Abstract
Cannabinoids form a singular family of plant-derived compounds (phytocannabinoids), endogenous signaling lipids (endocannabinoids), and synthetic derivatives with multiple biological effects and therapeutic applications in the central and peripheral nervous systems. One of these properties is the regulation of neuronal homeostasis and survival, which is the result of the combination of a myriad of effects addressed to preserve, rescue, repair, and/or replace neurons, and also glial cells against multiple insults that may potentially damage these cells. These effects are facilitated by the location of specific targets for the action of these compounds (e.g., cannabinoid type 1 and 2 receptors, endocannabinoid inactivating enzymes, and nonendocannabinoid targets) in key cellular substrates (e.g., neurons, glial cells, and neural progenitor cells). This potential is promising for acute and chronic neurodegenerative pathological conditions. In this review, we will collect all experimental evidence, mainly obtained at the preclinical level, supporting that different cannabinoid compounds may be neuroprotective in adult and neonatal ischemia, brain trauma, Alzheimer’s disease, Parkinson’s disease, Huntington’s chorea, and amyotrophic lateral sclerosis. This increasing experimental evidence demands a prompt clinical validation of cannabinoid-based medicines for the treatment of all these disorders, which, at present, lack efficacious treatments for delaying/arresting disease progression, despite the fact that the few clinical trials conducted so far with these medicines have failed to demonstrate beneficial effects.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-015-0381-7) contains supplementary material, which is available to authorized users.
Keywords: Cannabinoids, Endocannabinoid signaling system, CB1 and CB2 receptors, FAAH and MAGL enzymes, Neurodegenerative disorders, Neuroprotection
Overview on the Neuroprotective Properties of Cannabinoids
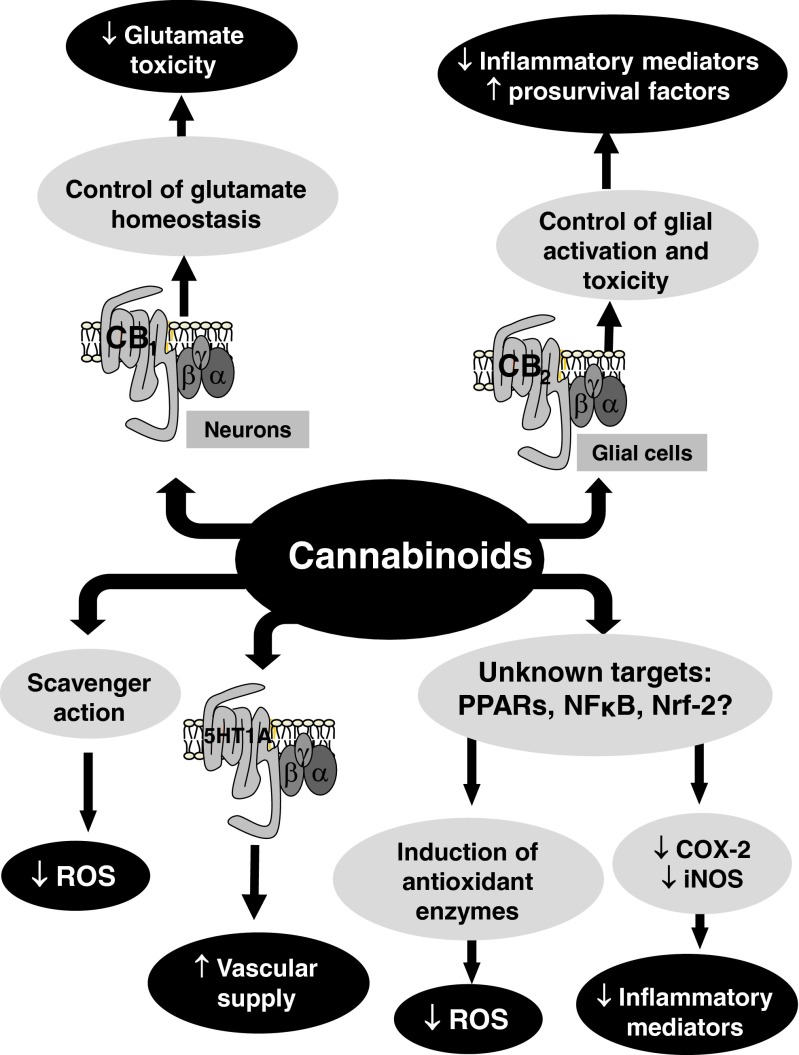
The neuroprotective potential of compounds targeting the endocannabinoid system (e.g., cannabinoid agonists, inhibitors of endocannabinoid inactivation, and allosteric modulators) has been extensively investigated over the last 15 years [1]. This potential is based on the ability of these compounds to limit the influence of multiple cytotoxic stimuli (e.g., excitotoxicity, oxidative stress, inflammation, etc.) on neuronal homeostasis and survival. It is now obvious that these stimuli collaborate for deteriorating neurons in most of neurodegenerative disorders, so a reliable strategy to preserve neurons from death needs the combination of protective effects on all or on most of these cytotoxic stimuli, this representing the major added-value of cannabinoids when compared with other types of compounds also investigated for their neuroprotective properties [e.g., antioxidants, N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid antagonists, calcium channel blockers, inhibitors of apoptosis, and anti-inflammatory agents] [1]. The advantage of cannabinoids in neuroprotection is their broad-spectrum profile determined by their activity at multiple molecular sites not only within the endocannabinoid system, but also outside this neuromodulatory system, and the location of those potential targets for cannabinoids in all key cellular elements in relation to the control of neuronal survival (e.g., neurons, astrocytes, resting and reactive microglia, oligodendrocytes and oligodendrocyte precursor cells, and neural progenitor cells) and also in key brain structures [e.g., blood–brain barrier (BBB)] [1]. This multiplicity of molecular sites allows for a unique cannabinoid (or a combination of cannabinoids with different profiles) to possibly reduce excitotoxicity by acting through neuronal cannabinoid type 1 receptors (CB1R), as well as the toxic influence of reactive microgliosis by acting through microglial cannabinoid type 2 receptors (CB2R), or enhancing the trophic and metabolic support to neurons by acting through astroglial CB1R or CB2R. These effects may also include actions through mechanisms that do not involve cannabinoid receptors/enzymes but interactions with transcription factors [e.g., nuclear factor (erythroid-derived)-like 2 (Nrf-2), nuclear factor kappa B (NFκB)] or nuclear receptors of the peroxisome proliferator-activated receptor (PPAR) family to limit oxidative stress/inflammatory responses, with elements of other transmission systems (e.g., 5-HT1A receptors) for the control of blood supply, or with components of the adenosine signaling pathway [1, 2] (see Fig. 1 for an overview of all molecular and cellular mechanisms proposed for the neuroprotective properties of cannabinoids).
Fig. 1.
Overview of the molecular and cellular mechanisms enabling the neuroprotective properties of cannabinoids. CB1 = cannabinoid type 1 receptor; CB2 = cannabinoid type 2 receptor; PPAR = peroxisome proliferator-activated receptor; NFkB = nuclear factor kappa B; Nrf-2 = nuclear factor (erythroid-derived)-like 2; ROS = reactive oxygen species; COX-2 = cyclooxygenase 2; iNOS = inducible nitric oxide synthase
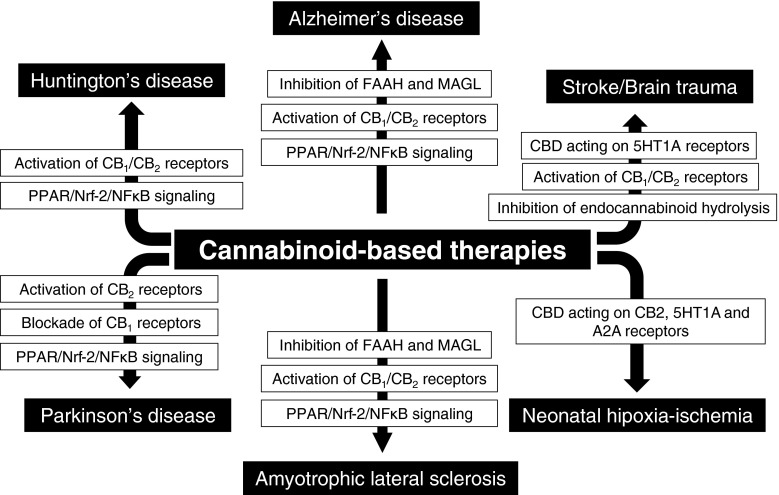
The objective of this review is to collect the preclinical evidence generated in the last 15 years, which support the need to develop cannabinoid-based therapies for the treatment of disease progression in experimental models of acute (e.g., adult and neonatal ischemia, brain trauma) or chronic (Alzheimer’s disease [AD], Parkinson’s disease [PD], Huntington’s disease [HD] and amyotrophic lateral sclerosis (ALS)] neurodegenerative disorders. In all cases, we will also review the changes observed in specific endocannabinoid elements of this signaling system during the progression of each pathology and will discuss how they can be relevant to pharmacology, for example they may indicate the most interesting and promising pharmacological targets for the development of specific neuroprotective therapies (see Fig. 2 for an overview on investigated targets for these disorders). We will end each section with a review of the clinical evidence (if it exists), reasons for their potential failures and proposals for a better development of these therapies in patients.
Fig. 2.
