Abstract
Introduction
While olfaction is one of the most important senses in most terrestrial mammals, it is absent in modern toothed whales (Odontoceti, Cetacea). Furthermore, behavioral evidence suggests that gustation is very limited. In contrast, their aquatic sistergroup, baleen whales (Mysticeti) retain small but functional olfactory organs, and nothing is known about their gustation. It is difficult to investigate mysticete chemosensory abilities because experiments in a controlled setting are impossible.
Results
Here, we use the functional regionalization of the olfactory bulb (OB) to identify the loss of specific olfactory functions in mysticetes. We provide the whole-genome sequence of a mysticete and show that mysticetes lack the dorsal domain of the OB, an area known to induce innate avoidance behavior against odors of predators and spoiled foods. Genomic and fossil data suggest that mysticetes lost the dorsal domain of the OB before the Odontoceti-Mysticeti split. Furthermore, we found that all modern cetaceans are revealed to have lost the functional taste receptors.
Conclusion
These results strongly indicate that profound changes in the chemosensory capabilities had occurred in the cetacean lineage during the period when ancestral whales migrated from land to water.
Electronic supplementary material
The online version of this article (doi:10.1186/s40851-014-0002-z) contains supplementary material, which is available to authorized users.
Keywords: Antarctic minke whale genome, Archaeoceti, Cetacea, Chemoreception, Olfactory bulb
Introduction
Terrestrial mammals usually have a well-developed sense of smell that can detect various odors using four kinds of G-protein coupled receptors (GPCRs) encoded by different multigene families to each other: olfactory receptors (ORs), trace amine-associated receptors (TAARs) and two types of vomeronasal receptors (V1Rs and V2Rs) [1]. But this sense was greatly reduced in the ancestors of modern cetaceans [2]. Modern cetaceans lack a large number of OR genes [3-5], and odontocetes lost the nervous system structures that mediate olfaction, such as the olfactory tract, olfactory bulb (OB) and cranial nerve I [6]. In addition to the four olfactory GPCRs, two GPCR families are involved in mammalian gustation: TAS1R (taste receptor type 1, the sweet and umami taste receptor) and TAS2R (taste receptor type 2, the bitter taste receptor) [1]. Most of the taste receptor genes have also been lost from dolphin genomes [7,8], though behavioral tests indicate that dolphins can detect several kinds of flavorants [9]. In contrast, mysticetes retained these anatomical structures, although they are small [10], and it has been suggested that mysticetes use olfaction in foraging [11]. Like those of terrestrial mammals, mysticetes’ olfactory nerves are concentrated in their nasal cavities [10], and their nasal passages remain filled with air when they dive and keep water out, indicating that mysticetes can smell in air but not underwater. Unfortunately, no mysticete species are kept in laboratories or aquariums, meaning that experiments in a controlled setting are impossible, and thus it is still a mystery how mysticetes use olfaction for their fully aquatic life. Regarding taste, most of their taste receptors have been lost [12,13], but it is not clear whether the remaining receptors are still functional or not.
Olfaction has been studied in laboratory mammals: olfactory sensory neurons (OSNs) are located in the olfactory epithelium of the nasal cavity and each OSN expresses only one chemosensory receptor gene [14]. The axons of the OSNs that express the same receptors converge to a set of glomeruli in the OB that are in a distinct topographic region of the OB [15]. Thus, odorous information received in the olfactory epithelium is converted to topographical maps of activated glomeruli of the OB. The glomerular layer of the OB can be divided into two non-overlapping areas, a dorsal domain (D domain) and a ventral domain (V domain) based on the expression patterns of several domain-specific marker genes [16,17]. D domain-ablated mice (ΔD mice) fail to show innate avoidance behavior against predator odors and spoiled smells [16].
We previously studied the anatomy and histology of the OB in a single mysticete (bowhead whale Balaena mysticetus) [10]. Olfactory nerves enter the OB from the ventral side in these mysticetes, and connect to glomeruli located on the ventral side. However, unlike OBs in most other mammals, dorsal OSN axons and glomeruli are absent or nearly absent. This distribution of glomeruli resembles that of ΔD mice, and this led us to hypothesize that mysticetes lack the D domain of the OB.
To test this hypothesis, we applied a whole-genome shotgun strategy and de Bruijn graph-based algorithms to sequence and assemble the Antarctic minke whale (Balaenoptera bonaerensis, Mysticeti) genome, and compared it to a dolphin (an odontocete) and a cow (an artiodactyl, the cetacean sister group). In addition, we investigated fossils to understand the evolution of whale OB from the morphological aspects. Genetic evidences about mysticete gustation are also examined based on the genome assembly.
Materials and methods
Genome sequencing and assembly
Muscle tissue of Antarctic minke whale was purchased from a fish market in Japan, and the genomic DNA was extracted following the protocol of our previous work [4]. A paired-end sequencing library with average insert size of 330 bp was constructed and sequenced on an Illumina HiSeq2000 sequencer, and then assembled into scaffolds using PLATANUS assembler [18] ver. 1.2.1. Details about genome sequencing and de novo assembly are described in Additional file 1 §1. The Antarctic minke whale genome assembly thus obtained was named KUjira_1.0.
Cow (Bos taurus, Artiodactyla) genome assembly (UMD_3.1 assembly) [19] were downloaded from the GenBank FTP site (ftp://ftp.ncbi.nlm.nih.gov/genbank/genomes/Eukaryotes/vertebrates_mammals/Bos_taurus/Bos_taurus_UMD_3.1/). Bottlenose dolphin (Tursiops truncatus, Odontoceti) genome assembly (Ttru_1.4 assembly) [20] were also downloaded from the GenBank FTP site (ftp://ftp.ncbi.nlm.nih.gov/genbank/genomes/Eukaryotes/vertebrates_mammals/Tursiops_truncatus/Ttru_1.4/).
