Abstract
Tumor-promoting effects of ethyl tertiary-butyl ether (ETBE) were investigated in a 2-stage carcinogenesis bioassay with regard to hepatic and renal carcinogenesis in rats. Male 6-week-old Wistar rats were given drinking water containing N-ethyl-N-(2-hydroxyethyl)nitrosamine (EHEN), as an initiator, at a dose of 500 ppm for 2 weeks. Starting one week thereafter, the animals were administered ETBE at dose levels of 0 (control), 100, 300, 500 or 1,000 mg/kg/day by gavage for 19 weeks from week 4 to 22. Necropsy of all rats was performed at week 23, and livers and kidneys were examined histopathologically. Incidences of hepatocellular adenomas, and those of combined hepatocellular adenomas and carcinomas were significantly elevated in rats given 1,000 mg/kg/day ETBE, but not 100‒500 mg/kg/day ETBE, and there was a significant increase in the average numbers of lesions. No significant differences in incidences and average numbers of renal tubule neoplasms were found in rats administered 100‒1,000 mg/kg/day ETBE. However, the average numbers of atypical tubule hyperplasias, considered to be preneoplastic lesions, were significantly increased in rats given ETBE at 1,000 mg/kg/day, but not in rats given 500 mg/kg/day or lower doses. Thus, these results imply that ETBE has hepatic and renal tumor-promoting activities that affect EHEN-induced carcinogenesis in male rats, and the no-observed-effect level is 500 mg/kg/day under the present experimental conditions.
Keywords: ethyl tertiary-butyl ether, tumor promotion, hepatic carcinogenesis, renal carcinogenesis, Wistar rat, N-ethyl-N-(2-hydroxyethyl)nitrosamine
Introduction
Ethers or other oxygen-containing compounds are used in place of lead in gasoline. Oxygenates enhance the octane number and improve combustion efficiency, thereby reducing emissions. In particular, methyl tertiary-butyl ether (MTBE) is used worldwide. While it was removed from the market in the United States due to contamination of ground water, this was based on its spoilage due to taste and odor issues rather than any toxicity1, 2. Other aliphatic ethers, such as ethyl tertiary-butyl ether (ETBE), tertiary-amyl methyl ether (TAME) and alcohols are alternatives to MTBE. The technical characteristics of ETBE are comparable to those of MTBE, and its very lower water solubility is considered an advantage, because the risk of contamination of underground water is low3. Another reason for the interest in ETBE is that it may serve to increase the market for biofuels, which can be manufactured from ethanol derived from agricultural and forestry feedstock.
In a toxicological review, it was concluded that ETBE was not genotoxic in in vitro studies using bacterial mutation tests and chromosomal aberration tests in cultured mammalian cells or in an in vivo micronucleus test3. Recently, it was clearly demonstrated that ETBE is not genotoxic by detailed micronucleus assay using the bone marrow of both sexes of rats in a subchronic drinking water toxicity study and an inhalation toxicity study4. However, there was equivocal evidence regarding carcinogenicity (tumor induction of the mouth epithelium, forestomach and schwannoma of the uterus and vagina) in rodents3. In addition, significant increases in quantitative values of glutathione S-transferase placental form (GST-P) positive foci, considered to be preneoplastic lesions, and hepatocellular adenomas were found in rats given 1,000 mg/kg/day ETBE in our previous medium-term multiorgan carcinogenesis bioassay5. Recently, development of hepatocellular adenoma was found in male F344 rats exposed to 5,000 ppm, but not less than 1,500 ppm, of ETBE in an inhalation carcinogenicity study6. However, it was reported that administration of ETBE (at doses of 625‒10,000 ppm) in drinking water for two years did not exert any carcinogenicity in both sexes of F344 rats7.
In the kidney, significant elevation of atypical tubule hyperplasia, also considered to be a preneoplastic lesion, was observed in rats given ETBE in our previous medium-term multiorgan carcinogenesis bioassay5. In addition, tertiary-butyl alcohol (TBA), a metabolite of ETBE, reportedly induces renal tubule adenomas associated with α2u-globulin nephropathy in male rats3, 8.
The medium-term hepatic and renal carcinogenesis bioassay of rats used in the present investigation is a rapid, reliable and practical tool for the early detection of carcinogenic agents and promoters targeting the liver and kidney9,10,11,12,13. The objectives of the present study were to determine any tumor-promoting effects of ETBE on hepatic and renal carcinogenesis induced by N-ethyl-N-(2-hydroxyethyl)nitrosamine (EHEN) in male rats and its no-effect dose level.