Summary of the targets and types of cannabinoid-based therapies that are being investigated in the different neurodegenerative conditions included in this review. FAAH = fatty acid amide hydrolase; MAGL = monoacylglycerol lipase; CB1 = cannabinoid type 1 receptor; CB2 = cannabinoid type 2 receptor; PPAR = peroxisome proliferator-activated receptor; Nrf-2 = nuclear factor (erythroid-derived)-like 2; NFkB = nuclear factor kappa B; CBD = cannabidiol
Cannabinoids and Acute Brain Damage: Stroke and Brain Trauma
Stroke remains the second most common cause of death and the third most common cause of disability worldwide. Approximately 80 % of strokes are attributable to the occlusion of a blood vessel (ischemic stroke), whilst the rest is mainly associated with vessel rupture (hemorrhagic stroke) [3]. When a blood vessel that irrigates the brain tissue is occluded, ischemic brain damage is triggered by excessive release of the excitatory neurotransmitter glutamate as a result of energy failure and ion gradient collapse, resulting in a reversal of glutamate uptake via glutamate transporters. Excessive glutamate-evoked Ca2+ entry via NMDA receptors further promotes cell death by triggering an excitotoxic cascade that involves the activation of Ca2+-dependent enzymes, the disruption of mitochondrial function, and cell necrosis or apoptosis. Ischemic brain injury is exacerbated by a robust inflammatory response that involves a local reaction, as well as an influx of blood-borne cells with production of inflammatory mediators, including cytokines, chemokines, proteases, reactive oxygen species, and vascular adhesion molecules (reviewed in [4]). For the acute phase of ischemic stroke, the only pharmacological treatment is the recanalization of the occluded vessel with thrombolytic therapy with tissue plasminogen activator. However, owing to its narrow time window, < 5 % of stroke patients receive this treatment. Although the use of mechanic thrombectomy is helping to expand this window, it is still imperative to pursue the search of new therapeutic targets amenable to pharmacological manipulation for stroke patients [5]. Traumatic brain injury (TBI) is another important focal form of acquired brain injury that occurs when a sudden trauma damages the brain. It is usually caused either by closed or by open, penetrating head injury, and is often the result of car accidents, firearms or falls [6]. Since its pathophysiology shares many of its mechanisms with stroke, we will address these 2 pathologies together. Both pathological conditions should be completed with the study of spinal injury, but owing to space constraints, we will not address the effects of cannabinoids in spinal injury here.
Cannabinoids have been proposed as promising neuroprotective agents for the treatment of stroke and TBI [7]. This possibility has been predominantly investigated in experimental models of both disorders in laboratory animals, although some of the studies supporting this promise have been conducted with the cannabinoid administered before the cytotoxic insults, a fact that is not possible to reproduce in the case of humans, so the results of these specific studies should be taken with the necessary caution. For stroke, most common models are those caused by middle cerebral artery occlusion (MCAO) in rats or mice, either permanent (pMCAO) or followed by reperfusion [transient MCAO (tMCAO)], as well as in vitro models of oxygen/glucose deprivation. In the case of TBI, damage is most commonly caused either by closed (concussion) or open head injury (stab wound). The cannabinoids having beneficial effects in these models included 1) dexanabinol (HU-211) [8–11], which is a synthetic compound having a chemical structure of a classic cannabinoid but no activity at cannabinoid receptors; 2) nonselective synthetic cannabinoid agonists such as HU-210, the active enantiomer of HU-211 [12], WIN 55,212-2 [13, 14], TAK-937 [15, 16], and BAY 38-7271 [17, 18]; 3) phytocannabinoids such as Δ9-tetrahydrocannabinol (Δ9-THC) [19], which binds not only CB1R and CB2R, but also cannabidiol (CBD), which has no affinity at these receptors but was highly active against brain ischemia [20–22]; 4) endocannabinoids such as 2-arachidonoylglycerol (2-AG), in particular in TBI induced by closed head injury [23–25], but also in experimental ischemia [26], and also anandamide [27] and its related signaling lipids palmitoylethanolamide (PEA) [28], oleoylethanolamide [27], and N-arachidonoyl-L-serine (AraS) [29]; and 5) selective CB2R targeting ligands such as O-3853, O-1966, and JWH-133 [30–35]. Most of these studies were conducted with the cannabinoid administered at least after the cytotoxic insult [12–19, 21–26, 28–35]. In most cases, the benefits obtained with these cannabinoid-related compounds (e.g., improved neurological performance, reduced infarct size, edema, BBB disruption, inflammation and gliosis, and control of immunomodulatory responses) involved the activation of CB1R (e.g., HU-210 [12], WIN55,212-2 [13, 14], TAK-937 [15, 16], BAY 38-7271 [17, 18], Δ9-THC [19], and PEA [36]) and/or CB2R (e.g., AraS [29], O-3853, O-1966, and JWH-133 [30–35]) . Similar findings derive from experiments using mice with a genetic deficiency in CB1R or, to a lesser extent, CB2R. For example, CB1–/– mice showed increased infarct size and neurological deficits after tMCAO, concomitant with a reduction in cerebral blood flow and NMDA excitotoxicity [37], and a similar greater vulnerability was also found in TBI models [24], then supporting the protective role of CB1R against both pathological conditions. In the case of CB2–/– mice, results were controversial, with a study reporting larger cerebral infarction and a worsened neurological function after tMCAO [30], but others describing no differences using permanent MCAO [32, 33], despite the notable effects found in pharmacological experiments with compounds selectively activating the CB2R [30–35]. These types of agonists are particularly interesting for a possible therapeutic application in stroke and TBI because of the lack of psychoactivity of their selective agonists. In addition, their strong anti-inflammatory profile appears to be one of the most consistent mechanisms leading to reduction of the lesion, by actions affecting resident, vascular, and peripheral cells. It is also important to remark that the benefits of certain cannabinoids in acute stroke and TBI also involve effects on other pharmacological targets, such as the blockade of NMDA receptors (e.g., HU-211 [8–11]), the activation of 5-HT1A receptors (e.g., CBD [20–22]), and the activation of transient receptor potential vanilloid-type 1 receptors (e.g., PEA [36] and AraS) [29]). It is also possible that part of these beneficial effects may be related to the hypothermic effects of cannabinoids, but it is well known that such effects are CB1R-mediated [12, 38, 39]. Lastly and apart from the acute phase, both stroke and TBI have in common a chronic phase characterized by severe functional sequelae. This late phase offers, at least theoretically, a broader window for promoting repair and decreasing disability, in which there might be some room for cannabinoids based on their capability to induce proliferation of neural progenitors cells [40, 41], their differentiation and migration at lesioned sites (Moro et al., unpublished results), or the differentiation of oligodendrocyte precursor cells to produce remyelination [42]—all these possibilities have already been investigated in experimental brain ischemia.
The neuroprotective and neurorepair effects of cannabinoids in stroke and TBI may be facilitated by the responses experienced by endocannabinoids and their receptors and enzymes during the progression of both pathological conditions. This may be the case, for example, of the transient accumulation of 2-AG at the site of injury in experimental TBI [23]. By contrast, in the neonatal rat brain, the exposure to concussive head trauma induced a moderate increase in the levels of anandamide and other N-acylethanolamines, but not of 2-AG and other 2-monoacylglycerols [43, 44]. Further studies demonstrated that these elevations are endogenous responses addressed to limit brain damage, as the inhibition of 2-AG and anandamide hydrolysis reduced brain damage and improved functional deficits in parallel to a reduction of proinflammatory responses in the mouse brain after TBI [45, 46]. Similar elevations of anandamide, 2-AG, and N-acylethanolamines have been detected in experimental cerebral ischemia [47–50]. As far as the cannabinoid receptors are concerned, most studies showed an upregulated expression of both CB1R and, in particular, CB2R in stroke, with neurons (for CB1R) and microglial/macrophages, astrocytes, and neutrophils (for CB2R) being the most common cellular substrates for these responses [33, 51–54]. However, some studies described downregulatory responses of both receptors at very early times after induction of ischemia [33, 55]. Upregulation of CB2R with no changes in CB1R have been found in TBI [56].
Despite the elevated number of preclinical studies, the number of clinical studies with cannabinoids in these pathological conditions is rather limited. The most relevant was a multicenter, placebo-controlled, phase III trial conducted 10 years ago, and that was addressed to determine safety and efficacy of dexanabinol in patients with TBI. The trial did show that dexanabinol was safe but not efficacious for the treatment of TBI [57]. It is important to remark that dexanabinol is a cannabinoid because of its chemical structure (it is the inactive enantiomer of HU-210) but it does not have any activity at the classic cannabinoid receptors, being active as a noncompetitive NMDA receptor antagonist, so there is an unmet need of repeating such clinical studies with compounds acting at CB1R and/or CB2R (e.g., Sativex; GWPharma, Cambridge, UK).
Cannabinoids and Brain Damage in the Immature Brain: Neonatal Hypoxia–Ischemia
Perinatal asphyxia affects 1–2 per 1000 live term newborns with one-third of them developing a severe neurological syndrome. About 25 % of severe cases result in lasting sequelae and about 20 % die, resulting in about 2 million babies dying or remaining severely disabled each year worldwide [58]. Immature brain show some characteristics that determine a higher vulnerability for hypoxic–ischemic (HI) damage, as well as some particular selectivity for the damage [58–61]: 1) a high metabolic rate and oxygen extraction together with immature glucose uptake mechanisms; 2) a highly developed excitotoxic system which overexpressed receptors responding faster and higher to glutamate; 3) hypersensitivity to inflammatory mediators, with a misbalance between pro- and antioxidant enzymes, differences in leukocyte–endothelial cell communication and distinct intracellular signaling within inflammatory pathways (NFκB and mitogen-activated protein kinase); 4) proapoptotic factor preponderancy because of the need for modeling the developing brain; and 5) antioxidant defenses only partially developed at birth. Therefore, excitotoxicity, inflammation, and oxidative stress constitute the triad of major factors leading to HI damage in immature brain [59]. However, immature brain has a huge plastic recovery potential after ischemia, increasing the proliferation in the subventricular zone of neural precursors that then migrate to the damaged areas in the neocortex [61], as well as of glial precursors that migrate similarly to support the newly created neurons [60]. The high vulnerability to oxidative stress of particularly active oligodendroglial cells because of the ongoing myelinization processes with enhanced iron metabolism, however, jeopardizes this process [61]. Thus, despite the fact that oligodendroglial precursors accumulate in brain after HI the absence of further progress to mature forms eventually results in hypomyelination [62]. In addition, despite the greater resistance of the BBB to the ischemic insult in newborn than in adult brain, the angiogenic response in immature brain is slower and weaker than in adults, which can compromise the postischemic neurorepair [63]. The existence of a lapse between primary and secondary energetic failure on brain after a HI insult offers an opportunity for treatment, a “therapeutic window”. However, currently available therapies for ischemic brain damage only afford partial protection. An example is therapeutic hypothermia for asphyxiated newborns. Although current evidence demonstrates that hypothermia reduces death and/or major sequelae, almost a half of babies with severe HI encephalopathy do not benefit from this treatment; in addition, its application in substandard environments might be troublesome or even dangerous [64]. Thus, to develop synergistic therapies is warranted.