Olfaction-related genes in the cow genome
The loci of the OMACS, NQO1 and OCAM genes in the cow UMD_3.1 genome assembly follow NCBI reference sequence (RefSeq) annotations. The gene ID of each gene is as follows: OMACS, 100299006; NQO1, 519632; OCAM, 535613. We confirmed the RefSeq annotations by comparing translated amino acid sequences with those of other mammals. The amino acid sequences of 15 mouse TAARs (TAAR1 (GenBank accession no. NP_444435.1), TAAR2 (NP_001007267.1), TAAR3 (NP_001008429.1), TAAR4 (NP_001008499.1), TAAR5 (NP_001009574.1), TAAR6 (NP_001010828.1), TAAR7a (NP_001010829.1), TAAR7b (NP_001010827.1), TAAR7d (NP_001010838.1), TAAR7e (NP_001010835.1), TAAR7f (NP_001010839.1), TAAR8a (NP_001010830.1), TAAR8b (NP_001010837.1), TAAR8c (NP_001010840.1), TAAR9 (NP_001010831.1)) and six human TAARs (TAAR2-1 (NP_001028252.1), TAAR2-2 (NP_055441.2), TAAR5 (NP_003958.2), TAAR6 (NP_778237.1), TAAR8 (NP_444508.1), TAAR9 (NP_778227.3)) were used as queries and TAAR sequences were searched against the cow genome assembly using TBLASTN program ver. 2.2.25 [21] with e-value cutoff of <1e-20 and without filtering query sequences. All overlapping sequences of hits with the same orientations were merged. The sequences thus obtained were searched against the mouse protein database (downloaded from the following URL on 14/Oct/2011: http://www.ncbi.nlm.nih.gov/protein/?term=%22Mus+musculus%22%5Bporgn%3A__txid10090%5D) using FASTY program ver. 35.04 [22] and the sequence was discarded if its best hit was not a TAAR gene. Then we aligned all the remaining sequences using the L-INS-i program in the MAFFT package ver. 6.240 [23,24] and looked for the initiation and termination codons. If we could not find initiation and/or termination codons in a sequence, we extended the sequence in the 5’ and/or 3’ direction to find them. If a sequence was interrupted by premature stop codon(s) and/or frame shift(s), or if it lacked one or more trans-membrane (TM) regions completely, the sequence was judged to be a functionless pseudogene. As a result, 17 intact TAAR genes and 14 pseudoegenes were found. The classification of intact cow TAAR genes into TAAR1-9 follows the phylogenetic tree shown in Additional file 1 §3. Deduced amino acid sequences of 142 class I and 828 class II intact OR genes were retrieved from Niimura and Nei [25].
Olfaction-related genes in the whale and dolphin genomes
For the multi-exon OMACS, NQO1 and OCAM genes, we used the DNA sequence of each exon of the corresponding cow genes as a query and searched against the minke whale and dolphin genome assemblies using BLASTN with e-value cutoff of <1e-20 and without filtering query sequences. The sequences thus obtained were searched against the cow genome assembly using BLASTN and the sequence was discarded if its best hit was not its query. Several exons of the OMACS gene cannot be found following this method, and therefore we compared the genomic regions encoding other exons with that of cow genome in order to confirm that these missing exons are actually deleted from whale and dolphin genomes. For dot-plot comparisons, GenomeMatcher ver. 1.75 [26] was used with default settings, and the bl2seq [27] option was chosen to output figures (Figure 1). We followed the same methods which we used to identify cow TAAR genes to identify whale and dolphin TAAR, OR and V1R genes, using the query amino acid sequences as follows: 17 intact cow TAARs for searching TAAR sequences, 970 intact cow ORs (142 class I and 828 class II) for OR sequences and 32 cow intact V1Rs identified by Grus et al. [28] for V1R sequences. Because of fragmented scaffolds, we could not find initiation and/or termination codons of several sequences which were not judged to be pseudogenes. We labeled such sequences as truncated genes. Under these criteria, we found 324 OR genes (60 intact, 19 truncated and 245 pseudo) and 34 V1R genes (two intact and 32 pseudo) in the KUjira_1.0 assembly, and 166 OR genes (twelve intact, two truncated and 152 pseudo) and 18 V1R genes (one intact and 17 pseudo) in the Ttru_1.4 assembly. However, only five TAAR genes and pseudogenes were found from the KUjira_1.0 assembly and two in the Ttru_1.4 assembly. Therefore, we compared the genomic regions encoding a cluster of TAAR genes with that of the cow genome using the GenomeMatcher program in order to confirm that the missing TAAR genes are actually deleted from whale and dolphin genomes. In the case of multi-exon V2R genes, we also followed the same methods as described above but we searched only 3rd exons using 3rd exons of 79 intact rat V2Rs identified by Young and Trask [29] as queries. As a result, we could not find any sequences in the Ttru_1.4 assembly but we found one sequence in the KUjira_1.0 assembly. However, premature stop codons interrupt its open reading frame. Therefore, we conclude that this exon is a part of a functionless pseudogene and that neither whale nor dolphin possesses intact V2R genes.
Figure 1.
