Abstract
The human motor system is active not only when actions are performed but also when they are observed. Experimenters often manipulate aspects of the action or context to examine factors that influence this “mirror” response. However, little is known about the role of the observer's own top-down intentions and motivation. In this exploratory study, we investigated whether observers are able to exert conscious control over their mirror response, when they are explicitly instructed to either increase or decrease mirroring. Transcranial magnetic stimulation (TMS) was used to elicit motor-evoked potentials (MEPs) in a thumb abductor muscle as participants (n = 13) watched a video of a hand squeezing a rubber ball. The size of these MEPs, relative to the size of MEPs elicited during fixation cross observation, was taken as an index of mirroring. In an initial block of trials, participants were instructed to merely observe the actions presented. After the first block, the concept of mirroring was explained to the participants, and in the second and third blocks participants were instructed to either increase or decrease their mirror response. We did not instruct them about how to achieve this increase or decrease. Our results showed no difference in either facilitation or absolute motor excitability (i.e., nonnormalized MEP size) between the three blocks, indicating that individuals do not seem to be able to exert control over motor excitability during action observation, at least in the absence of a specific and maintained strategy.
Keywords: motor excitability, mirror system, imagery, motor resonance, intention, control
during a typical day, we observe numerous movements performed by other people. Viewing the actions of others modulates activity in our own motor systems, and as a result might influence our own behavior. The activation of the motor system during action observation, hereafter referred to as “mirroring,” might play a role in our recognition and interpretation of others' actions (e.g., Buccino and Riggio 2004; Decloe and Obhi 2013; Iacoboni et al. 2005). Furthermore, the purported behavioral consequences of mirroring (i.e., mimicry) are thought to aid our affiliation with the person we are interacting with. Many studies have examined the role of factors such as action context (e.g., Iacoboni et al. 2005) and model type (e.g., Gazzola et al. 2007) on the mirror response, but little is known about the role of the observer themselves. More specifically, we do not know whether people are able to influence the mirror response directly through top-down intentions. If our mirroring of others' actions contributes to our ability to interpret the behavior of others, empathize with others, and even imitate actions that we observe, then having some control over the degree to which we mirror could be advantageous.
Direct evidence of changes in motor activity comes from studies using techniques such as electroencephalography (EEG; e.g., Muthukumaraswamy and McNair 2004) and transcranial magnetic stimulation (TMS; Fadiga et al. 1995), which have shown modulation of activity associated with movement execution when movements are simply observed. Activation of the motor system can also be seen when individuals are instructed to simply imagine themselves performing an action (e.g., Clark et al. 2004; Sakamoto et al. 2009). Indirect evidence of changes in the motor system during observation comes from behavioral studies showing that action execution is influenced by action observation. For example, we are faster to perform a movement if we simultaneously see the same action being performed but slower to perform movements when watching somebody perform the opposite action (e.g., opening our hand while watching a hand closing). These “automatic imitation” effects are thought to arise because the observed action is being mirrored by the observer's motor system, thus facilitating one's own execution of the action but impeding performance of different movements (e.g., Brass et al. 2000; Obhi and Hogeveen 2013; see Heyes 2011, for a discussion of the relationship between automatic imitation and the mirror system).
Automatic imitation is, as its name indicates, generally thought to occur automatically. As discussed by Heyes (2011), the automaticity of the response is reflected in the fact that imitation occurs despite the imitated stimulus being irrelevant to the experimental task. Furthermore, automatic imitation has been shown in the context of strategic games, in which imitating the action of another person is detrimental to the participant and not imitating is incentivized (Belot et al. 2013; Cook et al. 2012). While it has been shown that automatic imitation is influenced by factors such as the instructions given to participants (e.g., Longo and Bertenthal 2009), this does not necessarily imply that the imitation mechanism itself can be influenced or controlled by intention. Heyes (2011) refers to automatic imitation in terms of simple stimulus-response connections. Specifically, she hypothesized that automatic imitation arises from a link between the observed movement (the stimulus) and motor representations associated with that movement. The degree of automatic imitation can be influenced by 1) factors that affect the initial processing of the stimulus (e.g., attention, which can be influenced by task instructions) and 2) factors that influence the expression of the response (such as inhibition of motor activity). Importantly, however, the stimulus-response relationship itself cannot be influenced or overridden. Thus, according to the theory of Heyes (2011), automatic imitation is automatic, but the degree to which it is evident in a given context depends on factors affecting the input (stimulus) and output (motor response).