Materials and Methods
The present study was performed in compliance with the Good Laboratory Practice (GLP) Standards for Nonclinical Safety Studies of Drugs (Ministry of Health and Welfare Ordinance No. 21, March 26, 1997, Japan) and also in accordance with Section 3.2 (In vivo Additional Tests for Detection of Carcinogenicity) of the Guidelines for Carcinogenicity Studies of Drugs (Ministry of Health and Welfare Notification No.1607, November 1, 1999, Japan).
Initiator
EHEN (or N-Ethyl-N-hydroxyethylnitrosamine) (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) was used as an initiator in this bioassay, the specifications of which were as follows: lot numbers, HF2UF and IM01; CAS No., 13147-25-6; and storage condition, cool and shielded from light (in a refrigerator).
Test material
The ETBE used in the present study was manufactured by Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), and its specifications were as follows: appearance, colorless transparent liquid; boiling point, 70°C; vapor pressure, 17 Kpa (25°C); specific gravity, 0.74 (25°C/4°C); solubility, slightly soluble in water (1.2 g/100 g, 20°C); lot no., R74EE; purity, 99.3%; and storage conditions, store at room temperature and in a dark place. ETBE was stored in a steel can and kept at ambient temperature in a dark room.
Preparation of dosing solutions and analyses
The test material was accurately weighed, dissolved in olive oil (Nacalai Tesque, Inc., Kyoto, Japan), adjusted to produce10.0 w/v%, 5.0 w/v%, 3.0 w/v% and 1.0 w/v% ETBE dosing solutions, which were prepared more than once in each 7-day period, introduced into glass bottles and stored in a refrigerator. Dosing solutions of 10.0 w/v% and 0.05 w/v% were confirmed to be homogeneous and stable for 7 days (information from the Japanese Petroleum Energy Center and Petroleum Association of Japan). Analyses of concentrations of the test material in dosing preparations were performed twice during the course of the study, and each concentration of dosing solution was confirmed to be within the acceptable range (intended value ± 10%). All concentration analyses of the test material in the dosing solution were performed by gas chromatography with a flame ionization detector (GC-FID) at Nisso Chemical Analysis Service Co., Ltd. (Odawara, Kanagawa, Japan).
Animals and husbandry
Male, 5-week-old Crlj:WI (Wistar) rats (SPF animals) from Charles River Laboratories Japan Inc. (Atsugi, Kanagawa, Japan) were allowed an 8-day quarantine/acclimation period, during which health conditions and body weights were monitored. Only after confirmation of normal status were they entered into the study at the age of 6 weeks. The animals were housed 2 to a polycarbonate cage on hardwood chip bedding (Beta Chips, Northeastern Products Corp., Warrensburg, NY, USA) in an environmentally controlled room. Constant conditions of temperature (20–24°C), relative humidity (52–71%), and ventilation (more than 15 times/hr) were maintained, and the room was artificially illuminated to provide 12 hr of lighting (7:00–19:00) each day. Gamma ray irradiated (6.0 kGy) powder diet MF (Oriental Yeast Co. Ltd., Tokyo, Japan) and Ichinomiya city tap water were available ad libitum. Wistar rats were selected for this bioassay because of their high sensitivity to the initiator, EHEN, and their routine use for this bioassay10, 12, 13.
All experimental procedures were performed in accordance with the Law for the Humane Treatment and Management of Animals (Law No. 105, October 1, 1973, and amendment, December 21, 1999), Standards Relating to the Care and Management etc. of Experimental Animals (Notification No. 6, March 27, 1980, Prime Minister’s Office, Japan, and amendment, May 28, 2002) and Guideline for Animal Experimentation (May 22, 1987, Japanese Association for Laboratory Animal Science). The present study was conducted in accordance with the Standard for Care and Use of Laboratory Animals of DIMS Institute of Medical Science, Inc. (December 15, 2006).
Experimental design
An outline of the experimental design for the present study is shown in Fig. 1. A randomized block design (BrexNote Net, Yukms Co., Ltd., Tokyo, Japan) was used to allocate 150 rats to 5 groups (30/group). The animals were given drinking water containing 500 ppm EHEN from the commencement of the experiment to week 29,10,11 and maintained without treatment for 1 week9, 11. Reallocation was performed before the commencement of ETBE treatment, since the mean body weights were significantly different among the groups. Beginning in week 4, they were administered ETBE at dose levels of 0 mg/kg/day in the control group (group 1), or 100, 300, 500 or 1,000 mg/kg/day (groups 2‒5) by gavage (daily, 7 days/week) for 19 weeks (from week 4 to 22). The highest dose of ETBE was selected based on our previous study5. The administered volume (10 mL/kg) of ETBE dosing solution was adjusted based on the latest body weight of each rat. The animals were observed daily for abnormalities, and individual body weights were recorded weekly. Food and water consumption was measured over a 2-day period before each weighing. At experimental week 23, all animals were fasted overnight and euthanized for examination of preneoplastic and neoplastic lesion development in the liver and kidney.