Given that cannabinoids are able to attenuate excitotoxicity, inflammation, and oxidative stress, they have been proposed as promising candidates to become effective neuroprotective therapies, including the brain damage in neonatal ischemia [63]. This evidence derives from studies that were initiated >10 years ago using newborn rat forebrain slices subjected to oxygen glucose deprivation and exposed to the CB1R/CB2R agonist WIN55212-2, which reduced cell death, decreasing glutamate and cytokine release, as well as inducible nitric oxide synthase expression, effects that were abolished by either CB1R or CB2R antagonists [65]. In newborn rats exposed to severe anoxia or to acute hypoxia-ischemia [66, 67], postinsult administration of WIN55212-2 afforded a strong neuroprotective effect, abolished by either CB1R or CB2R antagonists, too, as well as increasing neuronal and oligodendroglial cell proliferation in the subventricular zone 7 days after neonatal HI in rats [68]. In term fetal lambs exposed to HI damage by umbilical cord occlusion, postinsult administration of WIN55212-2 improved cerebral blood flow and reduced astrocytic, as well as apoptotic neuronal, death—those effects relying on the preservation of mitochondrial integrity and functionality [69]. These neuroprotective effects were also afforded with CBD, the major nonpsychoactive component of Cannabis sativa, again in animal models of newborn HI encephalopathy [70–74]. CBD administered 15–30 min after an HI insult in newborn pigs reduced the death of neurons and astrocytes, preserved brain activity as measured by amplitude-integrated electroencephalography, prevented the increase in the concentration of H+ magnetic resonance spectroscopy biomarkers of brain damage (e.g., lactate/N-acetylaspartate ratio), prevented the appearance of seizures and improved neurobehavioral performance when examined 72 h after HI [70, 72, 74]. In the case of newborn rats, CBD administered 15 min after an HI insult led to long-lasting neuroprotective effects, reducing brain damage and restoring neurobehavioral function several weeks after the insult [73]. The neuroprotective effect of CBD included the prevention of necrotic and apoptotic cell death and was related to the modulation of excitotoxicity, inflammation, and oxidative stress, as demonstrated by in vitro and in vivo studies [71–74]. It is important to note that this neuroprotective action was associated with no significant side effects and even with some extracerebral benefits (e.g., improved hemodynamic stability and lung dynamics) [70, 72–74].
There are not too many data concerning the changes in endocannabinoid elements following a neonatal HI insult but the few available data support the findings derived from pharmacological studies. Thus, brain levels of endocannabinoids are increased in the newborn rat after acute injury and in the newborn pig after acute brain HI insult [74, 75], which has been interpreted as a part of an endogenous response of the endocannabinoid signaling system acting as a natural neuroprotective system.
Therefore, the preclinical evidence collected so far is highly suggestive of important benefits to be reached in newborns affected by HI encephalopathy with cannabinoid-based therapies, in particular with the nonpsychoactive phytocannabinoid CBD, which appears to be an adequate therapeutic option for the treatment of neonatal and infantile disorders. In fact, CBD has already been formulated as Epidiolex (GWPharma) and received the orphan designation from US and European regulatory agencies for the treatment infantile refractory epilepsies [76]. It may be a good choice for investigating the benefits of cannabinoid-based therapies in neonatal ischemia at the clinical level, alone or in combination with hypothermia, which is the only approved therapeutic strategy for this pathological condition.
Cannabinoids and Chronic Neurodegenerative Disorders: I. AD
AD represents the most prevalent chronic progressive neurodegenerative disorder. It may have a genetic origin with 3 major causal genes (APP, PSEN1, PSEN2) and numerous risk genes (APOE, SORL1, CLU, and others) but they account only <20 % of cases, most of them being of sporadic origin [77]. AD is characterized by a progressive cognitive deterioration leading to dementia, which affects mainly cortical and subcortical structures [78]. The major histopathological events are: 1) the formation of extracellular accumulations of β-amyloid (Aβ) protein called senile plaques; 2) the development of cytoskeleton abnormalities, so-called neurofibrillary tangles, caused by hyperphosphorylation of tau protein; and 3) an important degree of the neuritic dystrophy and neuronal death in affected structures [79]. Therapies for AD are still limited, in particular for delaying disease progression with most of them still under investigation (e.g., inhibitors for acetylcholinesterase, NMDA receptors, β- and γ-secretases, and tau protein hyperphosphorylation) [80], so there is an urgent need to discover novel targets and compounds.
Cannabinoids have also attracted interest in AD given their benefits in reducing classic neurotoxic events in the disease, such as excessive glutamatergic transmission, prolonged calcium influx, oxidative stress, and inflammation. They were successfully investigated in preclinical models (e.g., 5 × FAD, PS1/APP+ mice) [81–85], despite it being well known that these models do not completely reproduce the complex AD pathology in humans, and this may be an important factor to consider at the time of developing and analyzing the results in future clinical trials. Beneficial effects in preclinical studies involved CB1R and/or CB2R, the selective activation of which was found to be effective in improving cognitive impairment, preserving neuronal cells, and preventing Aβ-induced microglial activation and the generation of proinflammatory mediators, as well as removing pathological deposits in different in vivo and in vitro models of AD [86–90]. In addition, beneficial effects of cannabinoids in AD may also be, at least partially, related to the activation of PPAR nuclear receptors for which certain cannabinoids may serve as ligands [88, 91], whereas, in the case of some particular cannabinoids (e.g., antioxidant phytocannabinoids), they may exert some more specific effects in relation with AD pathogenesis, for example: 1) by preventing Aβ aggregation, thereby hindering plaque formation and reducing the density of neuritic plaques due to inhibition of acetylcholinesterase activity or increased expression of neprilysin, an enzyme in the Aβ degradation cascade [86, 91–94]; and 2) by inhibiting Aβ-induced tau protein hyperphosphorylation by glycogen synthase kinase-3β [82–84]. Some recent studies have also highlighted the interest of targeting endocannabinoid inactivation in AD, through strategies of genetic inactivation [e.g., mice deficient in monoacylglycerol lipase (MAGL) or fatty acid amide hydrolase (FAAH)] or by inhibiting these enzymes (e.g., JZL184, URB597, respectively) [95–98]. However, in some cases, these effects were not related to an increased CB1R and/or CB2R signaling, but to other pathways, for example PPAR signaling, alterations in arachidonic acid, and/or prostaglandin signaling [95, 96].
The neuroprotective effects of cannabinoids may be likely facilitated by the changes experienced by specific elements of the endocannabinoid signaling system during the progression of AD. This is the case, for example, of the upregulation of CB2R found in reactive microglial cells surrounding the Aβ plaques, a response found in postmortem brain tissues from patients with AD [87, 99], and also in some experimental models [87], which may facilitate the benefits found with compounds selectively targeting this receptor [86, 87, 90]. However, other effects are better explained as originated by a pharmacological correction of those changes in the endocannabinoid system that may contribute to the progression of AD pathogenesis, for example: 1) the reduction in CB1R observed in AD-affected areas [87, 100], which may aggravate excitotoxic events that are controlled by CB1R signaling, then enhancing neuronal losses; and 2) the elevation of the FAAH enzyme in astrocytes associated with the senile plaques in the postmortem human cortex, which would enhance endocannabinoid hydrolysis, then decreasing anandamide levels and elevating arachidonic acid levels, and contributing to the destructive inflammatory process that accompanies AD [99, 101], despite studies in PS1/APP+ mice describing an increase in brain levels of monoacylglycerols, N-acylethanolamines, free fatty acids, eicosanoids, and other lipid species [96].
Despite the positive results obtained with cannabinoids in preclinical models of AD, their clinical development for patients with AD is still very poor, and it will be a complicated task given that these preclinical models only partially reproduce the disease, as mentioned before. The few clinical studies conducted so far have concentrated on specific symptoms, for example dementia-induced loss of appetite [102], but they have not investigated any disease-modifying effect. An interesting formulation to be investigated at the clinical level is the recently licensed phytocannabinoid-based medicine Sativex (GWPharma), which, based on the activity of its 2 components, Δ9-THC and CBD, at different complementary targets identified as neuroprotective in AD, e.g. CB1R and CB2R, PPARs, could become a promising novel disease-modifying therapy for patients with AD, as has been recently demonstrated in a preclinical model of an AD-related disorder (frontotemporal dementia) [103].
