wannig et al., 2011). Furthermore, spatially attending to a stimulus will also boost the representation of objects with similar features across the visual field (McAdams and Maunsell, 2000; Treue and Trujillo, 1999).
Together, these results provide experimental support for a model in which top-down attention is broad and non-specific and only becomes focused through interactions with the anatomical connectivity embedded in sensory cortex and the bottom-up sensory drive.
Step #3: Biasing competition through normalization
As reviewed above, there is evidence that attention resolves competition between stimuli in a way that boosts selected representations while suppressing unselected ones. This finding was captured in the highly influential ‘biased competition’ theory of attention (Desimone and Duncan, 1995). In brief, the model proposes that stimuli are constantly competing with one another for greater representation; attention acts to bias this competition, allowing the selected stimulus to ‘win’.
Competition between stimuli is likely the result of the normalization process described in our second assertion (Reynolds et al., 1999). Recent work by Reynolds and Heeger (2009) showed that integrating a normalization model with attentional biasing mechanism, captures a wide variety of attentional effects. First, they were able to explain how spatial attention can increase the contrast gain of neurons (Reynolds et al., 2000), the response gain (Williford and Maunsell, 2006), or multiplicatively scale responses (McAdams and Maunsell, 2000). Second, they captured the sharpening of tuning curves with featural attention (David et al., 2008; Martinez-Trujillo and Treue, 2004). Finally, like the biased competition model, the normalization model of attention also predicts the response to multiple stimuli in a single receptive field (Moran and Desimone, 1985).
Lateral inhibition carried out by inhibitory interneurons is a candidate mechanism that may instantiate the normalization computation (Figure 5B). If so, one would expect significant attentional modulation of the inhibitory neurons that are computing the normalization signal. Indeed, attention has a much larger impact on the responses of putative inhibitory interneurons compared to putative pyramidal cells (Mitchell et al., 2007). Direct evidence for top-down targeting of inhibitory interneurons comes from a recent study showing that long-range projections from cingulate cortex to visual cortex in mice increase center-surround modulation via local inhibitory interneuron circuits (Zhang et al., 2014).
We propose that a model that centers on lateral inhibition has the potential to explain a diverse set of attention effects. For example, as noted by Reynolds and Heeger, such recurrent models of normalization may capture the temporal dynamics of attention effects (namely the lack of an attention effect on the visual transient) or the alterations of the size and center of receptive fields. Lateral inhibition has also been shown to increase the sparsity of neural representations (Schwartz and Simoncelli, 2001). As sparser signals are more likely to be independent to one another, this will lead to a reduction in noise correlations (as observed; Cohen and Maunsell, 2009).
Step #4: Synchrony is rhythmic inhibition
In addition to computing the normalization effect, lateral inhibition may also underlie synchronous oscillations (Figure 5C and 5D). As reviewed above, attention modulates local synchrony, possibly to increase the gain of a selected neural representation (Tiesinga et al., 2004) or to boost the transmission of information from one region to the next (Fries, 2005). However, such models that explain effects on synchronous firing in local populations are often separated from those that explain other effects of attention on single neurons. We propose a unifying mechanism relying on lateral inhibition: namely, that the same attentional modulation of inhibitory interneurons that leads to normalization also increases synchronous high-frequency oscillations.
Such a model makes several predictions about the nature of synchronous oscillations in cortex. First, it predicts high-frequency oscillations are generated by local inhibitory interneurons. As noted in our third assertion, a large body of modeling work suggests that this is true, either due to interactions between interneurons directly (so called “ING” models; Wang and Buzsáki, 1996) or between inhibitory interneurons and excitatory pyramidal neurons (so called “PING” networks; Börgers and Kopell, 2005). Furthermore, optogenetic stimulation of inhibitory interneurons produces high-frequency oscillations (Cardin et al., 2009). Second, the model predicts that attention should target inhibitory interneurons in a way that drives synchrony. Indeed, as noted above, experimental evidence suggests that attention has its greatest impact on inhibitory interneurons (Mitchell et al., 2007). More importantly, and as predicted, Vinck et al (2013) found inhibitory interneurons preferentially synchronized with local populations (measured via LFPs), with a phase relationship that suggested they were driving the high-frequency oscillations in LFP. Finally, according to our model, attention will increase firing rates (particularly in inhibitory interneurons) before increasing high-frequency oscillations. Although this has not been directly tested, there is some experimental evidence that attention effects on firing rate precede modulations in high-frequency oscillations in visual (Fries et al., 2008) and frontal cortex (Gregoriou et al., 2009b).