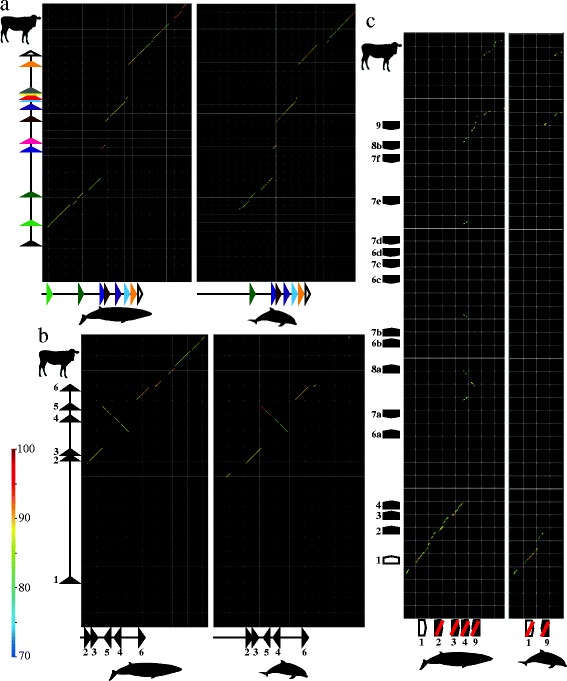
Dot-plot comparisons between Antarctic minke whale (horizontal, left) or dolphin (horizontal, right) and cow (vertical) sequences. Color scale bar indicates sequence similarity (%) of each dot. a. Comparisons of the genomic region where the OMACS gene is encoded. Cow sequence: chr. 25 (18,535,000-18,568,000 bp). Whale: scaffold100261 (1–18,000 bp, complement). Dolphin: scaffold4608 (95,000-115,000 bp, complement). The OMACS gene consists of 13 exons, and the position and the coding direction of each exon is shown as a triangle with an exon-specific color. Whale and dolphin have lost the genomic regions where the 5th, 9th, 10th and 11th exons are encoded. In addition, dolphin has lost the 1st and 2nd exons. Whale’s 1st exon is not included in this scaffold (see Additional file 1 for detail). Mesh size, 1 kbp × 1 kbp. b. Comparison of the genomic region where the NQO1 gene is encoded. Cow sequence: chr. 18 (36,908,355-36,927,688 bp). Whale: scaffold73885. Dolphin: scaffold317 (290,000-300,000 bp). The NQO1 gene consists of six exons, and the position and the coding direction of each exon are shown as a triangle with an exon number. Genomic inversion was confirmed in the whale and dolphin genomes around the region where the 4th and 5th exons are encoded. Whale’s 1st exon is not included in this scaffold (see Additional file 1 for detail). Mesh size, 1 kbp × 1 kbp. c. Comparison of the genomic region where the TAAR gene cluster is located. Cow sequence: chr. 9 (71,400,000-71,850,000 bp, complement). Whale: scaffold12993. Dolphin: scaffold181 (230,000-270,000 bp, complement). Positions and coding directions of TAAR1-9 genes are shown. Pseudogenes are indicated by red oblique lines (Cow TAAR pseudogenes are not shown). Mesh size, 10 kbp × 10 kbp.
Classification of cetacean OR genes into class I/class II
As Niimura and Nei pointed out [30], mammalian OR genes can clearly be classified into class I and class II based on the sequence similarity. Classification of OR genes into class I and II follows Glusman et al. [31] and Niimura and Nei [25], and the whale and dolphin intact OR genes identified in this study were classified into class I or II based on a phylogenetic tree which consists of deduced amino acid sequences of human (retrieved from HORDE database (http://genome.weizmann.ac.il/horde/) #43), cow, whale and dolphin intact OR genes (the phylogenetic tree is shown in Additional file 1 §3). In addition to 60 (minke whale) and twelve (dolphin) intact OR genes (Figure 2), we found 19 (minke whale) and two (dolphin) truncated OR genes. We added these truncated genes one by one to the OR phylogenetic tree and confirmed that all these truncated OR genes are classified into class II.
Figure 2.
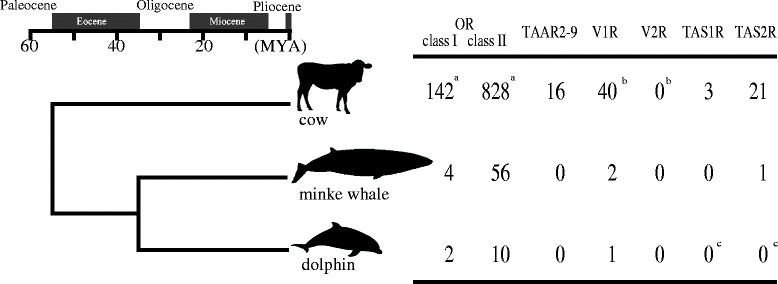
Phylogenetic relationships, divergence times and the numbers of intact chemosensory receptor genes of cow (Artiodactyla), Antarctic minke whale (Mysticeti) and bottlenose dolphin (Odontoceti). Notes: a. taken from Niimura and Nei [25]; b. taken from Shi and Zhang [52]; c. taken from Jiang et al. [7].
Genes for the sense of taste
TBLASTN searches with e-value cutoff of <1e-5 and without filtering query sequences were employed to identify TAS1R, TAS2R and GNAT3 genes. The amino acid sequence of cow GNAT3 is retrieved from GenBank (accession no. NP_001103452). Using all amniote GNAT3 sequences annotated in Ensembl database (http://www.ensembl.org/index.html) (release 73) as queries, GNAT3 genes were searched against KUjira_1.0 and Ttru_1.4 assemblies. TAS1R genes were also searched against UMD_3.1, KUjira_1.0 and Ttru_1.4 assemblies using all vertebrate TAS1R sequences annotated in Ensembl database (release 70) as queries. In the case of TAS2Rs, we used all intact Euarchontoglires TAS2Rs identified by Hayakawa et al. [32] as queries and searched against UMD_3.1, KUjira_1.0 and Ttru_1.4 assemblies. All overlapping sequences of hits with the same orientations were merged. The sequences thus obtained were searched against the human (GRCh37 assembly) [33,34] and the mouse (GRCm38 assembly) [35] genome assemblies using TBLASTX and the sequence was discarded if its best hit was not a GNAT3/TAS1R/TAS2R gene. Because TAS1Rs and GNAT3 are multi-exon genes, the results of TBLASTX were also utilized for subsequent exon annotations. Exon regions and splicing sites of the GNAT3 and TAS1R genes identified in this study were determined by comparing GNAT3 and TAS1R sequences of cetartiodactyls with that of humans and mice using E-INS-i program in the MAFFT package. A taste receptor gene was considered a pseudogene or truncated gene if the same criteria were met that we followed for odorant receptors.