Fig. 1.
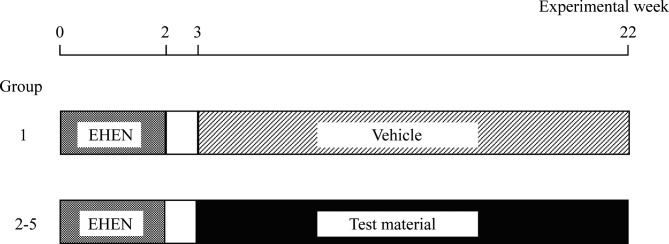
Experimental design. Animals were given drinking water containing 500 ppm EHEN for 2 weeks. Beginning in week 4, the animals were administered ETBE at dose levels of 0 (control), 100, 300, 500 or 1,000 mg/kg/day by gavage (daily, 7 days/week) for 19 weeks from week 4 to 22. All animals were euthanized at week 23.
Pathological examination
At necropsy, all tissues and organs were examined grossly, the liver, kidney, thoracic and abdominal organs and any other tissues with abnormal appearances were removed and preserved in 10% neutral buffered formalin solution. The livers and kidneys were weighed, and relative organ weights were calculated using the final body weights. The livers and kidneys from all rats and any other tissues with an abnormal appearance were routinely embedded in paraffin, sectioned and stained with hematoxylin and eosin solutions for histopathological examination.
Statistical analysis
The significance of differences for each parameter was analyzed and evaluated at P<0.05 or P<0.01. Statistical comparisons between group 1 and groups 2‒5 for numerical data obtained for body weights, food consumption, water consumption, organ weights, mean numbers of gross lesions for the liver and kidney and mean numbers of hyperplastic and neoplastic lesions for the liver and kidney were assessed using the Bartlett’s test. If homogeneous, the data were analyzed with Dunnett’s multiple comparison test (two sided), and if not, they were analyzed with Steel’s test (two sided). The significance of intergroup differences (between group 1 and groups 2 to 5) in incidences of findings from gross pathology and histopathology was analyzed using the Fisher’s exact probability test (two sided), and comparison of the grade of lesions was performed using the Mann-Whitney U-test (two sided). In addition, the Cochran-Armitage test was applied to analyze the increasing trend of incidences for hyperplastic and neoplastic lesions of the liver and kidney.
Results
Antemortem investigations
As no animals died during the experiment, the survival rates with 0 (group 1) and 100, 300, 500 and 1,000 mg/kg/day ETBE (groups 2‒5) were 100% at the end of the experiment. No abnormality in general condition was found in any animal during the EHEN treatment period (weeks 1 and 2) or nontreatment period (week 3). During the test material treatment period (weeks 4‒22), no clinical signs related to ETBE treatment were found in any ETBE-treated animal. The mean body weights with 100, 300, 500 and 1,000 mg/kg/day ETBE (groups 2‒5) were comparable to the control values throughout the experimental period. Significant increases in food consumption were observed with 1,000 mg/kg/day ETBE (group 5) at weeks 5 and 7‒22 as compared with the control values. During the ETBE treatment period, tendencies for increases in mean water consumption were noted with 1,000 mg/kg/day ETBE (group 5) at weeks 4, 5 and 22 when compared with the control values (group 1).
During the EHEN treatment period (weeks 1 and 2), the average EHEN intake, calculated from the nominal concentrations of EHEN, mean body weights and water consumption values, was 65 mg/kg/day.
Gross pathology and relative organ weights
A significantly increased incidence of discolored nodules of the liver was found in rats given 1,000 mg/kg/day ETBE (group 5) (data not shown). Cysts, discolored spots and discolored areas of the liver were sporadically observed in both the control and ETBE-treated groups. In the kidneys, cysts, discolored spots/areas and discolored nodules were also observed in each group, but there were no treatment-related increases.