By acting on inhibitory interneurons, attention increases local synchrony and, thus, increases the impact of a neuronal population on downstream brain regions (see dynamic functional connectivity section above for details). Attention also increases synchrony between regions, further boosting information transfer. However, if high-frequency oscillations are due to the activation of local circuits, then how are they synchronized across different brain regions? One possibility is that there is a controlling input that forces synchronization across regions (and could be modulated by attention). For example, high-frequency oscillations have been found to be coupled to low-frequency oscillations (Colgin, 2013; Schroeder and Lakatos, 2009) and so a synchronous low-frequency oscillations could organize the temporal dynamics of higher-frequency oscillations across regions.
Alternatively, synchronization across brain regions may be a passive process that only requires a phase reset to initially align local oscillations. This phase reset would occur with the onset of a strong input into the cortex, such as the appearance of a new stimulus in the world or an eye movement moving an existing stimulus into a receptive field. The propagation of this stimulus across brain regions (in a bottom-up manner), would then naturally align the local oscillations across regions. This predicts an increase in high-frequency synchrony with a strong stimulus drive, as seen following the onset of a pop-out stimulus (as seen by Buschman and Miller, 2007).
Step #5: Rhythmic oscillations of inhibition resets the neural network
Many of the above effects demonstrate how attention may increase synchrony to select specific representations. However, these effects do not strictly rely on synchrony being oscillatory in nature. Therefore, it is not clear what mechanistic function an oscillation may serve. We propose that oscillations modulate the attractor dynamics of local cortex by periodically ‘resetting’ the network through strong inhibition (Figure 5C and 5D).
For example, this may be crucial to disengaging attention. Suppose one deploys attention to a stimulus, which as a consequence, ‘wins’ the competition with other stimuli through lateral inhibition. In this way, the attended stimulus has captured the network; a state that will persist, even if attention is released and redeployed. One possible solution to this problem might be a strong negative, or inhibitory, signal that can counter the positive selection of attention. In support of this model, psychophysical studies have revealed a strong ‘inhibition of return’ (IOR) that inhibits re-selecting an already attended stimulus (Klein, 2000), an effect that reduces the neural representation of a previously selected stimulus (Mirpour et al., 2009). Alternatively, oscillations in global inhibition levels may serve this same purpose: every cycle of an oscillation effectively ‘resets’ the network, allowing a new stimulus to be captured. Such a mechanism has the advantage of not requiring a strong top-down inhibitory signal but rather relies on a local mechanism for generating inhibition. If true, our theory would predict that shifts in attention should be tied to ongoing oscillations in neural activity. Indeed, Buschman and Miller (2009) observed this effect during a visual search task. Covert shifts in attention (measured behaviorally and electrophysiologically) were locked to ongoing beta-band oscillations: on each cycle of the beta-band oscillation the animal attended to a new location in space. Similar effects have been observed in humans, although at lower frequencies (Busch and VanRullen, 2010; Fiebelkorn et al., 2013; Landau and Fries, 2012). Similarly, overt shifts in attention (i.e. eye movements) are phase-locked to lower frequency oscillations (Schroeder et al., 2010).
Note that, at the behavioral level, rhythmic attention will appear as the classic inhibition of return: stimuli are momentarily attended before being inhibited for a sustained period of time (as they are never returned to). Indeed, studies of IOR have found the onset of inhibition occurs around 225 ms (Klein, 2000), which is approximately the 4 Hz observed in rhythmic fluctuations of attention (Fiebelkorn et al., 2013). However, it remains to be seen which is the chicken and which is the egg: do oscillations structure the IOR or do we observe rhythmic IOR as oscillations?
Networks for attentional control: Interplay of spatial and featural attention
Directing attention to space of features appears to be controlled by individual sources in the brain. Spatial attention is likely directed by descending projections into extrastriate visual areas (e.g. FEF to V4 projections; Figure 6A). In contrast, featural attention is much broader, impacting the entire visual field. Therefore, featural attention likely begins in regions with larger receptive fields and more complex representations (Figure 6B). A ‘reverse hierarchy’ model of attention suggests selection begins at the highest, most abstract, level before filtering down to the details of an object (Hochstein and Ahissar, 2002). Such a model predicts the selection to begin in prefrontal/parietal cortex, where neurons represent abstract categories (Freedman et al., 2001), and then filters backwards along the cortical hierarchy to ‘simpler’ visual areas. Surprisingly, it also predicts that the ease of feature-based visual search should be directly related to whether the category of the sought-after stimulus is ‘natural’. Indeed, searching a cluttered natural scene for a complex object can be highly efficient if the object is typical to our everyday experiences, as noted above (e.g. “cars”, for review see Peelen and Kastner, 2014).