Gene annotation information thus obtained is available as Additional files 2, 3 and 4.
The 6th exon of GNAT3 genes in several cetaceans and artiodactyls (Additional file 1: Table S7) were sequenced using a pair of primers shown in Table 1.
Table 1.
Primers used for amplifying and sequencing the 6 th exon of GNAT3 gene
| Name | Sequence | loci |
|---|---|---|
| G3-6F | AGGTGGACAGAGATCTGARAG | Within 6th exon of GNAT3 gene |
| G3-6R | TATAAAAGATGAAAATGTGTAGGAT | Within 6th exon of GNAT3 gene |
| note: | This pair of primers are designed to amplify a 299 bp-length region including the partial amino coding region of the 6th exon of GNAT3 gene | |
Fossil investigation
Several fossil whale skulls were used in this study. A skull of the pakicetid cetacean Ichthyolestes pinfoldi (Howard - Geological Survey of Pakistan, H-GSP 98134) was described by Nummela et al. [36]. Further preparation of sediment from the olfactory region of this specimen using an airscribe and dental tools, revealed the cribriform plate of this specimen. A specimen of the remingtonocetid cetacean Remingtonocetus (Indian Institute of Technology, Roorkee, IITR-SB 2770) was described by Bajpai et al. [37], and this specimen was CT-scanned, and 3D reconstructed using AMIRA software (FEI Visualization Science Group) ver. 5.4.1 as described by Bajpai et al. [37]. 3D reconstructions presented in Figure 3 were produced by AMIRA.
Figure 3.

Cribriform plate and olfactory bulb in extinct cetaceans. a. Skull of the pakicetid cetacean Ichthyolestes pinfoldi (H-GSP 98134, described by Nummela et al. [36]) in ventral view, rectangle indicates detail shown in (b). b. Detail of (a), showing the dorsal side of the cribriform plate with some of its perforations encircled, and the lateral wall (LW) of the olfactory chamber. c. ventral view of the endocast of the cranial cavity of the remingtonocetid cetacean Remingtonocetus (IITR-SB 2770, described by Bajpai et al. [37]) based on 3D reconstruction of CT-scans, showing impressions of olfactory tract (OT) and olfactory bulb (OB), area in box is enlarged in (d). d-g. impression of olfactory bulb in ventral, dorsal, lateral, and cranial view respectively, dorsal and rostral view show midline dorsal crest (DC), and lateral view shows the contrast between convex ventral side where cranial nerve I pierces the cribriform plate (CP), and flat dorsal side.
Results
Loss of the D domain in all modern whales
The D domain is defined by the expression of the OMACS gene [16,38], and the unique expression of NQO1 gene is also reported [39]. We found that, in both minke whales and dolphins, the OMACS gene is not functional due to the loss of the 5th, 9th, 10th and 11th exons (Figure 1a). Both cetaceans also lost the functional NQO1 gene due to genomic inversion (Figure 1b). These findings suggest that both OMACS and NQO1 genes turned into pseudogenes before the Odontoceti-Mysticeti split.
The V domain is defined by the expression of the OCAM gene [16,40]. Minke whales have maintained the complete coding region of the OCAM gene. In addition to that, dolphins, even though they are anosmic, also have kept this gene under strict purifying selection (Additional file 1 §2), suggesting that this gene has unknown function besides olfaction.
The molecular basis of olfaction relies on the repertoires of four families of chemosensory receptors: TAAR, OR, V1R and V2R [1]. OSNs expressing TAARs project specifically to the D domain of the OB [41,42], and all of the TAAR genes are located in a single gene cluster with no interspersed genes [42]. We found that minke whales have lost the TAAR5, 6, 7 and 8 genes and dolphins have lost the TAAR2-8 genes. In addition, all of the remaining TAAR genes of whales and dolphins are functionless pseudogenes except for the whale TAAR1 gene (Figure 1c), which is not involved in olfaction [43]. Deletion of TAAR5-8 genes from the minke whale genome was also confirmed by PCR (Additional file 1 §4). This finding suggests that both minke whales and dolphins have lost all the olfactory TAARs.
Mammalian ORs can be classified into two subfamilies, class I and class II, based on sequence similarity [30]. Most OSNs expressing class I ORs project specifically to the D domain [44], whereas OSNs expressing class II ORs project to both the D and V domains [45]. We found only four intact class I OR genes in the minke whale genome and two in the dolphin genome (Figure 2). Both whales and dolphins have kept two intact class I ORs, OR51E1 and OR51E2 (Additional file 1 §3). The expression of these two ORs is highly restricted to prostate tissues [46,47], indicating that these ORs play roles that are not related to olfaction. Minke whales have kept two more intact class I OR genes, but it is difficult to judge whether these two remaining genes are still functional or not. In any case, all cetaceans underwent a significant loss of olfactory-functional class I ORs in evolution. In contrast, 56 intact class II OR genes were found in the minke whale genome, well below the 828 present in cow [25], but above the ten in dolphins (Figure 2). This is consistent with our previous findings that V domain of the baleen whale OB is small but functional and that baleen whales have a sense of smell [10].
All of these findings suggest that mysticetes have lost most of the D domain-specific markers and receptors. We conclude that the mysticete OB lacks a region homologous to the D domain of the mouse OB. Putting the loss of the D domain markers in evolutionary perspective, we hypothesize that whales lost the D domain of the OB during the Eocene epoch, which is the time when whale ancestors migrated from land to water.