Cannabinoids and Chronic Neurodegenerative Disorders: II. PD
The most important progressive neurodegenerative disorder affecting the basal ganglia is PD. With an incidence of 2 cases per 10,000 people, PD is caused preferentially by overexposure to different environmental factors (e.g., pesticides, insecticides, some medicines, metals) but also (<5 % of cases) by mutations in some genes encoding for proteins such as α-synuclein, parkin, PINK1 (phosphatase and tensin homolog-induced putative kinase 1) and LRRK2 (leucine-rich repeat kinase 2) [104]. PD affects numerous brain structures, then producing different nonmotor (e.g., drooling, changes in taste and smell, nausea and vomiting, constipation, bladder dysfunction, dementia and cognitive impairment, hallucinations, depression and anxiety, and others) and, in particular, motor (e.g., rigidity, bradykinesia, postural instability, tremor) anomalies [105]. Motor symptoms are predominantly caused by alterations in the basal ganglia circuitry triggered by the death of nigral dopaminergic neurons, the denervation of the striatum to which these degenerating neurons project, the formation of Lewy bodies in the cytosol of nigral neurons, and the loss of neuromelanin accumulated in the substantia nigra [106]. Current treatments include dopaminergic replacement therapies, which serve for symptom alleviation, but the disease lacks of efficacious disease-modifying therapies, despite the issue being widely investigated, even at the clinical level [107].
As in the case of AD, numerous cannabinoids have been investigated with the objective of developing novel neuroprotective therapies in experimental models of PD, for example 6-hydroxydopamine-lesioned rodents, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)- or lipopolysaccharide (LPS)-lesioned mice [108], despite these neurotoxin-based models reflecting only partial aspects of the complex PD pathology, with some important events (e.g., Lewy body formation) completely absent. These studies have concentrated on the antioxidant properties of phytocannabinoids, for example Δ9-THC [109], CBD [109–111], and Δ9-tetrahydrocannabivarin (Δ9-THCV) [111], which are cannabinoid receptor-independent, then reducing oxidative stress, which is a major hallmark in the pathogenesis of PD that may be experimentally reproduced in laboratory animals. Neuroprotection has also been provided by synthetic cannabinoids such as the endocannabinoid transporter inhibitor/vanilloid agonist AM404 [110], or the CB1R/CB2R agonist CP55,940 [112], which are also antioxidant and also work through cannabinoid receptor-independent mechanisms in this case. In contrast, cannabinoids that selectively target the CB2R were also active against local inflammation and gliosis in models of mitochondrial dysfunction or LPS insult [111, 113, 114], a fact also supported by studies conducted with classic parkinsonian neurotoxins administered to mice with genetic deletion of the CB2R (greater vulnerability against the insult) or overexpressing these receptors (lower susceptibility against the insult) [111, 115]. CB1R-activating compounds have also been studied, but with controversial results [110, 116]. Nevertheless, a neuroprotective strategy based on targeting CB1R might have some disadvantages in PD as the hypokinetic effects of CB1R may worsen bradykinesia and other parkinsonian symptoms [108], whereas the blockade of these receptors may reduce parkinsonian akinesia [117].
Again, these pharmacological effects may be influenced by the changes that the endocannabinoid system experienced in this disease, as revealed by the data collected in postmortem tissues and biological fluids [114, 118, 119], as well as those found in animal models [111, 113, 118, 120]. They include: 1) the upregulation of CB1R in striatal neurons under the control of dopaminergic neurons that degenerate in PD, as observed in postmortem tissue from patients and in different experimental models of the disease (reviewed in [108]); 1) the elevation of CB2R in glia recruited to the lesion sites in the postmortem substantia nigra of patients with PD and in mice lesioned with MPTP or LPS [111, 113, 114]; and 3) the loss of neuronal CB2R in postmortem tissues of patients with PD due to the degeneration of nigrostriatal dopaminergic neurons [121, 122].
Lastly, clinical studies with cannabinoids in patients with PD are still limited, with most of them addressing only the relief of specific symptoms, for example bradykinesia [123], tremor [124], and levodopa-induced dyskinesia [125]. No clinical data exist in relation to the neuroprotective effects of cannabinoids. However, the preclinical evidence collected so far supports that an effective therapy should be based on an adequate combination of compounds to ensure: 1) the antioxidant activity that would be exerted by cannabinoid receptor-independent mechanisms, possibly involving the activation of PPARs; 2) the control of inflammatory events by CB2R activation; and 3) the blockade of CB1R to reduce motor inhibition. The phytocannabinoid Δ9-THCV has such a profile, making it an interesting compound to be used therapeutically in PD, alone or in combination with CBD, and highlighting the need for a formulation that can be further evaluated in patients.
Cannabinoids and Chronic Neurodegenerative Disorders: III. HD
HD is a genetic disorder caused by an excessive number of CAG repeats in the gene encoding the regulatory protein huntingtin, being the most prevalent polyglutamine disorder, which also includes other diseases such as autosomal dominant hereditary ataxias [126]. The key symptoms in HD are choreic movements, which are produced by the degeneration of the striatum, and behavioral disturbances and dementia, which are caused by deterioration in cortical structures [127], whereas the key neuropathological features are the formation of intranuclear inclusions of the mutated huntingtin, ubiquitin, and other molecules, which have a critical influence in producing transcriptional dysregulation affecting a number of key genes (e.g., BDNF) and the death of a number of extremely vulnerable neuronal subpopulations (e.g., striatal projecting neurons) [128]. Pharmacological therapies for patients with HD are extremely limited, with only the inhibitor of the vesicular monoamine transporter 1 tetrabenazine (Xenazine; Lundbeck, Deerfield, IL, USA) approved for the treatment of choreic movements, but with poor results in most of patients [129]. There are no approved disease modifiers, although numerous antioxidants, minocycline, histone deacetylase inhibitors, and unsaturated fatty acids have been (or are being presently) investigated at the clinical level [129].
Cannabinoids in the form of Sativex (GWPharma) have been recently evaluated in patients with HD [130]. This was supported by an exhaustive preclinical work with positive results in a broad spectrum of animal models of HD (e.g., R6/2 mice, quinolinate-lesioned mice, 3-nitropropionate- or malonate-lesioned rats), which confirmed the benefits of cannabinoids against most of the cytotoxic stimuli acting in this disease (reviewed in [1, 2, 131]). For example, compounds targeting the CB1R preserved striatal neurons in studies conducted in a rat model that relies on quinolinate-induced excitotoxic damage [132]. The relevance of these receptors in HD was also demonstrated in a genetic model of the disease, R6/2 mice, in which CB1R activation again preserved striatal neurons from death, whereas striatal damage was aggravated in R6/2 mice having a genetic deficiency in CB1R [133]. Compounds that selectively activate the CB2R also appear to be effective in HD, preferentially ameliorating the inflammatory events and microglial activation that occurs after the striatum is damaged with malonate (a complex II inhibitor) in rats [134], in R6/2 mice [135], and following the excitotoxicity induced by striatal lesion with quinolinate in mice [136]. Antioxidant nonpsychoactive phytocannabinoids, such as CBD and cannabigerol (CBG), have also been investigated in experimental models of HD, even though its effects are independent of CB1R/CB2R. Their effects may be mediated by activation of PPARs or other nonendocannabinoid targets. CBD was very active in animal models characterized by mitochondrial damage, oxidative stress and calpain activation, such as rats intoxicated with the complex II inhibitor 3-nitropropionate [136], yet it was inactive in proinflammatory models like malonate-lesioned rats [134]. CBG was neuroprotective in 3-nitropropionate-lesioned and R6/2 mice [137]. Based on these beneficial effects, CBD combined with Δ9-THC, as in the cannabinoid-based medicine Sativex (GWPharma), has also been studied in animal models of HD given the wide spectrum of pharmacological actions produced by this combination. This combination preserved striatal neurons in malonate-lesioned mice and in 3-nitropropionate-lesioned rats [138, 139].
An important observation in relation to the changes experienced by the endocannabinoid signaling during the progression of HD is that early defects in CB1R signaling followed by a progressive loss of these receptors have been found even prior to neuronal death and the onset of choreic symptoms [140, 141]. This may explain why an early stimulation of these receptors may dampen their impairment, thereby maintaining their capacity to inhibit the excitotoxic events that initiate the damage to striatal neurons [132], although such an approach is unlikely to work at later symptomatic stages that are characterized by an important loss of CB1R-containing striatal neurons [117]. However, as recent study unequivocally demonstrated that CB1R-dependent neuroprotective activity in HD is predominantly derived from a restricted population of these receptors on cortical glutamatergic neurons that project to the striatum and that are preserved during the progression of HD rather than from the CB1R located on striatal projection γ-aminobutyric acid (GABA)-ergic neurons that are progressively lost during disease progression [142], supporting the relevance of these receptors as potential targets for a neuroprotective therapy with cannabinoids in HD. In addition, the benefits found after CB2R activation in HD may be facilitated by overexpression of these receptors in the striatal parenchyma, an effect that was first detected when striatal damage was provoked in rats with malonate [134], and in R6/2 mice and other genetic mouse models of HD [135, 143], as well as in postmortem tissues from patients with HD [134]. This upregulation appears to occur in astrocytes [134] (although no CB2R expression was found in these glial cells in human HD tissues [144]) and particularly in reactive microglia [134, 135].
Cannabinoids have been also examined in patients with HD, although these clinical trials concentrated on the alleviation of specific symptoms, particularly chorea and behavioral disturbances, with controversial results (reviewed in [1]). The only clinical trial aimed at validating a cannabinoid-based neuroprotective therapy in HD has been recently carried out in Spain using Sativex (GWPharma), and, although it successfully demonstrated that Sativex (GWPharma) was safe and well-tolerated in patients with HD, as previously found in controls, yet, unfortunately, it failed to provide any evidence that it may slow down disease progression in HD [130]. This may be related to the relatively short time (12 weeks) for the active treatment and an unexpected influence of the placebo effect, so it is possible that a longer time may be necessary for revealing neuroprotective effects in patients with HD treated with Sativex (GWPharma) or similar preparations.