Despite their independent sources, these two forms of attention do interact with one another. A network view of selection suggests that such interactions are mediated through the convergence of feature and spatial attention in visual cortex (Figure 6C). Attending to a spatial location will select an object (or a piece of an object) at that location. This selection will propagate up-and-down the visual hierarchy, acting to select associated representations. In turn, the more abstract, invariant representations in higher cortical regions will lead to the automatic selection of similar objects in the visual scene based on their featural properties. Indeed, psychophysical studies have shown that spatial attention can drive featural attention (e.g. spatially attending to a single object leads to increases in attention to its properties across the entire cortex, Summerfield et al., 2006). Conversely, featural attention can drive spatial attention (e.g. the detection of a car in a visual scene drives spatial attention to that location). In this way, spatial and featural attention can be flexibly combined to allow for the dynamic nature of attention.
Future directions
We have attempted to outline a parsimonious theoretical model that captures the diversity of attention effects on neural activity. In particular, we have focused on local cortical interactions as these are the most prevalent connections in the brain and therefore the most likely to impact neural processing. We have also attempted to avoid the need for precise top-down or controlling inputs, whether it is spatially precise (in the case of a spotlight of attention) or temporally precise (in the case of inter-areal synchronization). As we hope is clear, this model relies heavily on previous theoretical and experimental work. However, despite this strong basis, a more mechanistic model is needed to test the details of our theory.
In addition, there are many experimental details that need to be worked out. For example, many of the observed effects of attention can be explained by modulating the excitatory/inhibitory balance of this network, particularly by increasing the inhibitory gain in the network. However, we are only beginning to understand how this balance is modulated in the brain. For example, there are several (perhaps dozens) of different types of inhibitory interneurons. Recent work is beginning to unravel the relative roles of these interneurons, both in perception and attention (Lee et al., 2012; Wilson et al., 2012; Zhang et al., 2014) but future work must continue to detail the respective roles of these varied cell types.
A particularly intriguing avenue for further exploration is the role of neuromodulation in altering the computational properties of local cortical circuitry. In particular, acetylcholine (ACh) has been proposed to play a role in attention. Indeed, manipulating ACh receptors changes the effect of attention on V1 neurons (Disney et al., 2007; Herrero et al., 2008). This effect may be mediated by cholinergic midbrain regions that represent stimulus saliency (Asadollahi et al., 2010) and are themselves modulated by prefrontal cortex (Sarter et al., 2005).
Finally, the theory outlined here is focused on the effects of attention on perception (almost exclusively visual perception). However, attention is just one small part of cognition and it is becoming increasingly clear that attention interacts heavily with other cognitive domains, as we will review in the final section.
Attention and other cognitive processes
Attention, defined as the act of selecting task-relevant information, is a central component of cognition. Although research on attention has been largely focused on its impact on visual processing, there are close relationships between attention and other cognitive processes.
Attention and working memory
Working memory is the ability to hold items ‘in mind’, without relying on the external world. Working memory plays a central role in cognition: it acts as a dynamic mental ‘workspace’ in which thoughts are processed, manipulated, and transformed. Indeed, attention may rely on working memory workspaces to maintain the current ‘search template’ (Wolfe, 1994). Evidence for such a model comes from studies showing that attention can be biased by the current contents of working memory (Soto et al., 2008). Furthermore, brain regions involved in controlling attention are also strongly recruited during working memory, particularly the fronto-parietal network (Awh and Jonides, 2001). However, the relationship between attention and working memory is complicated. Models of working memory predict the existence of a ‘central executive’ that controls and manipulates the contents of working memory ‘sketchpads’ (Baddeley and Hitch, 1974). Recent studies underline the importance of this ‘central executive’: an individual’s general intelligence correlates highly with how effectively the contents of working memory are controlled (Fukuda and Vogel, 2011).
Attention may be this ‘central executive’ of working memory. In support of this idea, attention filters what enters working memory (Gazzaley, 2011) and plays a role in maintaining items in memory (Kuo et al., 2011). Attention may also pull together the distributed brain regions necessary to support working memory (Postle, 2006). There is also growing evidence that the capacity limitation of working memory is due to competition in a manner very similar to the competition observed during perception (Buschman et al., 2011), suggesting that some of the neural mechanisms limiting perception may also be limiting working memory. Indeed, the same brain regions involved in directing attention to external stimuli are activated when attending to ‘internal’ stimuli (Chun et al., 2011; Nobre et al., 2004).