The OB communicates with the nasal cavity via the cribriform plate. The cribriform plate fossilizes and its shape can be used to deduce the shape of the OB, thus tracing the reduction of the D domain in evolutionary time. Cetaceans originated around 50 million years ago (MYA) [48], and their basal family is Pakicetidae [49]. In this family, the orbits are close together near the midline and the OB is located just anterior to the orbit [36]. As a consequence, the OB is very small [50]. However, we investigated the skull of a pakicetid Ichthyolestes and found that a part of the cribriform plate faces dorsally, and is perforated by many small foramina, presumably for cranial nerve I (Figure 3ab). On the other hand, the cribriform plate of remingtonocetids, Eocene whales closer to the divergence of mysticetes and odontocetes, differs from that of pakicetids, in that the OB faces ventral, similar to bowhead whales and there is no indication for dorsally projecting fibers of cranial nerve I (Figure 3c-g). Thus, whereas pakicetids show connections of the cribriform plate on the dorsal side of the OB, these connections are lost in remingtonocetids. In effect, the olfactory anatomy of modern minke whales resembles that of late Eocene whales [51]. This suggests that the D domain was lost during the course of the Eocene, but was present in the earliest cetaceans.
Loss of the vomeronasal olfaction in basal cetaceans
No intact V2R genes exist in the cetaceans and cattle (Figure 2), suggesting that this gene family was lost in the cetartiodactyl lineage before the cow-cetacean split, congruent with a previous report [52]. In contrast, cattle have 40 intact V1R genes whereas mysticetes have only two and odontocetes just one (Figure 2). Absence of the VO, in which V1Rs are expressed [1,53], can be inferred from fossils, and this suggests that the organ was lost around 45 MYA, before the divergence of odontocetes and mysticetes. The vomeronasal ducts of mammals pass through the anterior palatine foramina, and the absence of these foramina implies that the organ is absent. Whereas these foramina are still present in the earliest whales pakicetids [36], they have been lost in remingtonocetids [37], and it is likely that the VO was lost at this node of the cladogram.
Loss of the sense of sweet, umami and bitter tastes in whales
Regarding taste receptors, no intact genes had not been found in cetacean genomes except for TAS2R16 gene in common minke whale genome [7,8,13], and Feng et al. [12] suggested that several mysticetes possess an intact TAS2R16 gene based on sequencing of partial coding regions. We confirmed that Antarctic minke whales have also lost all functional TAS1R and TAS2R genes except for one TAS2R gene, TAS2R67. We sequenced complete coding regions of TAS2R16 and TAS2R67 genes in several cetartiodactyl species and confirmed that the last common ancestor of odontocetes and mysticetes possessed intact TAS2R16 and TAS2R67 sequences (Additional file 1 §5). In addition, we found that the last common ancestors of odontocetes and mysticetes possessed an intact TAS1R2 gene among TAS1R genes (Additional file 1 §5). However, proper function of the TAS1Rs and TAS2Rs requires their interaction with gustducin, and the GNAT3 (gustducin α-subunit)-KO mice show highly reduced responses to sweet and bitter taste [54]. Mammalian GNAT3 gene consists of eight exons, and all exons of GNAT3 except for the 2nd and 3rd exons were found in both KUjira_1.0 and Ttru_1.4 assemblies. Several pseudogenization mutations (frame shift mutations and premature stop codons) were found in both the whale and dolphin GNAT3 sequences, indicating that both whales and dolphins lost the functional GNAT3. Especially among such mutations, whales and dolphins share two pseudogenization mutations (1 bp deletion and a premature stop codon) in the 6th exon. We sequenced 6th exon of the GNAT3 gene and found that all cetaceans have lost the GNAT3, by means of a frame shift mutation and a premature stop codon in the 6th exon (Figure 4). These findings suggest that the gustatory capability had been greatly reduced in cetaceans between the Artiodactyla-Cetacea split and the Odontoceti-Mysticeti split.
Figure 4.

A multiple alignment of partial nucleotide sequences of the 6th exon of GNAT3 . Human, mouse and cow GNAT3 sequences were shown in addition to that of cetaceans and artiodactyls sequenced (shown with red fonts) or annotated in this study. Numbers above the alignment are corresponding to the nucleotide positions in the human GNAT3. Sequences based on Sanger-sequencing technology are indicated by red fonts. Asterisks under the alignment indicate non-variable nucleotide positions. Disrupting mutations and their positions are shaded. ‘Hap1’ and ‘hap2’ indicate haplotypes. All cetaceans share two lineage-specific pseudogenization mutations (a 1-bp deletion in the position 643 and a premature stop codon in the position 658–660).
Discussion
Taken together, these results describe the outline of chemosensory evolution in cetaceans during the land to water transition. Cetaceans are derived from artiodactyls with well-developed olfactory and vomeronasal organs [55,56], although their V2Rs were already lost [52]. Amphibious basal cetaceans emerged around 50 MYA, when olfactory organs were reduced, but retained both D and V domains. Around 45 MYA, the cetacean family Remingtonocetidae underwent significant changes in their chemical senses, losing the VO and the D domain of the OB. At this time, V1Rs, OMACS, NQO1, olfactory TAARs as well as most of class I OR genes are speculated to have lost their functions. Remingtonocetus are considered to have been one of the earliest whales that acquired well-established underwater hearing systems [37], and it is possible that, at this point in evolution, the importance of olfaction as a sense decreased. Basically, these conditions were maintained in modern mysticete whales. It is not obvious that physiological studies using mice can be directly extended to other mammals. However, it is reasonable to assume that the olfactory capability of mysticetes is similar to that of ΔD mice, i.e., that mysticetes lack innate avoidance behavior against predator odors and spoiled smells. Terrestrial animals cannot prey on fully aquatic whales, and whales’ predators, such as sharks and killer whales, cannot be detected by smelling in air. In addition, unlike the nares of other mammals, whales’ nares are not located at the tip of their snout, and whales’ nasal passage is not connected directly to their oral cavity, indicating that it is difficult for whales to rely on olfaction to judge whether something they are about to swallow is edible or not. Further studies will test this assumption. The evolution of taste cannot be traced in mysticete evolution, but gene evidence indicates that all cetaceans lack functional receptors for sweet, bitter and umami flavors. Although some Neogene odontocetes had a large OB chamber and well-developed cribriform plate [57], modern odontocetes reduced their chemical senses even further, losing the entire OB with further loss of class II OR genes.