The view that automatic imitation is indeed automatic is also supported by the findings of Hogeveen and Obhi (2013). In their study, participants were required to lift either their index or middle finger on each trial, depending on a visual cue presented to them. The cue overlaid a video of a hand, in which either the index finger or middle finger was lifted. On each trial, the observed movement was either the same (“congruent”) or different (“incongruent”) to the movement that the participant was instructed to make. In this paradigm, it is typically found that responses are slower and more error-prone on incongruent trials. Hogeveen and Obhi examined the level of automaticity by manipulating participants' expectation of the congruence of observed and cued movement. Specifically, in one condition the congruent trials made up 75% of the trial set, and participants were told that the movement they saw would likely be the same as the movement they were required to perform. In another condition, only 25% of the trials were congruent and participants were told that the observed movement would likely be different to the required movement. It was found that automatic imitation was unaffected by this manipulation; accuracy and reaction times were similarly affected by the incongruent stimuli regardless of whether the participants expected the observed stimulus to be incongruent.
Interestingly, expectation has been found to affect performance on the Stroop task, in which performance on a color-naming task is impaired by participants' implicit processing of color words. More specifically, when participants expected that color words were going to differ from the color of the text itself, their performance (i.e., ability to ignore the irrelevant word meaning) improved, suggesting a top-down influence on performance (e.g., Tzelgov et al. 1992). The effects of incongruent word meaning and font color on the Stroop task are thought to occur automatically; however, this finding shows that the effects can be counteracted to some extent by the individual. If effects of action-viewing present in automatic imitation tasks are subject to similar top-down control, we would expect that performance on the automatic imitation task used by Hogeveen and Obhi would have improved when participants could predict the incongruence.
To our knowledge, only one study has looked specifically at individuals' ability to control the neural effects of action observation. Bardi et al. (2015) examined how mirror responses (as indexed by the size of motor-evoked responses in the hand) to the observation of hand movement were affected by instructing participants to prepare the same or different movement. It was found that preparing to imitate the observed movement (but not actually moving) resulted in a typical “mirror” pattern of modulation: greater activation in an index finger abductor muscle when index finger movement was viewed compared with when little finger movement was observed, and the opposite pattern of activation in a little finger abductor muscle. However, in a separate block in which participants prepared to perform the opposite movement (i.e., index finger movement when a little finger movement was observed), these differences (indicating mirroring) were eliminated. Thus it seems that the mirror response can be attenuated by the preparation of movement incongruent with that observed. However, it is not known whether participants would be able to attenuate (or enhance) their mirror responses in the absence of specific instructions telling them to prepare a different movement. There is evidence that people can be trained to control the amplitude of brain activity in the 8- to 13-Hz frequency range (Kuhlman 1978; Sheikh et al. 2003), which is often a taken as a measure of mirror system activity. Furthermore, individuals can learn to modulate activation of motor regions of the brain using movement execution and imagery, when provided with near real-time updates of localized brain activation using a functional MRI (Berman et al. 2012; deCharms et al. 2004; Yoo and Jolesz 2002; Yoo et al. 2004). These studies suggest that it is indeed possible to control activity in motor regions of the brain when feedback is provided, but it is not clear whether motor activity can be controlled in the absence of feedback. These findings also do not tell us whether activation arising from action observation specifically can be controlled in a similar manner. The fact that motor imagery can be used to control motor activation might suggest that effects of action observation can also be controlled, as motor imagery appears to affect motor excitability in a similar way to observation (e.g., Clark et al. 2004; Roosink and Zijdewind 2010).