Attention and reward learning
Attention is also intricately related to reward processing. Attention is attracted to salient, behaviorally-relevant, stimuli. Obviously, rewarding stimuli should be salient and therefore reward signals are likely closely tied to attention signals. Recent electrophysiological evidence has begun to tease apart the relationship between attention and reward in visual cortex, with some early evidence for partially overlapping representations (Foley et al., 2014). In addition, since reward information guides learning, it may aid in learning of where to direct attention (Rombouts et al., 2015).
Attention may also be critical to learning what is rewarding in the real world. Reinforcement learning is not efficient when a reward can be associated with too many possible sources. In this context, attention may act to select the most likely sources and therefore limit reinforcement learning to this subset (Niv et al., 2015). In this way, attention can act to guide learning towards task-relevant stimuli.
Together, these results suggest that attention, working memory, and rewards are closely intertwined, and therefore may share many of the same underlying neural mechanisms. Future work is needed to continue elucidating which mechanisms are shared and which are distinct. This entwinement also highlights the integrative nature of behavior. Attention is crucial to working memory and reward processing because both functions rely on selection of task-relevant stimuli. Indeed, as we review next, the selective nature of attention may underlie cognitive control more broadly.
Attention and cognitive control
Cognitive control is our ability to guide our actions based on our task, our internal goals, and the current context. Cognitive control is thought to operate by guiding activity throughout the brain in a task-dependent manner (Miller and Cohen, 2001). In reality, this is just a super-set of attention: instead of only acting upon sensory representations, cognitive control can operate more broadly to select relevant stimulus representations, decision making circuits, and motor planning regions (Norman and Shallice, 1986).
Indeed, there is significant overlap between the neural mechanisms supporting cognitive control and attention. For example, similar to attention, prefrontal cortex is thought to be the source of cognitive control: lesions in PFC disrupt cognitive control (Barceló and Knight, 2002) and single neurons in PFC represent the current task (Wallis et al., 2001; White and Wise, 1999). Furthermore, synchrony within prefrontal cortex carves out ensembles of task-related neurons (Buschman et al., 2012), much like in the way attention creates synchronous ensembles in posterior cortex.
In this light it seems that by studying attention we have been studying one specific form of cognitive control. Therefore, it is possible that many of the neural mechanisms underlying attention will apply more broadly. For example, cognitive control may resolve competition between motor plans in the same way attention resolves competition between sensory stimuli (a generalization of Figure 5B). Similarly, oscillations are observed throughout the brain and may play a similar role in moderating cortical dynamics in any cortical region (Figure 5C and 5D). This would make sense from an evolutionary perspective – once the brain solves one problem, it might as well apply the same solution to other, similar, problems.
Conclusions
Attention research has moved from laboratory scenes to the real world at the behavioral level, and from the single neuron to local populations and functional interactions across large-scale networks at the neural level (see Box 1: Current status of the field). We have outlined a unified theory of attention that begins to integrate these disparate effects. We propose broad top-down selection signals interact with the inherent knowledge embedded in sensory cortex and with bottom-up sensory drive. In addition, we suggest that these signals interact within the local cortical circuit to produce oscillatory synchrony. Such oscillations temporally parse neural activity in a way that facilitates selection of relevant representation, routes information through the brain, and modulates attractor dynamics of a network.
Box 1. Current status of the field.
Attention is used to select specific representations for greater neural representation. This resolves competition for limited neural resources.
Attention is controlled by a distributed fronto-parietal network that acts upon representations in sensory cortex.
Attention increases the strength of neural responses in several different ways to boost the attended representation, allowing it to win the competition for neural resources.
Attention interacts with many other cognitive domains, including learning, short- and long-term memory, and decision making.
Future work is needed to continue building a detailed, mechanistic, understanding of attention (see Box 2: Future directions). Detailed circuit models of attention will require continuing efforts to identify cell-types and quantify their role in attention. In addition, a unified theory must account for the dynamics of attention. Studying these dynamics will require accurately following the time-course of neural correlates of attention across large populations of neurons throughout the brain. Such large-scale recordings will require continued improvements in multi-electrode electrophysiology techniques and/or imaging approaches. This appears particularly important as neural dynamics may be key to understanding the enormous flexibility of attentional resources. Yet, the neural mechanisms supporting these dynamics remain largely unknown. Finally, we must continue to integrate attention with other cognitive domains. The parcellation of behavior and brain into different cognitive domains has yielded important insights into the neural mechanisms of many behaviors. However, cognition emerges from the interactions of these ‘cognitive domains’; thus, a complete understanding of cognition will require a more integrative approach.