Cannabinoids and Chronic Neurodegenerative Disorders: IV. ALS
ALS is a progressive neurodegenerative disease produced by the damage of the upper and lower motor neurons leading to muscle denervation, atrophy, and paralysis [145]. As in other disorders, the damage of these neurons occurs by the combination of excitotoxicity, chronic inflammation, oxidative stress, protein aggregation, and other cytotoxic events [146–148]. The most abundant cases of ALS are sporadic [149], but the disease may be also familiar, associated with mutations in genes encoding for superoxide dismutase-1 (SOD-1), TAR-DNA binding protein-43 (TDP-43) or FUS (fused in sarcoma) protein, as well as the more recent CCGGGG hexanucleotide expansion in C9orf72 [145, 148]. In familiar cases, which account for only 5 % of all ALS cases, depending on the mutated gene (e.g., TDP-43, FUS, C9orf72), ALS can be accompanied by features of frontotemporal lobar dementia, which supports the idea that, rather than being one disorder, ALS belongs to a spectrum of disorders having motor and cognitive deficits [150]. The disease still lacks an effective treatment for symptoms and/or disease progression, with the antiexcitotoxic agent riluzole (Rilutek, Sanofi Pharmaceuticals, Paris, France) as the only approved medicine [151].
Studies initiated in 2004 have situated cannabinoids as a possible and promising disease-modifying therapy in ALS [152, 153], based on solid evidence collected exclusively in the classic transgenic mouse that overexpresses a mutated form (G93A) of SOD-1, despite this model only representing a small percentage of ALS cases. The model was developed in the 1990s and was used to investigate the effects of Δ9-THC [154], cannabinol [155], WIN55,212-2 [156], and the selective CB2R agonist AM1241 [157, 158]. This solid pharmacological evidence is also supported by data collected from double mutants generated by crossing SOD-1 mutant mice with some of the different mice deficient in endocannabinoid genes (e.g., FAAH–/–, CB1–/–), which not only reinforced the interest of CB1R agonists, but also the elevation of endocannabinoid levels with FAAH inhibitors [156]. Collectively, it appears that the neuroprotective effects of cannabinoids in ALS were apparently caused by 11) a CB2R-mediated (and possibly involving PPAR-γ, too) reduction in microglial activation and neuroinflammation; 2) a CB1R-mediated reduction in excitotoxic damage; and 3) antioxidant effects that appear to be receptor-independent and/or related to PPAR-γ/Nrf-2 signaling [159].
As in other disorders, this efficacy of cannabinoid compounds in ALS may be determined by specific changes in endocannabinoid elements that are targeted by cannabinoids in the spinal cord, brainstem and cortical areas, the central nervous system structures more affected in this disease. Thus, the levels of anandamide and 2-AG are elevated in the spinal cord of SOD-1 mutant mice [156, 160], in parallel to an increase in the expression of N-acyl-phosphatidyl-ethanolamine-selective phospholipase D, the enzyme that synthesizes anandamide, but no changes in diacylglycerol lipase, the enzyme that synthesizes 2-AG, and in FAAH and MAGL, the 2 major degradative enzymes for the 2 major endocannabinoids [161]. In addition, CB2R experience an important upregulatory response in the spinal cord of SOD-1 mutant and TDP-43 transgenic mice [158, 161, 162], as well as in patients with ALS [163]. This upregulation appears to occur predominantly in microglial elements recruited at lesioned sites [162, 163], so that it may facilitate the beneficial effects derived from selectively targeting this receptor in the control of microglial toxicity for motor neurons. However, to determine the changes in CB1R remains controversial, with a study reporting downregulatory responses in the spinal cord of SOD-1 mutant mice, even at early presymptomatic phases [164], which may predispose motor neurons to excitotoxic events, given the role that CB1R play in the control of glutamate homeostasis. However, a further study conducted in the same mutant mice did not find any changes in CB1R in the spinal cord [161], and this has been recently confirmed in TDP-43 transgenic mice, too [162].
Cannabinoids have been also studied at the clinical level in ALS, although the number of clinical trials is still too small to get significant and reliable findings, thus stressing the urgent need for additional clinical investigation [152]. First studies were exclusively observational and based on patients with ALS who self-medicated with cannabis for attenuating specific ALS-related symptoms, for example cramps, spasticity, and drooling [165]. A randomized, double-blind crossover trial conducted with oral Δ9-THC studied its effects on cramps [166], which are an important symptom experienced by patients with ALS during the course of the disease. However, despite Δ9-THC being well-tolerated, there was no reduction in cramp frequency and intensity [166]. Two additional studies again indicated good tolerability of Δ9-THC in patients with ALS and a nonsignificant attenuation on cramps and fasciculations [167, 168]. There are no clinical studies so far that have tried to investigate the potential of cannabinoids as disease-modifying therapies, for example with the recently licensed cannabinoid-based medicine Sativex (GWPharma), which, given its broad-spectrum profile, may be adequate for clinical studies in patients with ALS following the results obtained in the preclinical studies (reviewed in [159]). In support of this possibility, we recently conducted a pharmacological study with a Sativex-like combination of phytocannabinoids in postsymptomatic SOD-1 mutant mice [161]. However, although the treatment preserved the motor neurons in the spinal cord, it did not completely preserve the neuron–muscle joint, producing a poor neurological recovery and no changes in animal survival [161], thus suggesting the need to use additional phytocannabinoid combinations for clinical studies.
Concluding Remarks and Future Perspectives
The studies reviewed here are all concordant with the view that cannabinoid-based medicines may serve as a novel therapy able to delay/arrest neurodegeneration in acute and chronic neurodegenerative conditions, owing to their capability of normalizing glutamate homeostasis, reducing oxidative injury, and/or attenuating local inflammatory events, and possibly also by their capability of activating cellular responses (e.g., induction of autophagy) in controlling the toxicity of protein aggregates, although this has not been addressed here. However, most of the studies that have examined the neuroprotective potential of these compounds in neurodegeneration have been conducted in animal or cellular models, whereas the few clinical trials that have investigated cannabinoid-based medicines were focused on the alleviation of specific symptoms and not on the control of disease progression. This latter aspect remains the major challenge for the future and it may be facilitated by the recent approval of the first cannabinoid-based medicines [e.g., Sativex (GWPharma), Epidiolex (GWPharma)] available for clinical use. These formulations, and additional combination of phytocannabinoids, present 2 important advantages: 2) its safety demonstrated in previous studies [169], despite the fact that manipulation of the endocannabinoid system may be harmful in certain circumstances [1], a fact deserving additional investigation; and 2) its broad-range profile that appears to be adequate for diseases in which different cytotoxic mechanisms cooperate to damage specific neuronal subpopulations. Therefore, it is expected and desirable that the issue recruits an important amount of clinical research in the future, which will allow for the promising expectation generated around the progress of these molecules from the present preclinical evidence to a real clinical exploitation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 498 kb)
Acknowledgments
This work was supported by grants from MINECO “Programa Nacional de Biomedicina” (SAF2012-39173; SAF2012-33216; CSD2010-00045); “Instituto de Salud Carlos III (ISCiii)”, according to the “Plan Nacional de I+D+I 2008-2011”; and the “Plan Estatal de Investigación Científica y Técnica y de Innovación 2013-2016”. Co-funding was from the European Regional Development Funds (FEDER) (FIS-PS09/01900, PS12/00192 and PI1301722); “Programa de Biomedicina de la Comunidad de Madrid” (S2010/BMD-2308, S2010/BMD-2336); CIBERNED (CB06/05/0089); and GW Pharmaceuticals Ltd. We thank all colleagues that contributed to the studies conducted in the groups that have been mentioned here.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Fernández-Ruiz J, Romero J, Ramos JA. Endocannabinoids and neurodegenerative disorders: Parkinson’s disease, Huntington’s chorea, Alzheimer’s disease and others. In: Pertwee RG (ed.) Handbook of experimental pharmacology: endocannabinoids. Germany, Dortmund: Springer; 2015. [DOI] [PubMed]
- 2.Fernández-Ruiz J, de Lago E, Gómez-Ruiz M, et al. Neurodegenerative disorders other than multiple sclerosis. In: Pertwee RG, et al., editors. Handbook of cannabis. Oxford: Oxford University Press; 2014. pp. 505–525. [Google Scholar]
- 3.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther. 2015;146:23–34. doi: 10.1016/j.pharmthera.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.England TJ, Hind WH, Rasid NA, O'Sullivan SE. Cannabinoids in experimental stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2015;35:348–358. doi: 10.1038/jcbfm.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belayev L, Busto R, Zhao W, Ginsberg MD. HU-211, a novel noncompetitive N-methyl-D-aspartate antagonist, improves neurological deficit and reduces infarct volume after reversible focal cerebral ischemia in the rat. Stroke. 1995;26:2313–2319. doi: 10.1161/01.STR.26.12.2313. [DOI] [PubMed] [Google Scholar]
- 9.Shohami E, Novikov M, Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674:55–62. doi: 10.1016/0006-8993(94)01433-I. [DOI] [PubMed] [Google Scholar]
- 10.Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/S0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 11.Lavie G, Teichner A, Shohami E, Ovadia H, Leker RR. Long term cerebroprotective effects of dexanabinol in a model of focal cerebral ischemia. Brain Res. 2001;901:195–201. doi: 10.1016/S0006-8993(01)02356-3. [DOI] [PubMed] [Google Scholar]