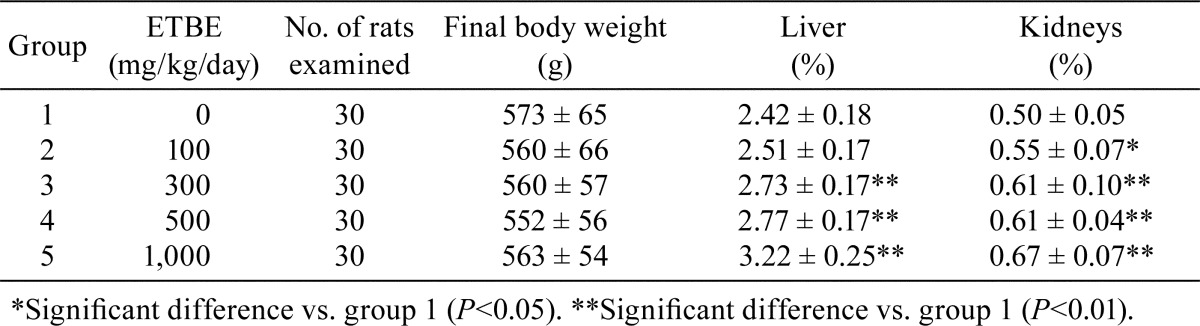
Final body weight and relative organ weight data are summarized in Table 1. Statistically significant increases in relative liver weights were noted with 300, 500 and 1,000 mg/kg/day ETBE (groups 3‒5). Significant increases in relative kidney weights were found with 100‒1,000 mg/kg/day ETBE (groups 2‒5). These increases in relative liver and kidney weights were dose dependent.
Table 1. Final Body Weight and Relative Organ Weight Data for Rats Initiated with EHEN and then Given ETBE.
Histopathology
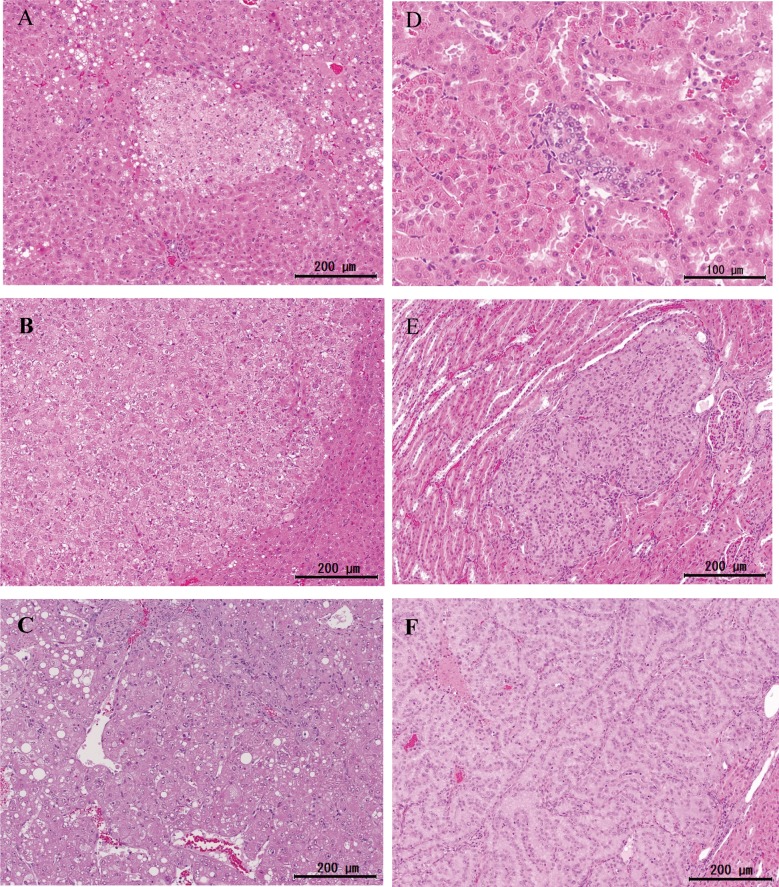
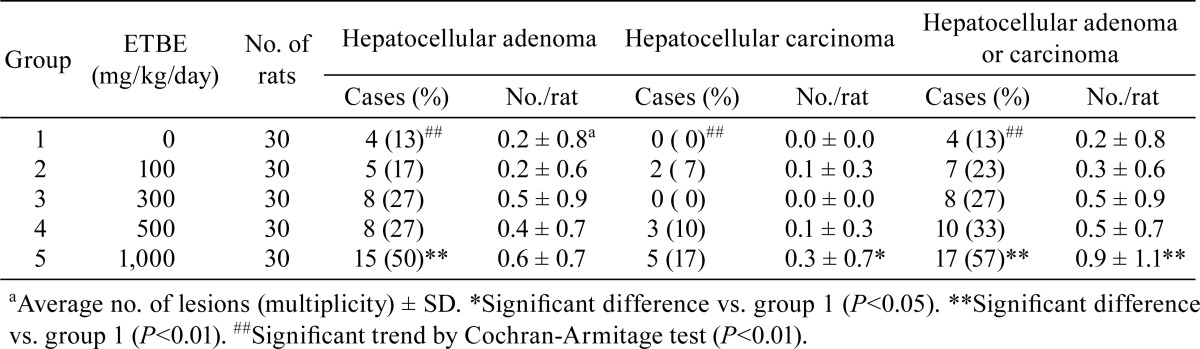
In the liver, foci (areas) of cellular alterations (Fig. 2A) were observed in all animals in each group, including the control group (data not shown). The incidence of hepatocellular adenomas (Fig. 2B) and combined incidence of hepatocellular adenomas and carcinomas were significantly increased with 1,000 mg/kg/day ETBE (group 5) (Table 2). However, the incidence of hepatocellular carcinomas (Fig. 2C) in this group was comparable to the control (group 1) value. The Cochran-Armitage test indicated a significant positive trend in the incidences of animals with hepatocellular adenoma and hepatocellular carcinoma and in the combined incidence of hepatocellular adenoma and carcinoma. The average numbers of hepatocellular adenomas in ETBE-treated animals (groups 2‒5) were comparable to the control (group 1) value. However, the average numbers of hepatocellular carcinomas and neoplastic lesions (hepatocellular adenomas plus carcinomas) were significantly increased with 1,000 mg/kg/day ETBE (group 5), but not 100‒500 mg/kg/day ETBE (groups 2‒4), when compared with the control (group 1) values.
Fig. 2.