Box 2. Future Directions.
Attention is highly dynamic; changing rapidly between locations and/or features. These dynamics have been associated with local population oscillations, yet the exact neural mechanisms remain unknown.
The recruitment of network parts within the fronto-parietal network is highly flexible, depending on changing behavioral demands. However, the neural mechanisms utilized to couple these parts for a given task are unknown, nor is it clear how a network pattern produces the desired behavior.
Attention may boost neural representations at several different levels, from increasing single neuron responses to boosting population selectivity. However, it is unknown how these effects interact and whether they share common circuit mechanisms.
Recent work has highlighted the existence of domain-specific neural networks. However, it remains unknown how these networks interact in order to support the integrative nature of cognition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JC, Kennedy H, Martin KAC. Pathways of Attention: Synaptic Relationships of Frontal Eye Field to V4, Lateral Intraparietal Cortex, and Area 46 in Macaque Monkey. J Neurosci. 2011;31:10872–10881. doi: 10.1523/JNEUROSCI.0622-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcizet F, Mirpour K, Bisley JW. A Pure Salience Response in Posterior Parietal Cortex. Cereb Cortex. 2011;21:2498–2506. doi: 10.1093/cercor/bhr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadollahi A, Mysore SP, Knudsen EI. Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci. 2010;13:889–895. doi: 10.1038/nn.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proc Natl Acad Sci. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G. Working Memory. In: Bower Gordon H., editor. Psychology of Learning and Motivation. Academic Press; 1974. pp. 47–89. [Google Scholar]
- Barceló F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–356. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Res. 2009;49:1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci. 1999;2:549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Vallar G. Hemineglect in humans. In: Boller F, Grafman J, Rizzolatti G, Goodglass H, editors. Handbook of Neuropsychology. Vol. 1. New York, NY, US: Elsevier Science; 1988. pp. 195–222. [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal Activity in the Lateral Intraparietal Area and Spatial Attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neural Correlates of Attention and Distractibility in the Lateral Intraparietal Area. J Neurophysiol. 2006;95:1696–1717. doi: 10.1152/jn.00848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börgers C, Kopell N. Effects of Noisy Drive on Rhythms in Networks of Excitatory and Inhibitory Neurons. Neural Comput. 2005;17:557–608. doi: 10.1162/0899766053019908. [DOI] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional Stimulus Selection through Selective Synchronization between Monkey Visual Areas. Neuron. 2012;75:875–888. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL. Interareal synchronization in the visual cortex. Behav Brain Res. 1996;76:37–49. doi: 10.1016/0166-4328(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. A backward progression of attentional effects in the ventral stream. Proc Natl Acad Sci. 2010;107:361–365. doi: 10.1073/pnas.0907658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, VanRullen R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proc Natl Acad Sci. 2010;107:16048–16053. doi: 10.1073/pnas.1004801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-Down Versus Bottom-Up Control of Attention in the Prefrontal and Posterior Parietal Cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Serial, Covert Shifts of Attention during Visual Search Are Reflected by the Frontal Eye Fields and Correlated with Population Oscillations. Neuron. 2009;63:386–396. doi: 10.1016/j.neuron.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Siegel M, Roy JE, Miller EK. Neural substrates of cognitive capacity limitations. Proc Natl Acad Sci. 2011 doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Denovellis EL, Diogo C, Bullock D, Miller EK. Synchronous Oscillatory Neural Ensembles for Rules in the Prefrontal Cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Goldberg ME, Robinson DL. Behavioral enhancement of visual responses in monkey cerebral cortex. I Modulation in posterior parietal cortex related to selective visual attention J Neurophysiol. 1981;46:755–772. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- Busse L, Wade AR, Carandini M. Representation of Concurrent Stimuli by Population Activity in Visual Cortex. Neuron. 2009;64:931–942. doi: 10.1016/j.neuron.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A Taxonomy of External and Internal Attention. Annu Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Mechanisms and Functions of Theta Rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- Connor CE, Preddie DC, Gallant JL, Essen DCV. Spatial Attention Effects in Macaque Area V4. J Neurosci. 1997;17:3201–3214. doi: 10.1523/JNEUROSCI.17-09-03201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Çukur T, Nishimoto S, Huth AG, Gallant JL. Attention during natural vision warps semantic representation across the human brain. Nat Neurosci. 2013;16:763–770. doi: 10.1038/nn.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Chui HC. Neglect following damage to frontal lobe or basal ganglia. Neuropsychologia. 1980;18:123–132. doi: 10.1016/0028-3932(80)90058-5. [DOI] [PubMed] [Google Scholar]