In this exploratory study, we examined whether people can increase or decrease mirroring when they are given an explicit instruction to do so but are not given any specific strategy to employ. Thus, in our study we merely asked participants, after explaining the mirror response to them, to try to increase mirroring or refrain from mirroring the observed action. The same action (a hand squeezing a rubber ball between the thumb and index finger) was observed across all blocks, while motor-evoked potentials (MEPs) were elicited in a thumb abductor muscle using TMS. We predicted that if people are able control the mirror response, MEP facilitation (i.e., MEP amplitude during action observation relative to during fixation cross viewing) would be greater in the “increase” block than in the “decrease” block. Depending on the degree to which MEPs can be controlled, we might expect MEP facilitation in the increase and decrease blocks to be larger and smaller (respectively) than those elicited in the baseline (no instruction) block.
METHOD
Participants.
Our sample consisted of 13 students from McMaster University (10 females, 3 males), who participated for course credits or monetary reimbursement. Participants were aged 18–23 yr (means = 19.7, SD = 1.25), and all were right-handed by self-report. The study was approved by the McMaster University Research Ethics Board and conformed to the guidelines of the Declaration of Helsinki. All participants gave written informed consent before beginning the experiment.
Design.
Our experiment followed a within-subject design. Participants watched the same video stimuli in three conditions separated into blocks. In the first block, the participants were given no instructions other than to watch the video and keep their hand and arm as relaxed as possible. After the first block, the experimenter explained the concept of mirroring to the participants, before disclosing to them the purpose of the experiment (to investigate people's ability to exert control over this mirror response). In the second and third experimental blocks, participants were instructed to increase or decrease their mirror responses, using whatever strategy they felt appropriate. The order of the increase and decrease blocks was counterbalanced between participants. Each of the three blocks consisted of 96 trials: 48 action trials and 48 fixation cross trials, presented in a randomized order. TMS was delivered on 50% of the fixation cross trials and 50% of the action trials; thus 24 MEPs were collected for each condition within each block. All three blocks were identical apart from the instruction given to the participant, and the trial order (which was randomized for each block and each participant).
Apparatus and stimuli.
Visual stimuli were presented using MATLAB (version 2013b), which also controlled the timing of TMS delivery. TMS was delivered using a Magstim Rapid2 stimulator with a figure-of-eight coil (Magstim, Wales, UK), and muscle activity recorded using self-adhesive snap electrodes (23 × 22 mm). The data were acquired at a sampling rate of 5 kHz, amplified, and filtered (band-pass 10–500 Hz), using Biopac MP150 hardware and Acqknowledge software (version 4.2.0; Biopac Systems). Offline analysis was performed using MATLAB and SPSS (version 16.0).
The action stimuli showed a female Caucasian right hand squeezing a rubber ball between the thumb and index finger. On each trial, the hand squeezed the ball five times. TMS was delivered (on 50% of trials) on one of these five squeezes. The trial duration was ∼11 s, and each of the three blocks lasted ∼6–8 min. Participants were invited to take breaks in between the blocks.
Procedure.
Two self-adhesive electrodes were positioned in a belly-tendon arrangement to record activity from the abductor pollicis brevis (APB) of the right (dominant) hand. The ground electrode was placed over the ulnar styloid process of the wrist for 10 of the participants and over the lateral epicondyle of the elbow for the final three participants. This change was made to minimize stimulation artifacts occurring in the data.1 The “hand” area of the motor cortex was found by first measuring a distance 4 cm lateral and 1 cm anterior to the vertex, and eliciting MEPs in this approximate area to find the position from which the largest responses could be elicited in the APB. The resting motor threshold (RMT) was defined as the lowest intensity of stimulation required to evoke responses of above 0.05 mV in at least 5 out of 10 consecutive trials, and stimulation intensity during the experiment was set at 120% of this RMT. Stimulation intensity during the experiment ranged from 65 to 89% of the maximum stimulator output (mean = 77.8, SD = 8.56).