Representative hyperplastic and neoplastic lesions. Focus (area) of cellular alteration (A), hepatocellular adenoma (B) and carcinoma (C) of the liver noted in rats initiated with EHEN and then treated with 1,000 mg/kg/day ETBE. Atypical tubule hyperplasia (D), renal tubule adenoma (E) and carcinoma (F) of the kidney found in rats in the same group. A‒F: H&E staining.
Table 2. Incidences and Multiplicities of Neoplastic Lesions of the Liver in Rats Initiated with EHEN and then Given ETBE.
In the kidneys, atypical tubule hyperplasia (Fig. 2D) was observed in all animals of each group, including the control group. The incidences of renal tubule adenoma (Fig. 2E) were significantly increased with 500 and 1,000 mg/kg/day ETBE (groups 4 and 5) (Table 3). The incidence of renal tubule carcinoma (Fig. 2F) was also significantly elevated with 300 mg/kg/day ETBE (group 3). The Cochran-Armitage test indicated a significant positive trend in the incidences of renal tubule adenoma and renal tubule adenoma plus carcinoma. However, the incidences of renal tubule adenomas plus carcinomas with 100, 300, 500 and 1,000 mg/kg/day ETBE (groups 2‒5) were comparable to the control (group 1) value.
Table 3. Incidences and Multiplicities of Preneoplastic and Neoplastic Lesions of the Kidney in Rats Initiated with EHEN and then Given ETBE.
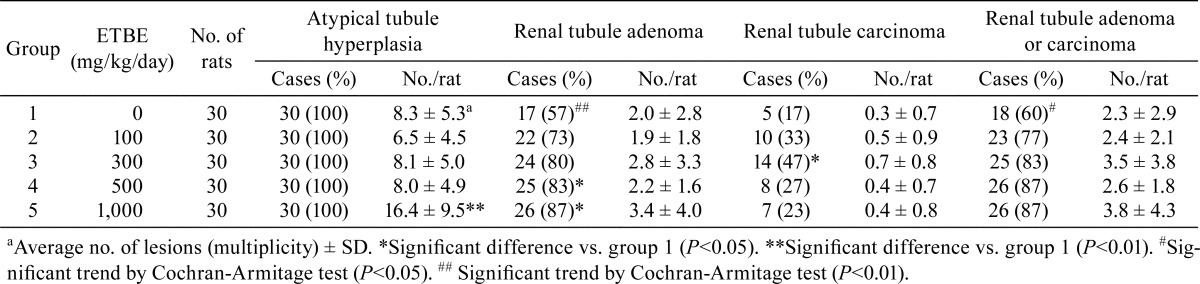
The average number of atypical tubule hyperplasias was significantly increased with 1,000 mg/kg/day ETBE (group 5), but not 100, 300 and 500 mg/kg/day ETBE (groups 2‒4), when compared with the control value (Table 3). However, the average numbers of renal tubule adenomas, renal tubule carcinomas and renal tubule adenomas plus carcinomas were comparable to the control values.
Increased incidences of hyaline droplets (accumulation of α2u-globulin) were also noted with 100–1,000 mg/kg/day ETBE (groups 2‒5) in a dose-dependent manner (data not shown).