- 12.Leker RR, Gai N, Mechoulam R, Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34:2000–2006. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- 13.Nagayama T, Sinor AD, Simon RP, et al. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi OZ, Barsoum S, Grayson J, et al. Effects of cannabinoid receptor agonist WIN 55,212-2 on blood–brain barrier disruption in focal cerebral ischemia in rats. Pharmacology. 2012;89:333–338. doi: 10.1159/000338755. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki N, Suzuki M, Murakami K, et al. Cerebroprotective effects of TAK-937, a cannabinoid receptor agonist, on ischemic brain damage in middle cerebral artery occluded rats and non-human primates. Brain Res. 2012;1430:93–100. doi: 10.1016/j.brainres.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Murakami K, Suzuki M, Suzuki N, et al. Cerebroprotective effects of TAK-937, a novel cannabinoid receptor agonist, in permanent and thrombotic focal cerebral ischemia in rats: therapeutic time window, combination with t-PA and efficacy in aged rats. Brain Res. 2013;1526:84–93. doi: 10.1016/j.brainres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Mauler F, Hinz V, Augstein KH, Fassbender M, Horváth E. Neuroprotective and brain edema-reducing efficacy of the novel cannabinoid receptor agonist BAY 38-7271. Brain Res. 2003;989:99–111. doi: 10.1016/S0006-8993(03)03376-6. [DOI] [PubMed] [Google Scholar]
- 18.Mauler F, Mittendorf J, Horváth E, De Vry J. Characterization of the diarylether sulfonylester (-)-(R)-3-(2-hydroxymethylindanyl-4-oxy)phenyl-4,4,4-trifluoro-1-sulfonate (BAY 38-7271) as a potent cannabinoid receptor agonist with neuroprotective properties. J Pharmacol Exp Ther. 2002;302:359–368. doi: 10.1124/jpet.302.1.359. [DOI] [PubMed] [Google Scholar]
- 19.Zani A, Braida D, Capurro V, Sala M. Δ9-tetrahydrocannabinol (THC) and AM404 protect against cerebral ischaemia in gerbils through a mechanism involving cannabinoid and opioid receptors. Br J Pharmacol. 2007;152:1301–1311. doi: 10.1038/sj.bjp.0707514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa K, Mishima K, Abe K, et al. Cannabidiol prevents infarction via the non-CB1 cannabinoid receptor mechanism. Neuroreport. 2004;15:2381–2385. doi: 10.1097/00001756-200410250-00016. [DOI] [PubMed] [Google Scholar]
- 21.Hayakawa K, Mishima K, Irie K, et al. Cannabidiol prevents a post-ischemic injury progressively induced by cerebral ischemia via a high-mobility group box1-inhibiting mechanism. Neuropharmacology. 2008;55:1280–1286. doi: 10.1016/j.neuropharm.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 22.Mishima K, Hayakawa K, Abe K, et al. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke. 2005;36:1077–1082. doi: 10.1161/01.STR.0000163083.59201.34. [DOI] [PubMed] [Google Scholar]
- 23.Panikashvili D, Simeonidou C, Ben-Shabat S, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- 24.Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J Cereb Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- 25.Panikashvili D, Shein NA, Mechoulam R, et al. The endocannabinoid 2-AG protects the blood-brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol Dis. 2006;22:257–264. doi: 10.1016/j.nbd.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hillard CJ, Ho WS, Thompson J, et al. Inhibition of 2-arachidonoylglycerol catabolism modulates vasoconstriction of rat middle cerebral artery by the thromboxane mimetic, U-46619. Br J Pharmacol. 2007;152:691–698. doi: 10.1038/sj.bjp.0707468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hind WH, Tufarelli C, Neophytou M, et al. Endocannabinoids modulate human blood–brain barrier permeability in vitro. Br J Pharmacol. 2015;172:3015–3027. doi: 10.1111/bph.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schomacher M, Müller HD, Sommer C, Schwab S, Schäbitz WR. Endocannabinoids mediate neuroprotection after transient focal cerebral ischemia. Brain Res. 2008;1240:213–220. doi: 10.1016/j.brainres.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Yeshurun A, Trembovler V, Alexandrovich A, et al. N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J Cereb Blood Flow Metab. 2011;31:1768–1777. doi: 10.1038/jcbfm.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Adler MW, Abood ME, et al. CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res. 2009;78:86–94. doi: 10.1016/j.mvr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Martin BR, Adler MW, et al. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol. 2009;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murikinati S, Jüttler E, Keinert T, et al. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J. 2010;24:788–798. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 33.Zarruk JG, Fernández-López D, García-Yébenes I, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke. 2012;43:211–219. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- 34.Amenta PS, Jallo JI, Tuma RF, Elliott MB. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J Neurosci Res. 2012;90:2293–2305. doi: 10.1002/jnr.23114. [DOI] [PubMed] [Google Scholar]
- 35.Elliott MB, Tuma RF, Amenta PS, Barbe MF, Jallo JI. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J Neurotrauma. 2011;28:973–981. doi: 10.1089/neu.2010.1672. [DOI] [PubMed] [Google Scholar]
- 36.Garg P, Duncan RS, Kaja S, Koulen P. Intracellular mechanisms of N-acylethanolamine-mediated neuroprotection in a rat model of stroke. Neuroscience. 2010;166:252–262. doi: 10.1016/j.neuroscience.2009.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA. Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci. 2002;22:9771–9775. doi: 10.1523/JNEUROSCI.22-22-09771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonfils PK, Reith J, Hasseldam H, Johansen FF. Estimation of the hypothermic component in neuroprotection provided by cannabinoids following cerebral ischemia. Neurochem Int. 2006;49:508–518. doi: 10.1016/j.neuint.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki N, Suzuki M, Hamajo K, et al. Contribution of hypothermia and CB1 receptor activation to protective effects of TAK-937, a cannabinoid receptor agonist, in rat transient MCAO model. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguado T, Romero E, Monory K, et al. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J Biol Chem. 2007;282:23892–23898. doi: 10.1074/jbc.M700678200. [DOI] [PubMed] [Google Scholar]
- 41.Cohen-Yeshurun A, Willner D, Trembovler V, et al. N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1242–1250. doi: 10.1038/jcbfm.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun J, Fang Y, Chen T, et al. WIN55, 212-2 promotes differentiation of oligodendrocyte precursor cells and improve remyelination through regulation of the phosphorylation level of the ERK 1/2 via cannabinoid receptor 1 after stroke-induced demyelination. Brain Res. 2013;1491:225–235. doi: 10.1016/j.brainres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Hansen HH, Ikonomidou C, Bittigau P, Hansen SH, Hansen HS. Accumulation of the anandamide precursor and other N-acylethanolamine phospholipids in infant rat models of in vivo necrotic and apoptotic neuronal death. J Neurochem. 2001;76:39–46. doi: 10.1046/j.1471-4159.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- 44.Hansen HH, Schmid PC, Bittigau P, et al. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J Neurochem. 2001;78:1415–1427. doi: 10.1046/j.1471-4159.2001.00542.x. [DOI] [PubMed] [Google Scholar]
- 45.Tchantchou F, Tucker LB, Fu AH, et al. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology. 2014;85:427–439. doi: 10.1016/j.neuropharm.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tchantchou F, Zhang Y. Selective inhibition of α/β-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma. 2013;30:565–579. doi: 10.1089/neu.2012.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger C, Schmid PC, Schabitz WR, et al. Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem. 2004;88:1159–1167. doi: 10.1046/j.1471-4159.2003.02244.x. [DOI] [PubMed] [Google Scholar]
- 48.Amantea D, Spagnuolo P, Bari M, et al. Modulation of the endocannabinoid system by focal brain ischemia in the rat is involved in neuroprotection afforded by 17β-estradiol. FEBS J. 2007;274:4464–4475. doi: 10.1111/j.1742-4658.2007.05975.x. [DOI] [PubMed] [Google Scholar]
- 49.Muthian S, Rademacher DJ, Roelke CT, Gross GJ, Hillard CJ. Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience. 2004;129:743–750. doi: 10.1016/j.neuroscience.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 50.Degn M, Lambertsen KL, Petersen G, et al. Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem. 2007;103:1907–1916. doi: 10.1111/j.1471-4159.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- 51.Ashton JC, Rahman RM, Nair SM, et al. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 52.Jin KL, Mao XO, Goldsmith PC, Greenberg DA. CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol. 2000;48:257–261. doi: 10.1002/1531-8249(200008)48:2<257::AID-ANA18>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt W, Schäfer F, Striggow V, Fröhlich K, Striggow F. Cannabinoid receptor subtypes 1 and 2 mediate long-lasting neuroprotection and improve motor behavior deficits after transient focal cerebral ischemia. Neuroscience. 2012;227:313–326. doi: 10.1016/j.neuroscience.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 54.Vandeputte C, Casteels C, Struys T, et al. Small-animal PET imaging of the type 1 and type 2 cannabinoid receptors in a photothrombotic stroke model. Eur J Nucl Med Mol Imaging. 2012;39:1796–1806. doi: 10.1007/s00259-012-2209-6. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Martin BR, Adler MW, et al. Modulation of the balance between cannabinoid CB1 and CB2 receptor activation during cerebral ischemic/reperfusion injury. Neuroscience. 2008;152:753–760. doi: 10.1016/j.neuroscience.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donat CK, Fischer F, Walter B, et al. Early increase of cannabinoid receptor density after experimental traumatic brain injury in the newborn piglet. Acta Neurobiol Exp (Wars) 2014;74:197–210. doi: 10.55782/ane-2014-1985. [DOI] [PubMed] [Google Scholar]
- 57.Maas AI, Murray G, Henney H, 3rd, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5:38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Orgado J, Fernández-Lopez D, Lizasoain I, Romero J. The seek of neuroprotection: introducing cannabinoids. Recent Patents CNS Drug Discov. 2007;2:131–139. doi: 10.2174/157488907780832724. [DOI] [PubMed] [Google Scholar]
- 59.Johnston MV, Fatemi A, Wilson MA, Northington F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011;10:372–382. doi: 10.1016/S1474-4422(11)70016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villapol S, Gelot A, Renolleau S, Charriaut-Marlangue C. Astrocyte responses after neonatal ischemia: the yin and the yang. Neuroscientist. 2008;14:339–344. doi: 10.1177/1073858408316003. [DOI] [PubMed] [Google Scholar]
- 61.Volpe JJ. Hypoxic-ischemic encephalopathy: clinical aspects. In: Volpe JJ (ed.) Neurology of the newborn. Saunders-Elsevier, Philadelphia, PA, 2008, pp. 400-479.