Participants were seated in front of a computer monitor on which the stimuli were displayed, with their right hand and forearm resting on a cushion on the table in front of them. Before the first block, they were told that they would see a hand squeezing a rubber ball and that their task was to simply watch the stimuli and keep their hand and arm as relaxed as possible. After the first block, the experimenter gave them a brief written description of mirroring, and also explained it to them verbally. The participants were told that we were interested in whether people are able to voluntarily control the degree to which they mirror actions, and that in the next two blocks they would be asked to either increase or decrease mirroring. If the participant asked how they should do this, they were told that there were no instructions and that they should “just try” to do it, using whatever strategy they liked. They were told both verbally and on the written instructions that the only stipulations were that they must watch the hand throughout the block and that they must keep their own hand and arm relaxed. Participants were not instructed to look at any specific part of the stimulus. The experimenter monitored the muscle recording throughout the blocks and reminded participants to relax their hand and arm if there was a visible increase in muscle activity. At the end of the experiment, participants wrote down any strategies that they used in each of the blocks.
Data analysis.
MEP amplitude was defined as the difference between the minimum and maximum values of the electromyography (EMG) signal in the 20- to 40-ms window following the TMS pulse. For each block per participant, we calculated the average background EMG amplitude in the 100 ms preceding the TMS pulse and excluded any trials for which the EMG amplitude during this prestimulus period was more than 3 SDs away from the block mean. This resulted in the exclusion of an average of 2.14% of trials (across all 3 blocks in each subject). In addition, we excluded trials for which the peak-to-peak MEP amplitude was greater than 3 SDs away from the average MEP amplitude for that block and participant. This resulted in the exclusion of a further 1.39% of trials. The average number of trials per block included in the final dataset was 46.3 (SD = 0.855, range = 44–48).
Following outlier exclusion, MEPs for each block were normalized by dividing by the average amplitude of MEPs elicited during fixation cross presentation within that block, such that these normalized values represented modulation of MEPs during movement observation. MEP facilitation was then compared between the three conditions (no instruction, increase mirroring, decrease mirroring) by means of a one-way ANOVA. We conducted the same analysis to look at the effect of instruction on nonnormalized MEP data collapsed across the fixation and action trials. To check that prestimulus EMG activity did not differ between the conditions, we calculated the average root mean square (RMS) of the EMG signal for the 100 ms preceding the stimulation pulse. These values were entered into the same one-way ANOVA as the MEP data to ensure no differences existed between the blocks.
All values were Greenhouse-Geisser-corrected, as the assumptions of sphericity were violated (as indicated by Mauchly's test values significant at the P < 0.05 level).
RESULTS
The one-way ANOVA showed no significant effect of instruction condition on MEP modulation [F(1.31,15.7) = 0.060, P = 0.871, η2 = 0.005; see Fig. 1]. To examine the possibility that the increase or decrease instructions lead to a modulation of general motor excitability (i.e., not specific to action observation), we conducted a further one-way ANOVA with overall MEP amplitude as the dependent variable. This analysis also showed no differences between the conditions [F(1.39,16.6) = 1.21, P = 0.306, η2 = 0.092]. Individual subject data can be seen in Fig. 2.
Fig. 1.
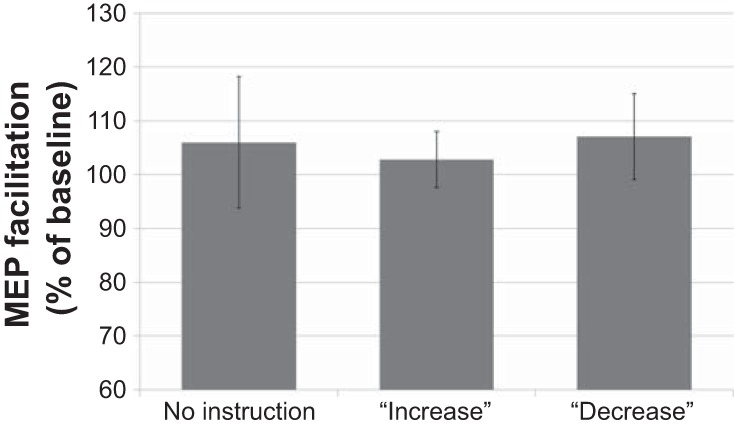
Motor-evoked potential (MEP) facilitation, displayed as percentage increase during action observation trials relative to fixation cross trials, for each of the 3 instruction conditions. Plotted data show the mean across participants, and error bars represent SE. Note that there were no significant differences among the 3 conditions.