- David SV, Hayden BY, Mazer JA, Gallant JL. Attention to Stimulus Features Shifts Spectral Tuning of V4 Neurons during Natural Vision. Neuron. 2008;59:509–521. doi: 10.1016/j.neuron.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural Mechanisms of Selective Visual Attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain Modulation by Nicotine in Macaque V1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. Selective attention and the organization of visual information. J Exp Psychol Gen. 1984;113:501–517. doi: 10.1037//0096-3445.113.4.501. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychol Rev. 1989;96:433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G, Ward R. Competitive brain activity in visual attention. Curr Opin Neurobiol. 1997;7:255–261. doi: 10.1016/s0959-4388(97)80014-1. [DOI] [PubMed] [Google Scholar]
- Egly R, Driver J, Rafal RD. Shifting visual attention between objects and locations: Evidence from normal and parietal lesion subjects. J Exp Psychol Gen. 1994;123:161–177. doi: 10.1037//0096-3445.123.2.161. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, Saalmann YB, Kastner S. Rhythmic Sampling within and between Objects despite Sustained Attention at a Cued Location. Curr Biol. 2013;23:2553–2558. doi: 10.1016/j.cub.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley NC, Jangraw DC, Peck C, Gottlieb J. Novelty Enhances Visual Salience Independently of Reward in the Parietal Lobe. J Neurosci. 2014;34:7947–7957. doi: 10.1523/JNEUROSCI.4171-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical Representation of Visual Stimuli in the Primate Prefrontal Cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The Effects of Visual Stimulation and Selective Visual Attention on Rhythmic Neuronal Synchronization in Macaque Area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. Individual Differences in Recovery Time From Attentional Capture. Psychol Sci. 2011;22:361–368. doi: 10.1177/0956797611398493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49:1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Esterman M, Wilson D, Serences JT, Yantis S. Control of Spatial and Feature-Based Attention in Frontoparietal Cortex. J Neurosci. 2010;30:14330–14339. doi: 10.1523/JNEUROSCI.4248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-Frequency, Long-Range Coupling Between Prefrontal and Visual Cortex During Attention. Science. 2009a;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-Frequency, Long-Range Coupling Between Prefrontal and Visual Cortex During Attention. Science. 2009b;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenny PE, Schiller PH. State dependent activity in monkey visual cortex. Exp Brain Res. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- Hasegawa RP, Matsumoto M, Mikami A. Search Target Selection in Monkey Prefrontal Cortex. J Neurophysiol. 2000;84:1692–1696. doi: 10.1152/jn.2000.84.3.1692. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory Postsynaptic Potentials Carry Synchronized Frequency Information in Active Cortical Networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of Functional Connectivity in Frontoparietal Networks Underlies Behavioral Deficits in Spatial Neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention The mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–327. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S, Ahissar M. View from the Top: Hierarchies and Reverse Hierarchies in the Visual System. Neuron. 2002;36:791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M, Sawaguchi T. Involvement of the Dorsolateral Prefrontal Cortex of Monkeys in Visuospatial Target Selection. J Neurophysiol. 2003;89:587–599. doi: 10.1152/jn.00148.2002. [DOI] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- James W. The principles of psychology. New York, NY: Holt and Company; 1890. [Google Scholar]
- Janer KW, Pardo JV. Deficits in Selective Attention Following Bilateral Anterior Cingulotomy. J Cogn Neurosci. 1991;3:231–241. doi: 10.1162/jocn.1991.3.3.231. [DOI] [PubMed] [Google Scholar]
- Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE. Prioritized Maps of Space in Human Frontoparietal Cortex. J Neurosci. 2012;32:17382–17390. doi: 10.1523/JNEUROSCI.3810-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased Activity in Human Visual Cortex during Directed Attention in the Absence of Visual Stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–49. [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends Cogn Sci. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Kuo B-C, Stokes MG, Nobre AC. Attention Modulates Maintenance of Representations in Visual Short-term Memory. J Cogn Neurosci. 2011;24:51–60. doi: 10.1162/jocn_a_00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of Neuronal Oscillations as a Mechanism of Attentional Selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lakatos P, O’Connell MN, Barczak A, Mills A, Javitt DC, Schroeder CE. The Leading Sense: Supramodal Control of Neurophysiological Context by Attention. Neuron. 