- 62.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernández-López D, Lizasoain I, Moro MA, Martínez-Orgado J. Cannabinoids: well-suited candidates for the treatment of perinatal brain injury. Brain Sci. 2013;3:1043–1059. doi: 10.3390/brainsci3031043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Lopez D, Martínez-Orgado J, Núñez E, et al. Characterization of the neuroprotective effect of the cannabinoid agonist WIN-55212 in an in vitro model of hypoxic-ischemic brain damage in newborn rats. Pediatr Res. 2006;60:169–173. doi: 10.1203/01.pdr.0000228839.00122.6c. [DOI] [PubMed] [Google Scholar]
- 66.Martínez-Orgado J, Fernández-Frutos B, González R, et al. Neuroprotection by the cannabinoid agonist WIN-55212 in an in vivo newborn rat model of acute severe asphyxia. Mol Brain Res. 2003;114:132–139. doi: 10.1016/S0169-328X(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 67.Fernández-López D, Pazos MR, Tolón RM, et al. The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats. Pediatr Res. 2007;62:255–260. doi: 10.1203/PDR.0b013e318123fbb8. [DOI] [PubMed] [Google Scholar]
- 68.Fernández-López D, Pradillo JM, García-Yébenes I, et al. The cannabinoid WIN55212-2 promotes neural repair after neonatal hypoxia-ischemia. Stroke. 2010;41:2956–2964. doi: 10.1161/STROKEAHA.110.599357. [DOI] [PubMed] [Google Scholar]
- 69.Alonso-Alconada D, Alvarez A, Alvarez FJ, Martínez-Orgado JA, Hilario E. The cannabinoid WIN 55212-2 mitigates apoptosis and mitochondrial dysfunction after hypoxia ischemia. Neurochem Res. 2012;37:161–170. doi: 10.1007/s11064-011-0594-z. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez FJ, Lafuente H, Rey-Santano MC, et al. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr Res. 2008;64:653–658. doi: 10.1203/PDR.0b013e318186e5dd. [DOI] [PubMed] [Google Scholar]
- 71.Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol Dis. 2010;37:434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 72.Lafuente H, Alvarez FJ, Pazos MR, et al. Cannabidiol reduces brain damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr Res. 2011;70:272–277. doi: 10.1203/PDR.0b013e3182276b11. [DOI] [PubMed] [Google Scholar]
- 73.Pazos MR, Cinquina V, Gómez A, et al. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology. 2012;63:776–783. doi: 10.1016/j.neuropharm.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 74.Pazos MR, Mohammed N, Lafuente H, et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT1A and CB2 receptors. Neuropharmacology. 2013;71:282–291. doi: 10.1016/j.neuropharm.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 75.Mechoulam R, Lichtman AH. Stout guards of the central nervous system. Science. 2003;302:65–67. doi: 10.1126/science.1091256. [DOI] [PubMed] [Google Scholar]
- 76.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lewczuk P, Mroczko B, Fagan A, Kornhuber J. Biomarkers of Alzheimer's disease and mild cognitive impairment: a current perspective. Adv Med Sci. 2015;60:76–82. doi: 10.1016/j.advms.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Braskie MN, Thompson PM. Understanding cognitive deficits in Alzheimer's disease based on neuroimaging findings. Trends Cogn Sci. 2013;17:510–516. doi: 10.1016/j.tics.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thal DR, Attems J, Ewers M. Spreading of amyloid, tau, and microvascular pathology in Alzheimer's disease: findings from neuropathological and neuroimaging studies. J Alzheimer Dis. 2014;42:S421–S429. doi: 10.3233/JAD-141461. [DOI] [PubMed] [Google Scholar]
- 80.Berk C, Paul G, Sabbagh M. Investigational drugs in Alzheimer's disease: current progress. Expert Opin Investig Drugs. 2014;23:837–846. doi: 10.1517/13543784.2014.905542. [DOI] [PubMed] [Google Scholar]
- 81.Karl T, Cheng D, Garner B, Arnold JC. The therapeutic potential of the endocannabinoid system for Alzheimer's disease. Expert Opin Ther Targets. 2012;16:407–420. doi: 10.1517/14728222.2012.671812. [DOI] [PubMed] [Google Scholar]
- 82.Iuvone T, Esposito G, De Filippis D, Scuderi C, Steardo L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci Ther. 2009;15:65–75. doi: 10.1111/j.1755-5949.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits β-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med. 2006;84:253–258. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 84.Esposito G, Scuderi C, Savani C, et al. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br J Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci Ther. 2011;17:637–644. doi: 10.1111/j.1755-5949.2010.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tolón RM, Núñez E, Pazos MR, et al. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro β-amyloid removal by human macrophages. Brain Res. 2009;1283:148–154. doi: 10.1016/j.brainres.2009.05.098. [DOI] [PubMed] [Google Scholar]
- 87.Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fakhfouri G, Ahmadiani A, Rahimian R, et al. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology. 2012;63:653–666. doi: 10.1016/j.neuropharm.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 89.Aso E, Palomer E, Juvés S, et al. CB1 agonist ACEA protects neurons and reduces the cognitive impairment of AβPP/PS1 mice. J Alzheimer Dis. 2012;30:439–459. doi: 10.3233/JAD-2012-111862. [DOI] [PubMed] [Google Scholar]
- 90.Aso E, Juvés S, Maldonado R, Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J Alzheimer Dis. 2013;35:847–858. doi: 10.3233/JAD-130137. [DOI] [PubMed] [Google Scholar]
- 91.Scuderi C, Steardo L, Esposito G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of β-amyloid expression in SHSY5YAPP+ cells through PPARγ involvement. Phytother Res. 2014;28:1007–1013. doi: 10.1002/ptr.5095. [DOI] [PubMed] [Google Scholar]
- 92.Martín-Moreno AM, Brera B, Spuch C, et al. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J Neuroinflammation. 2012;9:8. doi: 10.1186/1742-2094-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eubanks LM, Rogers CJ, Beuscher AE, et al. A molecular link between the active component of marijuana and Alzheimer's disease pathology. Mol Pharmacol. 2006;3:773–777. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen R, Zhang J, Fan N, et al. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155:1154–1165. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen R, Zhang J, Wu Y, et al. Monoacylglycerol lipase is a therapeutic target for Alzheimer's disease. Cell Rep. 2012;2:1329–1339. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piro JR, Benjamin DI, Duerr JM, et al. A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer's disease. Cell Rep. 2012;1:617–623. doi: 10.1016/j.celrep.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benito C, Tolon RM, Castillo AI, et al. β-Amyloid exacerbates inflammation in astrocytes lacking fatty acid amide hydrolase through a mechanism involving PPARα, PPARγ and TRPV1, but not CB1 or CB2 receptors. Br J Pharmacol. 2012;166:1474–1489. doi: 10.1111/j.1476-5381.2012.01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vázquez C, Tolón RM, Pazos MR, et al. Endocannabinoids regulate the activity of astrocytic hemichannels and the microglial response against an injury: In vivo studies. Neurobiol Dis. 2015;79:41–50. doi: 10.1016/j.nbd.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Benito C, Nuñez E, Tolon RM, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 101.Jung K, Astarita G, Yasar S, et al. An amyloid β42-dependent deficit in anandamide mobilization is associated with cognitive dysfunction in Alzheimer’s disease. Neurobiol Aging. 2012;33:1522–1532. doi: 10.1016/j.neurobiolaging.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 1997;12:913–919. doi: 10.1002/(SICI)1099-1166(199709)12:9<913::AID-GPS663>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 103.Casarejos MJ, Perucho J, Gómez A, et al. Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J Alzheimer Dis. 2013;35:525–539. doi: 10.3233/JAD-130050. [DOI] [PubMed] [Google Scholar]
- 104.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lima MM, Martins EF, Delattre AM, et al. Motor and non-motor features of Parkinson's disease—a review of clinical and experimental studies. CNS Neurol Disord Drug Targets. 2012;11:439–449. doi: 10.2174/187152712800792893. [DOI] [PubMed] [Google Scholar]
- 106.Dickson DW. Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2:a009258. doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson’s disease. Nat Rev Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 108.García-Arencibia M, García C, Fernández-Ruiz J. Cannabinoids and Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:432–439. doi: 10.2174/187152709789824642. [DOI] [PubMed] [Google Scholar]
- 109.Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 110.García-Arencibia M, González S, de Lago E, et al. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–170. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 111.García C, Palomo-Garo C, García-Arencibia M, et al. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson's disease. Br J Pharmacol. 2011;163:1495–1506. doi: 10.1111/j.1476-5381.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiménez-Del-Rio M, Daza-Restrepo A, Velez-Pardo C. The cannabinoid CP55,940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: implications in Parkinson's disease. Neurosci Res. 2008;61:404–411. doi: 10.1016/j.neures.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 113.Price DA, Martinez AA, Seillier A, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Eur J Neurosci. 2009;29:2177–2186. doi: 10.1111/j.1460-9568.2009.06764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gómez-Gálvez Y, Palomo-Garo C, Fernández-Ruiz J, García C. Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry 2015 Apr 9 [Epub ahead of print]. [DOI] [PubMed]
- 115.Ternianov A, Pérez-Ortiz JM, Solesio ME, et al. Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol Aging. 2012;33:e1–e16. doi: 10.1016/j.neurobiolaging.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 116.Chung YC, Bok E, Huh SH, et al. Cannabinoid receptor type 1 protects nigrostriatal dopaminergic neurons against MPTP neurotoxicity by inhibiting microglial activation. J Immunol. 2011;187:6508–6517. doi: 10.4049/jimmunol.1102435. [DOI] [PubMed] [Google Scholar]
- 117.Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol. 2009;156:1029–1040. doi: 10.1111/j.1476-5381.2008.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lastres-Becker I, Cebeira M, de Ceballos M, et al. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson's disease and MPTP-treated marmosets. Eur J Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- 119.Pisani A, Fezza F, Galati S, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson's disease patients. Ann Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- 120.Gubellini P, Picconi B, Bari M, et al. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.García MC, Cinquina V, Palomo-Garo C, Rábano A, Fernández-Ruiz J. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson's disease. Neurosci Lett. 2015;587:1–4. doi: 10.1016/j.neulet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 122.Sierra S, Luquin N, Rico AJ, et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct 2014 Jun 28 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 123.Mesnage V, Houeto JL, Bonnet AM, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004;27:108–110. doi: 10.1097/00002826-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 124.Frankel JP, Hughes A, Lees AJ, Stern GM. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990;53:436. doi: 10.1136/jnnp.53.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fox SH, Lang AE, Brotchie JM. Translation of nondopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase IIa clinical studies: keys to success and roads to failure. Mov Disord. 2006;21:1578–1594. doi: 10.1002/mds.20936. [DOI] [PubMed] [Google Scholar]
- 126.Zielonka D, Mielcarek M, Landwehrmeyer GB. Update on Huntington's disease: advances in care and emerging therapeutic options. Parkinsonism Relat Disord. 2015;21:169–178. doi: 10.1016/j.parkreldis.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 127.Jankovic J, Roos RA. Chorea associated with Huntington's disease: to treat or not to treat? Mov Disord. 2014;29:1414–1418. doi: 10.1002/mds.25996. [DOI] [PubMed] [Google Scholar]
- 128.Waldvogel HJ, Thu D, Hogg V, Tippett L, Faull RL. Selective neurodegeneration, neuropathology and symptom profiles in Huntington's disease. Adv Exp Med Biol. 2012;769:141–152. doi: 10.1007/978-1-4614-5434-2_9. [DOI] [PubMed] [Google Scholar]
- 129.Sampaio C, Borowsky B, Reilmann R. Clinical trials in Huntington's disease: interventions in early clinical development and newer methodological approaches. Mov Disord. 2014;29:1419–1428. doi: 10.1002/mds.26021. [DOI] [PubMed] [Google Scholar]
- 130.García de Yébenes J. Neuroprotection by cannabinoids in Huntington’s disease. Available at: https://clinicaltrials.gov/ct2/show/NCT01502046?term=NCT01502046&rank=1. Accessed August 5, 2015.