Fig. 2.
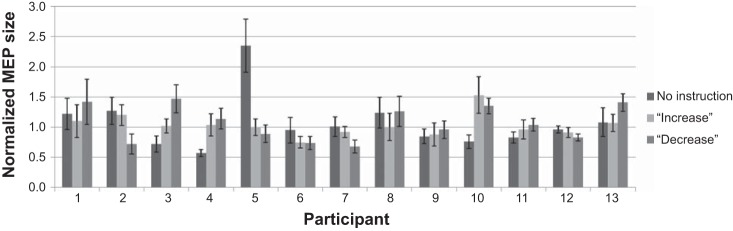
Bar plot showing normalized MEP amplitude for each participant for each condition. Data represent the mean normalized amplitude of action observation trials for each condition; error bars represent SE.
Our analysis also revealed no significant differences between conditions in prestimulus EMG activation: RMS of the EMG signal in the 100 ms preceding stimulation was comparable between the three blocks [F(1.45,21.7) = 0.316, P = 0.662, η2 = 0.021]. Interestingly, there was a nearly significant (P = 0.056) effect of condition on the number of trials excluded due to above-average prestimulus EMG activity (i.e., more than 3 SDs away from the block mean) in the 100 ms before the pulse. Specifically, a larger number of trials were excluded in the increase and decrease blocks (2.56% exclusion rate for both) compared with the no instruction block [1.27% trials excluded; F(2,24) = 3.27, P = 0.056, η2 = 0.214].
Self-reported strategies.
The most common strategy (reported by nine participants) for increasing mirroring was to imagine oneself performing the squeezing action. Three participants reported trying to keep their hand fully relaxed, two reported repeating the words “squeeze,” “grab,” or “increase” in their head while watching the action, and two reported that they focused their visual attention on the movement of the hand or parts of the hand that showed the most motion. Strategies reported by only one participant was tensing the other (left) hand, visualizing someone throwing a ball towards them so that they had to catch it, trying to anticipate the TMS pulse, and conversely, “relaxing the mind” so that the pulse came as more of a surprise. Two participants referred to the fixation cross trials specifically: one reported that they repeated to themselves “don't move” during these trials, and the other reported visualizing the action video during these trials.
For the “decrease” mirroring block, three participants reported focusing on the part of the stimulus with least movement: either the ball itself or the little finger. Three said that they repeated the words “don't move” or “decrease” in their heads, and two participants stated that they tried to not imagine themselves performing the action. Other strategies included trying to stay as relaxed as possible (n = 2), counting the number of squeezes (n = 2), imagining performing the opposite movement (finger extension; n = 2), paying attention to their left hand (n = 1), imagining performing a movement that did not involve the hands (n = 1), and thinking about other things (n = 1).
DISCUSSION
This study set out to determine whether people are able to exert conscious control over the response of the motor system when an action is observed (i.e., mirroring), in the absence of feedback or specific strategy instructions. Previous research has shown that mirroring is influenced by factors such as action context (Iacoboni et al. 2005), familiarity (Calvo-Merino et al. 2006), and prior social interactions (Hogeveen and Obhi 2012), but it was not known whether the explicit intentions of an observer can modulate the response. Our results suggest that people are not able to control motor cortical activity when they observe an action, at least in a context with no feedback and no explicit instruction of what approach to take. Specifically, in this experiment, motor excitability was not affected by the instruction to participants to increase or decrease mirroring.