This study indicates that all modern cetaceans lack innate avoidance behavior against spoiled smells, and the sense of tastes. This could be one of the reasons why cetaceans often die from ingesting inedible debris [58], and has implications for whale conservation.
Conclusion
We showed that, though mysticetes possess functional olfactory bulbs, they had lost the D domain of the olfactory bulb during the Eocene Epoch. In addition, all modern whales have lost the functional sweet, umami and bitter taste receptors before the Odontoceti-Mysticeti split (Figure 5). These findings suggest that profound changes in the chemosensory capabilities occurred in the cetacean lineage before the Odontoceti-Mysticeti split, during the period when ancestral whales migrated from land to water.
Figure 5.
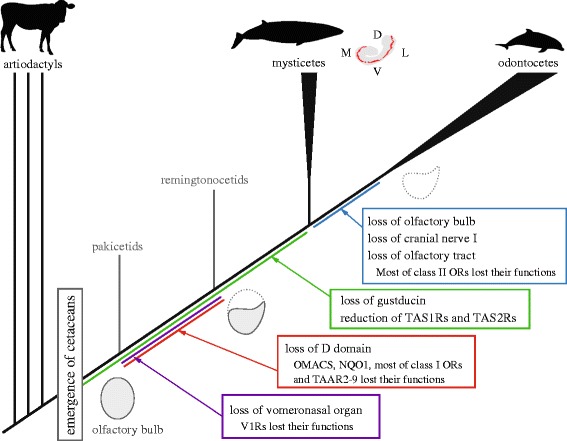
Simplified phylogeny of cetaceans discussed here with evolutionary events indicated. Distribution pattern of the glomeruli on the coronal section of OB of a modern mysticete (modified from Thewissen et al. [10]; D, dorsal; M, medial; V, ventral; L, lateral) , and evolutionary changes of the speculated shapes of the coronal section of OB, are also shown. Our findings indicate that profound changes on olfactory systems occurred just before the modern whales-remingtonocetids split. Remingtonocetus are suggested to have been ambush predators that were one of the earliest whales living in seawater [37]. They had well-established capabilities of underwater hearing, but their ecology remains largely elusive.
Availability of supporting data
The Antarctic minke whale genome data sets supporting the results of this article are available in the DDBJ/EBI/NCBI databases under the following BioProject ID: PRJDB1465. The 6th exons of the GNAT3 genes amplified and sequenced in this study are available in the DDBJ/EMBL/GenBank databased under the following accession numbers: AB897678, AB897679, AB897680, AB897682 and AB897683.
Acknowledgements
This work was supported by Excellent Graduate Schools of Biodiversity & Evolution of Kyoto University, Leading Graduate Program of Primatology & Wildlife Science of Kyoto University, Yamada Science Foundation and JSPS KAKENHI (24770075 to TK and 12J04270 to TH). The super computer system of National Institute of Genetics (NIG), Research Organization of Information and Systems (ROIS) was used in part for assembling the whale genome. We are grateful to Rei Kajitani, Osamu Nishimura, Yasuhiro Go and Elizabeth Nakajima for technical advices and helpful comments. Muscle tissue of a hippopotamus was kindly provided by Takeshi Wada, Osaka Museum of Natural History.
Abbreviations
- D domain
Dorsal domain
- GNAT3
Guanine nucleotide binding protein, alpha transducing 3
- GPCR
G-protein coupled receptor
- MYA
Million years ago
- NQO1
NAD(P)H dehydrogenase quinone 1
- OB
Olfactory bulb
- OCAM
Olfactory cell adhesion molecule
- OMACS
Olfactory medium-chain acyl-CoA synthetase
- OR
Olfactory receptor
- OSN
Olfactory sensory neuron
- TAAR
Trace amine-associated receptor
- TAS1R
Taste receptor type 1
- TAS2R
Taste receptor type 2
- TM
Trans-membrane
- V domain
Ventral domain
- V1R
Vomeronasal receptor type 1
- V2R
Vomeronasal receptor type 2
- VO
Vomeronasal organ
Additional files
This additional file provides the supplementary information on 1) Antarctic minke whale (Balaenoptera bonaerensis) genome assembly; 2) Evolution of OCAM gene in the cetacean lineage; 3) Phylogenetic analyses and classification of TAAR , OR and V1R genes; 4) Amplification of a genomic region between TAAR4 and TAAR9 genes in the minke whale genome; and 5) Evolution of the taste receptor genes in cetaceans.
Gene annotation on Balaenoptera bonaerensis genome assembly (KUjira_1.0).
Gene annotation on Tursiops truncatus genome assembly (Ttru_1.4).
Gene annotation on Bos taurus genome assembly (UMD_3.1).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TK designed this study, sequenced and assembled the whale genome, performed the molecular experiment, analyzed the data and drafted the manuscript. JGMT investigated fossils and drafted the manuscript. TH performed the molecular experiments, analyzed the data and drafted the manuscript related to taste genes. HI performed the molecular experiments. KA sequenced the whale genome. All authors read and approved the final manuscript.
Contributor Information
Takushi Kishida, Email: takushi@zoo.zool.kyoto-u.ac.jp.
JGM Thewissen, Email: thewisse@neomed.edu.
Takashi Hayakawa, Email: hayakawa.takashi.46s@st.kyoto-u.ac.jp.
Hiroo Imai, Email: imai.hiroo.5m@kyoto-u.ac.jp.
Kiyokazu Agata, Email: agata@mdb.biophys.kyoto-u.ac.jp.