2009;64:419–430. doi: 10.1016/j.neuron.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, Fries P. Attention Samples Stimuli Rhythmically. Curr Biol. 2012;22:1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FF, VanRullen R, Koch C, Perona P. Rapid natural scene categorization in the near absence of attention. Proc Natl Acad Sci U S A. 2002;99:9596–9601. doi: 10.1073/pnas.092277599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gratton C, Yao D, Knight RT. Role of frontal and parietal cortices in the control of bottom-up and top-down attention in humans. Brain Res. 2010;1344:173–184. doi: 10.1016/j.brainres.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural Mechanisms of Spatial Selective Attention in Areas V1, V2, and V4 of Macaque Visual Cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-Based Attention Increases the Selectivity of Population Responses in Primate Visual Cortex. Curr Biol. 2004;14:744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Martínez-Trujillo JC, Treue S. Attentional Modulation Strength in Cortical Area MT Depends on Stimulus Contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JHR. Attention to Both Space and Feature Modulates Neuronal Responses in Macaque Area V4. J Neurophysiol. 2000;83:1751–1755. doi: 10.1152/jn.2000.83.3.1751. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional Modulation of Thalamic Reticular Neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An Integrative Theory of Prefrontal Cortex Function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mirpour K, Arcizet F, Ong WS, Bisley JW. Been There, Seen That: A Neural Mechanism for Performing Efficient Visual Search. J Neurophysiol. 2009;102:3481–3491. doi: 10.1152/jn.00688.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential Attention-Dependent Response Modulation across Cell Classes in Macaque Visual Area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial Attention Decorrelates Intrinsic Activity Fluctuations in Macaque Area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the Frontal Eye Field and Its Effects on Covert Spatial Attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective Attention Gates Visual Processing in the Extrastriate Cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Nassi J, Gomez-Laberge C, Kreiman G, Born R. Corticocortical feedback increases the spatial extent of normalization. Front Syst Neurosci. 2014;8:105. doi: 10.3389/fnsys.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daniel R, Geana A, Gershman SJ, Leong YC, Radulescu A, Wilson RC. Reinforcement Learning in Multidimensional Environments Relies on Attention Mechanisms. J Neurosci. 2015;35:8145–8157. doi: 10.1523/JNEUROSCI.2978-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Maquet P, Frith CD, Vandenberghe R, Mesulam MM. Orienting Attention to Locations in Perceptual Versus Mental Representations. J Cogn Neurosci. 2004;16:363–373. doi: 10.1162/089892904322926700. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to Action. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self-Regulation. Springer US; 1986. pp. 1–18. [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature. 1996;381:607–609. doi: 10.1038/381607a0. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Kastner S. A neural basis for real-world visual search in human occipitotemporal cortex. Proc Natl Acad Sci. 2011 doi: 10.1073/pnas.1101042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Kastner S. Attention in the real world: toward understanding its neural basis. Trends Cogn Sci. 2014;18:242–250. doi: 10.1016/j.tics.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelen MV, Fei-Fei L, Kastner S. Neural mechanisms of rapid natural scene categorization in human visual cortex. Nature. 2009;460:94–97. doi: 10.1038/nature08103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol Gen. 1980;109:160–174. [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premereur E, Vanduffel W, Roelfsema PR, Janssen P. Frontal eye field microstimulation induces task-dependent gamma oscillations in the lateral intraparietal area. J Neurophysiol. 2012;108:1392–1402. doi: 10.1152/jn.00323.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premereur E, Janssen P, Vanduffel W. Effector Specificity in Macaque Frontal and Parietal Cortex. J Neurosci. 2015;35:3446–3459. doi: 10.1523/JNEUROSCI.3710-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The Normalization Model of Attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive Mechanisms Subserve Attention in Macaque Areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention Increases Sensitivity of V4 Neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Rombouts JO, Bohte SM, Martinez-Trujillo J, Roelfsema PR. A learning rule that explains how rewards teach attention. Vis Cogn. 2015;0:1–27. [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes J-D, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and Psychophysics Reveal Frontal Influences on Human Retinotopic Visual Cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural Mechanisms of Visual Attention: How Top-Down Feedback Highlights Relevant Locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science. 2012;337:753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci. 2002;5:631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nat Neurosci. 2001;4:819–825. doi: 10.1038/90526. [DOI] [PubMed] [Google Scholar]