Discussion
The present study was conducted to determine whether no-effect levels exist regarding the hepatic and renal tumor-promoting effects of ETBE on EHEN-induced carcinogenesis using male Wistar rats10, 12, 13. As end points, hyperplastic and neoplastic lesions in the liver and kidney were employed in this 2-stage carcinogenesis bioassay. As a result, the test material was judged as positive for tumor-promoting activity when significantly increased incidences and/or multiplicities (average numbers/rat) of preneoplastic and neoplastic lesions were detected in the liver or kidney in comparison with control values.
Regarding liver carcinogenesis, liver tumor-promoting effects of ETBE observed in a medium-term multiorgan carcinogenesis bioassay5 were reconfirmed by the significant increased in incidences of hepatocellular adenomas and hepatocellular adenomas or carcinomas and significantly elevated average numbers of hepatocellular carcinomas and hepatocellular adenomas or carcinomas in rats given 1,000 mg/kg/day ETBE in the present study. However, there were no increased incidences of hyperplastic and neoplastic lesions of the liver in rats given ETBE at doses of 500 mg/kg/day or less. It has been reported that ETBE is a non-genotoxic substance3, 4 that induces liver microsome enzymes3. In addition, 5-bromo-2’-deoxy-uridine labeling indices in hepatocytes were found to be significantly increased in rats exposed to ETBE in an inhalation toxicity study14. Recently, the possible mode of action (MOA) for ETBE hepatotumorigenicity in rats was investigated, and the results indicated that liver tumor development could be related to induction of cell proliferation due to induction of oxidative stress and DNA modifications, which depend on activation of constitutive androstane receptor, pregnane-X-receptor and peroxisome proliferator-activated receptors15, 16. The available evidence thus strongly indicates that stimulation of cell proliferation is involved in liver tumor-promoting effects of ETBE15, 16.
Recently, a significant increase in the incidence of hepatocellular adenoma was found in male rats exposed to 5,000 ppm ETBE for 6 hrs/day, 5 days/week, but not in those exposed to less than 1,500 ppm, in a two-year carcinogenicity test (inhalation study)6. The calculated amount of ETBE uptake, based on a minute volume for male adult rats of 561 mL/min/kg body weight17, by inhalation exposure (5,000 ppm) was approximately 4,222 mg/kg/day6. As the estimated absorption rate (respiratory uptake) was approximately 32–34%18, ETBE intake by inhalation exposure (5,000 ppm) was equivalent to approximately 1,350–1,435 mg/kg/day. However, no liver tumors developed in male rats given drinking water containing 10,000 ppm ETBE (calculated ETBE intake: 542 mg/kg/day) for 2 years7. In the present study, increased incidences of hepatocellular adenoma and combined hepatocellular adenoma and carcinoma were evident in male rats initiated with EHEN and then treated with 1,000 mg/kg/day ETBE, but not less than 500 mg/kg/day, by gavage for 19 weeks. Thus, the liver tumor promotion dose of 1,000 mg/kg/day ETBE in our previous initiation/promotion study by oral administration5 and that in the present study were roughly equal to a hepatotumorigenic dose of 5,000 ppm by inhalation exposure6.
A significant increase in the average number of atypical tubule hyperplasias, regarded as preneoplastic lesions, was found in rats given ETBE at a dose of 1,000 mg/kg/day, but not 500 mg/kg/day or less, which was in agreement with the results of our previous medium-term multiorgan carcinogenesis bioassay5. A significant increase in the incidences of renal tubule adenomas was found in rats given ETBE at 500 and 1,000 mg/kg/day, and a significant increase in renal tubule carcinomas was noted in rats given ETBE at 300 mg/kg/day. Unequivocal distinction between renal tubule adenomas and carcinomas is difficult in many cases, and there is an evolutionary continuum from adenoma to carcinoma19. Therefore, the renal tumor-promoting effects of ETBE were evaluated with reference to combined adenomas and carcinomas. The incidence of renal tubule adenoma plus carcinoma showed a significant positive trend. However, the incidences and average numbers of renal tubule adenomas plus carcinomas in the treated groups were comparable to control values. It was reported that accumulation of α2u-globulin and increased DNA synthesis in renal tubules were evident in male rats treated with ETBE3, 14. The increased incidences of hyaline droplets (accumulation of α2u-globulin) noted in rats given ETBE in the present study were also observed in male rats given 10,000 ppm ETBE (drinking water study) for 13 weeks7. MTBE, a chemical structurally related to ETBE, and TBA, a common metabolite of ETBE and MTBE, induces similar renal lesions20,21,22,23. In carcinogenicity studies of MTBE and TBA, increased incidences of atypical tubule hyperplasia and renal tubule adenoma were found in male rats8, 21, 24. From the available evidence, the renal tumor promotion activity noted in male rats given ETBE can be considered related to accumulation of α2u-globulin and a consequent increase of DNA synthesis, which is specific to male rats25, 26. Thus, the renal tumor-promoting effects of ETBE cannot be extrapolated to humans. Furthermore, renal cell tumor induction was not evident in 2 carcinogenicity studies (inhalation study and drinking water study) published recently6, 7.