References
- 1.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nat Rev Genet. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 2.Pihlström H. Comparative anatomy and physiology of chemical senses in aquatic mammals. In: Thewissen JGM, Nummela S, editors. Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates. Berkeley: University of California Press; 2008. pp. 95–109. [Google Scholar]
- 3.Hayden S, Bekaert M, Crider TA, Mariani S, Murphy WJ, Teeling EC. Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res. 2010;20:1–9. doi: 10.1101/gr.099416.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kishida T, Kubota S, Shirayama Y, Fukami H. The olfactory receptor gene repertoires in secondary-adapted marine vertebrates: evidence for reduction of the functional proportions in cetaceans. Biol Lett. 2007;3:428–430. doi: 10.1098/rsbl.2007.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGowen MR, Clark C, Gatesy J. The vestigial olfactory receptor subgenome of odontocete whales: phylogenetic congruence between gene-tree reconciliation and supermatrix methods. Syst Biol. 2008;57:574–590. doi: 10.1080/10635150802304787. [DOI] [PubMed] [Google Scholar]
- 6.Oelschläger HHA, Ridgway SH, Knauth M. Cetacean brain evolution: Dwarf sperm whale (Kogia sima) and common dolphin (Delphinus delphis) - An investigation with high-resolution 3D MRI. Brain Behav Evol. 2010;75:33–62. doi: 10.1159/000293601. [DOI] [PubMed] [Google Scholar]
- 7.Jiang P, Josue J, Li X, Glaser D, Li W, Brand JG, Margolskee RF, Reed DR, Beauchamp GK. Major taste loss in carnivorous mammals. Proc Natl Acad Sci U S A. 2012;109:4956–4961. doi: 10.1073/pnas.1118360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Sun F, Xu S, Fan G, Zhu K, Liu X, Chen Y, Shi C, Yang Y, Huang Z, et al. Baiji genomes reveal low genetic variability and new insights into secondary aquatic adaptations. Nat Commun. 2013;4:2708. doi: 10.1038/ncomms3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachtigall P, Hall R. Taste reception in the bottlenosed dolphin. Acta Zoologica Fennica. 1984;172:147–148. [Google Scholar]
- 10.Thewissen JGM, George J, Rosa C, Kishida T. Olfaction and brain size in the bowhead whale (Balaena mysticetus) Mar Mamm Sci. 2011;27:282–294. doi: 10.1111/j.1748-7692.2010.00406.x. [DOI] [Google Scholar]
- 11.Kishida T, Thewissen JGM. Evolutionary changes of the importance of olfaction in cetaceans based on the olfactory marker protein gene. Gene. 2012;492:349–353. doi: 10.1016/j.gene.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Feng P, Zheng J, Rossiter SJ, Wang D, Zhao H. Massive Losses of Taste Receptor Genes in Toothed and Baleen Whales. Genome Biol Evol. 2014;6:1254–1265. doi: 10.1093/gbe/evu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yim H-S, Cho YS, Guang X, Kang SG, Jeong J-Y, Cha S-S, Oh H-M, Lee J-H, Yang EC, Kwon KK, et al. Minke whale genome and aquatic adaptation in cetaceans. Nat Genet. 2014;46:88–92. doi: 10.1038/ng.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serizawa S, Miyamichi K, Sakano H. One neuron–one receptor rule in the mouse olfactory system. Trends Genet. 2004;20:648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/S0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- 16.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 17.Imai T, Sakano H. Roles of odorant receptors in projecting axons in the mouse olfactory system. Curr Opin Neurobiol. 2007;17:507–515. doi: 10.1016/j.conb.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Kajitani R, Toshimoto K, Noguchi H, Toyoda A, Ogura Y, Okuno M, Yabana M, Harada M, Nagayasu E, Maruyama H, et al. Efficient de novo assembly of highly heterozygous genomes from whole-genome shotgun short reads. Genome Res. 2014;24:1384–1395. doi: 10.1101/gr.170720.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimin AV, Delcher AL, Florea L, Kelley DR, Schatz MC, Puiu D, Hanrahan F, Pertea G, Van Tassell CP, Sonstegard TS, et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10:R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindblad-Toh K, Garber M, Zuk O, Lin MF, Parker BJ, Washietl S, Kheradpour P, Ernst J, Jordan G, Mauceli E, et al. A high-resolution map of human evolutionary constraint using 29 mammals. Nature. 2011;478:476–482. doi: 10.1038/nature10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson WR, Wood T, Zhang Z, Miller W. Comparison of DNA sequences with protein sequences. Genomics. 1997;46:24–36. doi: 10.1006/geno.1997.4995. [DOI] [PubMed] [Google Scholar]
- 23.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niimura Y, Nei M. Extensive gains and losses of olfactory receptor genes in Mammalian evolution. PLoS ONE. 2007;2:e708. doi: 10.1371/journal.pone.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtsubo Y, Ikeda-Ohtsubo W, Nagata Y, Tsuda M. GenomeMatcher: a graphical user interface for DNA sequence comparison. BMC Bioinform. 2008;9:376. doi: 10.1186/1471-2105-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatusova TA, Madden TL. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 28.Grus WE, Shi P, Zhang YP, Zhang J. Dramatic variation of the vomeronasal pheromone receptor gene repertoire among five orders of placental and marsupial mammals. Proc Natl Acad Sci U S A. 2005;102:5767–5772. doi: 10.1073/pnas.0501589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JM, Trask BJ. V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet. 2007;23:212–215. doi: 10.1016/j.tig.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Niimura Y, Nei M. Evolutionary dynamics of olfactory and other chemosensory receptor genes in vertebrates. J Hum Genet. 2006;51:505–517. doi: 10.1007/s10038-006-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glusman G, Bahar A, Sharon D, Pilpel Y, White J, Lancet D. The olfactory receptor gene superfamily: data mining, classification, and nomenclature. Mamm Genome. 2000;11:1016–1023. doi: 10.1007/s003350010196. [DOI] [PubMed] [Google Scholar]