- 131.Sagredo O, Pazos MR, Valdeolivas S, Fernandez-Ruiz J. Cannabinoids: novel medicines for the treatment of Huntington's disease. Recent Pat CNS Drug Discov. 2012;7:41–48. doi: 10.2174/157488912798842278. [DOI] [PubMed] [Google Scholar]
- 132.Pintor A, Tebano MT, Martire A, et al. The cannabinoid receptor agonist WIN 55,212-2 attenuates the effects induced by quinolinic acid in the rat striatum. Neuropharmacology. 2006;51:1004–1012. doi: 10.1016/j.neuropharm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 133.Blázquez C, Chiarlone A, Sagredo O, et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington's disease. Brain. 2011;134:119–136. doi: 10.1093/brain/awq278. [DOI] [PubMed] [Google Scholar]
- 134.Sagredo O, González S, Aroyo I, et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for Huntington's disease. Glia. 2009;57:1154–1167. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Palazuelos J, Aguado T, Pazos MR, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain. 2009;132:3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- 136.Sagredo O, Ramos JA, Decio A, Mechoulam R, Fernández-Ruiz J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci. 2007;26:843–851. doi: 10.1111/j.1460-9568.2007.05717.x. [DOI] [PubMed] [Google Scholar]
- 137.Valdeolivas S, Navarrete C, Cantarero I, et al. Neuroprotective properties of cannabigerol in Huntington's disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12:185–199. doi: 10.1007/s13311-014-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valdeolivas S, Satta V, Pertwee RG, Fernández-Ruiz J, Sagredo O. Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington's disease: role of CB1 and CB2 receptors. ACS Chem Neurosci. 2012;3:400–406. doi: 10.1021/cn200114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sagredo O, Pazos MR, Satta V, et al. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington's disease. J Neurosci Res. 2011;89:1509–1518. doi: 10.1002/jnr.22682. [DOI] [PubMed] [Google Scholar]
- 140.Glass M, Dragunow M, Faull RLM. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA-A receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience. 2000;97:505–519. doi: 10.1016/S0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 141.Lastres-Becker I, Berrendero F, Lucas JJ, et al. Loss of mRNA levels, binding and activation of GTP-binding proteins for cannabinoid CB1 receptors in the basal ganglia of a transgenic model of Huntington's disease. Brain Res. 2002;929:236–242. doi: 10.1016/S0006-8993(01)03403-5. [DOI] [PubMed] [Google Scholar]
- 142.Chiarlone A, Bellocchio L, Blázquez C, et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc Natl Acad Sci USA. 2014;111:8257–8262. doi: 10.1073/pnas.1400988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bouchard J, Truong J, Bouchard K, et al. Cannabinoid receptor 2 signaling in peripheral immune cells modulates disease onset and severity in mouse models of Huntington's disease. J Neurosci. 2012;32:18259–18268. doi: 10.1523/JNEUROSCI.4008-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dowie MJ, Grimsey NL, Hoffman T, Faull RL, Glass M. Cannabinoid receptor CB2 is expressed on vascular cells, but not astroglial cells in the post-mortem human Huntington's disease brain. J Chem Neuroanat. 2014;59-60:62–71. doi: 10.1016/j.jchemneu.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 145.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 146.Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 148.Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- 150.Cruts M, Gijselinck I, Van Langenhove T, van der Zee J, Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci. 2013;36:450–459. doi: 10.1016/j.tins.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 151.Habib AA, Mitsumoto H. Emerging drugs for amyotrophic lateral sclerosis. Expert Opin Emerg Drugs. 2011;16:537–558. doi: 10.1517/14728214.2011.604312. [DOI] [PubMed] [Google Scholar]
- 152.Carter GT, Abood ME, Aggarwal SK, Weiss MD. Cannabis and amyotrophic lateral sclerosis: hypothetical and practical applications, and a call for clinical trials. Am J Hosp Palliat Care. 2010;27:347–356. doi: 10.1177/1049909110369531. [DOI] [PubMed] [Google Scholar]
- 153.Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp Neurol. 2010;224:92–102. doi: 10.1016/j.expneurol.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 154.Raman C, McAllister SD, Rizvi G, et al. Amyotrophic lateral sclerosis: delayed disease progression in mice by treatment with a cannabinoid. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:33–39. doi: 10.1080/14660820310016813. [DOI] [PubMed] [Google Scholar]
- 155.Weydt P, Hong S, Witting A, et al. Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6:182–184. doi: 10.1080/14660820510030149. [DOI] [PubMed] [Google Scholar]
- 156.Bilsland LG, Dick JR, Pryce G, et al. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J. 2006;20:1003–1005. doi: 10.1096/fj.05-4743fje. [DOI] [PubMed] [Google Scholar]
- 157.Kim K, Moore DH, Makriyannis A, Abood ME. AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. Eur J Pharmacol. 2006;542:100–105. doi: 10.1016/j.ejphar.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 158.Shoemaker JL, Seely KA, Reed RL, Crow JP, Prather PL. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem. 2007;101:87–98. doi: 10.1111/j.1471-4159.2006.04346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de Lago E, Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J. Endocannabinoids and amyotrophic lateral sclerosis. In: Fattore L, editor. Cannabinoids in neurologic and mental disease. Amsterdam: Elsevier; 2015. pp. 99–124. [Google Scholar]
- 160.Witting A, Weydt P, Hong S, et al. Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J Neurochem. 2004;89:1555–1557. doi: 10.1111/j.1471-4159.2004.02544.x. [DOI] [PubMed] [Google Scholar]
- 161.Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1G93A transgenic mice and evaluation of a Sativex®-like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther. 2014;20:809–815. doi: 10.1111/cns.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Espejo-Porras F, Piscitelli F, Verde R, et al. Changes in the endocannabinoid signaling system in CNS structures of TDP-43 transgenic mice: relevance for a neuroprotective therapy in TDP-43-related disorders. J Neuroimmune Pharmacol. 2015;10:233–244. doi: 10.1007/s11481-015-9602-4. [DOI] [PubMed] [Google Scholar]
- 163.Yiangou Y, Facer P, Durrenberger P, et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao P, Ignacio S, Beattie EC, Abood ME. Altered presymptomatic AMPA and cannabinoid receptor trafficking in motor neurons of ALS model mice: implications for excitotoxicity. Eur J Neurosci. 2008;27:572–579. doi: 10.1111/j.1460-9568.2008.06041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Amtmann D, Weydt P, Johnson KL, Jensen MP, Carter GT. Survey of cannabis use in patients with amyotrophic lateral sclerosis. Am J Hosp Palliat Care. 2004;21:95–104. doi: 10.1177/104990910402100206. [DOI] [PubMed] [Google Scholar]
- 166.Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry. 2010;81:1135–1140. doi: 10.1136/jnnp.2009.200642. [DOI] [PubMed] [Google Scholar]
- 167.Gelinas D, Miller R, Abood M. A pilot study of safety and tolerability of Δ9-THC (marinol) treatment for ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3:23. doi: 10.1080/146608202320374318. [DOI] [PubMed] [Google Scholar]
- 168.Joerger M, Wilkins J, Fagagnini S, et al. Single-dose pharmacokinetics and tolerability of oral Δ9-tetrahydrocannabinol in patients with amyotrophic lateral sclerosis. Drug Metab Lett. 2012;6:102–108. doi: 10.2174/1872312811206020102. [DOI] [PubMed] [Google Scholar]
- 169.Flachenecker P, Henze T, Zettl UK. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol. 2014;72:95–102. doi: 10.1159/000360285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 498 kb)