In conclusion, the present investigation clearly demonstrated tumor-promoting effects on hepatic and renal carcinogenesis at 1,000 mg/kg/day. It was decided that the no-observed-effect level for tumor-promoting effects of ETBE on hepatic and renal carcinogenesis was 500 mg/kg/day, taking account of the non-genotoxicity of ETBE. However, the tumor-promoting effect of ETBE on renal carcinogenesis is not relevant to humans, since it is related to accumulation of α2u-globulin in renal tubules, which is a species-specific response.
Acknowledgments
The present studies were contracted with and financially supported by the Japan Petroleum Energy Center and Petroleum Association of Japan.
Footnotes
Disclosure of Potential Conflicts of Interest: There are no conflicts of interest to declare.
References
- 1.American Cancer Society MTBE. 2011. Available from http://www.cancer.org/cancer/cancercauses/othercarcinogens/pollution/mtbe.
- 2.McCarthy JE, and Tiemann M. MTBE in gasoline: Clean air and drinking water issues., Congressional Reseach Service Reports, Paper 26, University of Nebraska. 2006. Available from http://digitalcommons.unl.edu/crsdocs/26.
- 3.McGregor D. Ethyl tertiary-butyl ether: a toxicological review. Crit Rev Toxicol. 37: 287–312. 2007. [DOI] [PubMed] [Google Scholar]
- 4.Noguchi T, Kamigaito T, Katagiri T, Kondou H, Yamazaki K, Aiso S, Nishizawa T, Nagano K, and Fukushima S. Lack of micronucleus induction activity of ethyl tertiary-butyl ether in the bone marrow of F344 rats by sub-chronic drinking-water treatment, inhalation exposure, or acute intraperitoneal injection. J Toxicol Sci. 38: 913–924. 2013. [DOI] [PubMed] [Google Scholar]
- 5.Hagiwara A, Doi Y, Imai N, Nakashima H, Ono T, Kawabe M, Furukawa F, Tamano S, Nagano K, and Fukushima S. Medium-term multi-organ carcinogenesis bioassay of ethyl tertiary-butyl ether in rats. Toxicology. 289: 160–166. 2011. [DOI] [PubMed] [Google Scholar]
- 6.Saito A, Sasaki T, Kasai T, Katagiri T, Nishizawa T, Noguchi T, Aiso S, Nagano K, and Fukushima S. Hepatotumorigenicity of ethyl tertiary-butyl ether with 2-year inhalation exposure in F344 rats. Arch Toxicol. 87: 905–914. 2013. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Yamazaki K, Kano H, Aiso S, Nagano K, and Fukushima S. No carcinogenicity of ethyl tertiary-butyl ether by 2-year oral administration in rats. J Toxicol Sci. 37: 1239–1246. 2012. [DOI] [PubMed] [Google Scholar]
- 8.NTP Toxicology and carcinogenesis studies of t-butyl alcohol (CAS No. 75–65–0) in F344/N rats and B6C3F1 mice (Drinking water studies) (Tech. Rep. Ser. No.436; NIH Publ. No. 95–3167). National Toxicology Program, Research Triangle Park, NC. 1995. [PubMed] [Google Scholar]
- 9.Dietrich DR, and Swenberg JA. The presence of α2u-globulin is necessary for d-limonene promotion of male rat kidney tumors. Cancer Res. 51: 3512–3521. 1991. [PubMed] [Google Scholar]
- 10.Furukawa F, Nishikawa A, Miyauchi M, Nakamura H, Son H-Y, Yamagishi M, and Hirose M. Concurrent administration of fish meal and sodium nitrite does not promote renal carcinogenesis in rats after initiation with N-ethyl-N-hydroxyethylnitrosamine. Cancer Lett. 154: 45–51. 2000. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa Y, Aoki S, Imazawa T, Hayashi Y, Matsushima Y, and Takamura N. Dose-related enhancing effect of potassium bromate on renal tumorigenesis in rats initiated with N-ethyl-N-hydroxyethyl-nitrosamine. Jpn J Cancer Res. 76: 583–589. 1985. [PubMed] [Google Scholar]