- 32.Hayakawa T, Suzuki-Hashido N, Matsui A, Go Y: Frequent Expansions of the Bitter Taste Receptor Gene Repertoire during Evolution of Mammals in the Euarchontoglires Clade. Mol Biol Evol. 2014;31:2018-2031. [DOI] [PubMed]
- 33.International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 34.International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- 35.Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 36.Nummela S, Hussain ST, Thewissen JGM. Cranial anatomy of Pakicetidae (Cetacea, Mammalia) J Vertebr Paleontol. 2006;26:746–759. doi: 10.1671/0272-4634(2006)26[746:CAOPCM]2.0.CO;2. [DOI] [Google Scholar]
- 37.Bajpai S, Thewissen JGM, Conley RW. Cranial Anatomy of Middle Eocene Remingtonocetus (Cetacea, Mammalia) from Kutch, India. J Paleontol. 2011;85:703–718. doi: 10.1666/10-128.1. [DOI] [Google Scholar]
- 38.Oka Y, Kobayakawa K, Nishizumi H, Miyamichi K, Hirose S, Tsuboi A, Sakano H. O-MACS, a novel member of the medium-chain acyl-CoA synthetase family, specifically expressed in the olfactory epithelium in a zone-specific manner. Eur J Biochem. 2003;270:1995–2004. doi: 10.1046/j.1432-1033.2003.03571.x. [DOI] [PubMed] [Google Scholar]
- 39.Gussing F, Bohm S. NQO1 activity in the main and the accessory olfactory systems correlates with the zonal topography of projection maps. Eur J Neurosci. 2004;19:2511–2518. doi: 10.1111/j.0953-816X.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoshihara Y, Kawasaki M, Tamada A, Fujita H, Hayashi H, Kagamiyama H, Mori K. OCAM: a new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacifico R, Dewan A, Cawley D, Guo C, Bozza T. An olfactory subsystem that mediates high-sensitivity detection of volatile amines. Cell Reports. 2012;2:76–88. doi: 10.1016/j.celrep.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewan A, Pacifico R, Zhan R, Rinberg D, Bozza T. Non-redundant coding of aversive odours in the main olfactory pathway. Nature. 2013;497:486–489. doi: 10.1038/nature12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 44.Tsuboi A, Miyazaki T, Imai T, Sakano H. Olfactory sensory neurons expressing class I odorant receptors converge their axons on an antero-dorsal domain of the olfactory bulb in the mouse. Eur J Neurosci. 2006;23:1436–1444. doi: 10.1111/j.1460-9568.2006.04675.x. [DOI] [PubMed] [Google Scholar]
- 45.Miyamichi K, Serizawa S, Kimura HM, Sakano H. Continuous and Overlapping Expression Domains of Odorant Receptor Genes in the Olfactory Epithelium Determine the Dorsal/Ventral Positioning of Glomeruli in the Olfactory Bulb. J Neurosci. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu LL, Stackhouse BG, Florence K, Zhang W, Shanmugam N, Sesterhenn IA, Zou Z, Srikantan V, Augustus M, Roschke V. PSGR, a novel prostate-specific gene with homology to a G protein-coupled receptor, is overexpressed in prostate cancer. Cancer Res. 2000;60:6568–6572. [PubMed] [Google Scholar]
- 47.Weng J, Wang J, Hu X, Wang F, Ittmann M, Liu M. PSGR2, a novel G-protein coupled receptor, is overexpressed in human prostate cancer. Int J Cancer. 2006;118:1471–1480. doi: 10.1002/ijc.21527. [DOI] [PubMed] [Google Scholar]
- 48.Thewissen JGM, Cooper LN, George JC, Bajpai S. From Land to Water: the Origin of Whales, Dolphins, and Porpoises. Evo Edu Outreach. 2009;2:272–288. doi: 10.1007/s12052-009-0135-2. [DOI] [Google Scholar]
- 49.Thewissen JGM, Williams EM, Roe LJ, Hussain ST. Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature. 2001;413:277–281. doi: 10.1038/35095005. [DOI] [PubMed] [Google Scholar]
- 50.Thewissen JGM, Nummela S. Sensory evolution in aquatic tetrapods: toward and integrative approach. In: Thewissen JGM, Nummela S, editors. Sensory biology on the threshold: Adaptations in secondarily aquatic vertebrates. Berkeley: University of California Press; 2008. pp. 333–340. [Google Scholar]
- 51.Godfrey SJ, Geisler J, Fitzgerald EMG. On the Olfactory Anatomy in an Archaic Whale (Protocetidae, Cetacea) and the Minke Whale Balaenoptera acutorostrata (Balaenopteridae, Cetacea) Anat Rec. 2013;296:257–272. doi: 10.1002/ar.22637. [DOI] [PubMed] [Google Scholar]
- 52.Shi P, Zhang J. Comparative genomic analysis identifies an evolutionary shift of vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Res. 2007;17:166–174. doi: 10.1101/gr.6040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohara H, Nikaido M, Date-Ito A, Mogi K, Okamura H, Okada N, Takeuchi Y, Mori Y, Hagino-Yamagishi K. Conserved repertoire of orthologous vomeronasal type 1 receptor genes in ruminant species. BMC Evol Biol. 2009;9:233. doi: 10.1186/1471-2148-9-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- 55.Spaulding M, O'Leary MA, Gatesy J. Relationships of Cetacea (Artiodactyla) among mammals: increased taxon sampling alters interpretations of key fossils and character evolution. PLoS ONE. 2009;4:e7062. doi: 10.1371/journal.pone.0007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatesy J, Geisler JH, Chang J, Buell C, Berta A, Meredith RW, Springer MS, McGowen MR. A phylogenetic blueprint for a modern whale. Mol Phylogenet Evol. 2013;66:479–506. doi: 10.1016/j.ympev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Godfrey SJ. On the olfactory apparatus in the Miocene odontocete Squalodon sp. (Squalodontidae) Comptes Rendus Palevol. 2013;12:519–530. doi: 10.1016/j.crpv.2013.03.002. [DOI] [Google Scholar]
- 58.Simmonds MP. Cetaceans and Marine Debris: The Great Unknown. J Mar Biol. 2012;2012:684279. doi: 10.1155/2012/684279. [DOI] [Google Scholar]