- Serences JT, Boynton GM. Feature-Based Attentional Modulations in the Absence of Direct Visual Stimulation. Neuron. 2007;55:301–312. doi: 10.1016/j.neuron.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S. Control of Object-based Attention in Human Cortex. Cereb Cortex. 2004;14:1346–1357. doi: 10.1093/cercor/bhh095. [DOI] [PubMed] [Google Scholar]
- Sharma J, Angelucci A, Sur M. Induction of visual orientation modules in auditory cortex. Nature. 2000;404:841–847. doi: 10.1038/35009043. [DOI] [PubMed] [Google Scholar]
- Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc B Biol Sci. 2003;358:1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal Synchronization along the Dorsal Visual Pathway Reflects the Focus of Spatial Attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Siegel M, Buschman TJ, Miller EK. Cortical information flow during flexible sensorimotor decisions. Science. 2015;348:1352–1355. doi: 10.1126/science.aab0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncelli EP, Olshausen BA. Natural Image Statistics and Neural Representation. Annu Rev Neurosci. 2001;24:1193–1216. doi: 10.1146/annurev.neuro.24.1.1193. [DOI] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends Cogn Sci. 2008;12:342–348. doi: 10.1016/j.tics.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Summerfield JJ, Lepsien J, Gitelman DR, Mesulam MM, Nobre AC. Orienting Attention Based on Long-Term Memory Experience. Neuron. 2006;49:905–916. doi: 10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Kastner S. Shifting Attentional Priorities: Control of Spatial Attention through Hemispheric Competition. J Neurosci. 2013;33:5411–5421. doi: 10.1523/JNEUROSCI.4089-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci Off J Soc Neurosci. 2010;30:148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Tiesinga PHE, Fellous J-M, Salinas E, Jose JV, Sejnowski TJ. Synchronization as a mechanism for attentional gain modulation. Neurocomputing. 2004;58–60:641–646. doi: 10.1016/j.neucom.2004.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cognit Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Effects of Attention on the Processing of Motion in Macaque Middle Temporal and Medial Superior Temporal Visual Cortical Areas. J Neurosci. 1999;19:7591–7602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Trujillo JCM. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Kastner S. Mechanisms of Visual Attention in the Human Cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Vinck M, Womelsdorf T, Buffalo EA, Desimone R, Fries P. Attentional Modulation of Cell-Class-Specific Gamma-Band Synchronization in Awake Monkey Area V4. Neuron. 2013;80:1077–1089. doi: 10.1016/j.neuron.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. 2001;411:953–956. doi: 10.1038/35082081. [DOI] [PubMed] [Google Scholar]
- Wang X-J, Buzsaki G. Gamma Oscillation by Synaptic Inhibition in a Hippocampal Interneuronal Network Model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannig A, Stanisor L, Roelfsema PR. Automatic spread of attentional response modulation along Gestalt criteria in primary visual cortex. Nat Neurosci. 2011;14:1243–1244. doi: 10.1038/nn.2910. [DOI] [PubMed] [Google Scholar]
- Ward R, Danziger S, Owen V, Rafal R. Deficits in spatial coding and feature binding following damage to spatiotopic maps in the human pulvinar. Nat Neurosci. 2002;5:99–100. doi: 10.1038/nn794. [DOI] [PubMed] [Google Scholar]
- White IM, Wise SP. Rule-dependent neuronal activity in the prefrontal cortex. Exp Brain Res. 1999;126:315–335. doi: 10.1007/s002210050740. [DOI] [PubMed] [Google Scholar]
- Wilke M, Turchi J, Smith K, Mishkin M, Leopold DA. Pulvinar Inactivation Disrupts Selection of Movement Plans. J Neurosci. 2010;30:8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford T, Maunsell JHR. Effects of Spatial Attention on Contrast Response Functions in Macaque Area V4. J Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature. 2012;488:343–348. doi: 10.1038/nature11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM. Guided Search 2.0 A revised model of visual search. Psychon Bull Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Cave KR, Franzel SL. Guided search: An alternative to the feature integration model for visual search. J Exp Psychol Hum Percept Perform. 1989;15:419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Võ ML-H, Evans KK, Greene MR. Visual search in scenes involves selective and nonselective pathways. Trends Cogn Sci. 2011;15:77–84. doi: 10.1016/j.tics.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006a;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nat Neurosci. 2006b;9:1156–1160. doi: 10.1038/nn1748. [DOI] [PubMed] [Google Scholar]
- Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012 doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Do JPH, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre Anna, Kastner Sabine., editors. The Oxford Handbook of Attention. Oxford Library of Psychology; Oxford, UK: 2014. [Google Scholar]