- 12.Nakagawa Y, Kitahori Y, Cho M, Konishi N, Tsumatani K, Ozono S, Okajima E, Hirao Y, and Hiasa Y. Effect of hexachloro-1,3-butadiene on renal carcinogenesis in male rats pretreated with N-ethyl-N-hydroxyethylnitrosamine. Toxicol Pathol. 26: 361–366. 1998. [DOI] [PubMed] [Google Scholar]
- 13.Takashima K, Ozono S, Nakanou I, Ota M, Tanaka M, Tani M, Hirao K, Hirao Y, Kuwashima S, and Hiasa Y. Strain variation in renal carcinogenesis by N-ethyl-N-hydroxyethylnitrosamine in F1 (Wistar-Fischer) rats. Cancer Lett. 170: 125–130. 2001. [DOI] [PubMed] [Google Scholar]
- 14.Medinsky MA, Wolf DC, Cattley RC, Wong B, Janszen DB, Farris GM, Wright GA, and Bond JA. Effects of a thirteen-week inhalation exposure to ethyl tertiary butyl ether on fischer-344 rats and CD-1 mice. Toxicol Sci. 51: 108–118. 1999. [DOI] [PubMed] [Google Scholar]
- 15.Kakehashi A, Hagiwara A, Imai N, Nagano K, Nishimaki F, Banton M, Wei M, Fukushima S, and Wanibuchi H. Mode of action of ethyl tertiary-butyl ether hepatotumorigenicity in the rat: evidence for a role of oxidative stress via activation of CAR, PXR and PPAR signaling pathways. Toxicol Appl Pharmacol. 273: 390–400. 2013. [DOI] [PubMed] [Google Scholar]
- 16.Kakehashi A, Hagiwara A, Imai N, Wei M, Fukushima S, and Wanibuchi H. Induction of cell proliferation in the rat liver by the short-term administration of ethyl tertiary-butyl ether. J Toxicol Pathol. 28: 27–32. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauderly JL, Tesarek JE, Sifford LJ, and Sifford LJ. Respiratory measurements of unsedated small laboratory mammals using nonrebreathing valves. Lab Anim Sci. 29: 323–329. 1979. [PubMed] [Google Scholar]
- 18.Nihlén A, Löf A, and Johanson G. Controlled ethyl tert-butyl ether (ETBE) exposure of male volunteers. I. Toxicokinetics. Toxicol Sci. 46: 1–10. 1998. [DOI] [PubMed] [Google Scholar]
- 19.IARC International classification of rodent tumours. Part I - The rat, 3. Urinary system. In IARC Scientific Publications No. 122. (U Mohr, ed.) Oxford University Press, International Agency for Research on Cancer, Lyon. 1992. [Google Scholar]
- 20.Borghoff SJ, Prescott JS, Janszen DB, Wong BA, and Everitt JI. α2u-globulin nephropathy, renal cell proliferation, and dosimetry of inhaled tert-butyl alcohol in male and female F-344 rats. Toxicol Sci. 61: 176–186. 2001. [DOI] [PubMed] [Google Scholar]
- 21.McGregor D. Methyl tertiary-butyl ether: studies for potential human health hazards. Crit Rev Toxicol. 36: 319–358. 2006. [DOI] [PubMed] [Google Scholar]
- 22.Poet TS, and Borghoff SJ. In vitro uptake of methyl tert-butyl ether in male rat kidney: use of a two-compartment model to describe protein interactions. Toxicol Appl Pharmacol. 145: 340–348. 1997. [DOI] [PubMed] [Google Scholar]
- 23.Williams TM, and Borghoff SJ. Characterization of tert-butyl alcohol binding to alpha2u-globulin in F-344 rats. Toxicol Sci. 62: 228–235. 2001. [DOI] [PubMed] [Google Scholar]
- 24.Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, and Andrews LS. Oncogenicity studies of inhaled methyl tertiary-butyl ether (MTBE) in CD-1 mice and F-344 rats. J Appl Toxicol. 17(Suppl 1): S45–S55. 1997. [DOI] [PubMed] [Google Scholar]
- 25.Swenberg JA, Short B, Borghoff S, Strasser J, and Charbonneau M. The comparative pathobiology of α2u-globulin nephropathy. Toxicol Appl Pharmacol. 97: 35–46. 1989. [DOI] [PubMed] [Google Scholar]
- 26.Swenberg JA. α 2u-globulin nephropathy: review of the cellular and molecular mechanisms involved and their implications for human risk assessment. Environ Health Perspect. 101(Suppl 6): 39–44. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]