Our findings are somewhat consistent with the results of Hogeveen and Obhi (2013), who found that automatic imitation, thought to be closely linked to neural mirroring, was not affected by participants' expectation that an on-screen stimulus would affect their own action execution. Research finding that people are better able to ignore irrelevant stimuli when they expect it to be presented (Tzelgov et al. 1992) suggests that people can control seemingly bottom-up implicit processing if they are aware of it. Based on this work, we should expect that if individuals are able to control their motor excitability during action observation, less automatic imitation would occur when participants were expecting to see incongruent visual stimuli. On the contrary, Hogeveen and Obhi (2013) found that the degree to which participants' performance was disrupted by observing an irrelevant movement was not influenced by their anticipation of the irrelevant, and thus distracting, stimuli. Thus the current finding and that of Hogeveen and Obhi are complementary.
Perhaps the most relevant recent study to the current work is that of Bardi et al. (2015) who showed that mirror responses can be eliminated by instructions given to participants. It could be argued that their results are at odds with the findings in the current study. However, task differences between Bardi et al. and the present experiment can explain the seemingly discrepant results. It is clear that, although the mirror response appears to occur relatively automatically (Hogeveen and Obhi 2013), the modulation characterizing it does not always strictly reflect the observed action (e.g., Sartori et al. 2013). The nonrigidity of the mirror response has also been demonstrated in research by Catmur et al. (2011) showing that the mirror response (as indexed by MEP facilitation) associated with viewing a specific action can be modified by training people to perform a different movement whenever they see the action (e.g., lift the index finger when a little finger lift is observed). In Bardi et al. (2015) experiment, participants were instructed to prepare to perform either the same or different (in different blocks) movement in response to seeing index and little finger lifts. In the congruent condition, in which participants prepared to imitate the observed movement, the pattern of MEP modulation reflected the observed movement (i.e., larger MEPs in the index finger abductor when the index finger was observed). However, preparing to perform the opposite movement in response to seeing the movement (e.g., preparing to lift the index when the on-screen little finger moved) eliminated this mirror pattern. These results show that participant intentional motor preparation can influence (in this case eliminate) the mirror response as measured by peripheral muscle activation. However, this does not mean that automatic mirroring did not occur. Rather, as discussed by Bardi et al., it could suggest that the motor representations associated with the prepared (opposite) movement simply overrode the representations elicited (via automatic mirror processes) by the observed stimulus. That is, the instruction to prepare the opposite movement led to the formation of new stimulus-response associations between the observed movement and the neural activation associated with preparing the opposite movement.
In contrast, in the present study, the task did not specifically require participants to form new associations, so there was no alternative representation to overshadow the automatic mirror response. Again, in the study by Bardi et al., mirror responses were seemingly overridden by a new short-term association between the observed and the opposite movement. This overshadowing of the mirror response makes sense if one assumes that actions serving current goals have privileged access to motor output (and a current goal was to activate a particular motor representation). This explanation could be consistent with the idea of output modulation described by Heyes (2011). In contrast, in our study, there was no specific goal to prepare one action over another as we simply required participants to volitionally “try” to affect the mirror response, which they could not do. Thus our results and those of Bardi et al. can be viewed as complementary in that they address related but different questions surrounding voluntary control over the mirror response.
Although no specific instructions were given in the current study, a number of participants reported that they attempted to increase mirroring by imagining themselves performing the action. Imagery of an action has indeed been found to increase motor excitability in muscles involved in the imagined movement (e.g., Clark et al. 2004; Rossini et al. 1999; Sakamoto et al. 2009; Wright et al. 2014), so it is surprising that this strategy did not increase MEP size. A few studies have shown that viewing an action whilst simultaneously imagining performing it enhances motor activity to a greater extent than does viewing or imagining the movement alone (Ohno et al. 2011; Sakamoto et al. 2009; Tsukazaki et al. 2012; Wright et al. 2014). This suggests that action observation and imagery can be performed simultaneously and in fact have additive effects on motor excitability facilitation. One possible reason for the apparent lack of effect of motor imagery in this study could be that the requirement and reminders to participants to keep their muscles as relaxed as possible (which was reiterated if the experimenter saw a visible increase in EMG activity) prevented or overrode the expected facilitative effects of imagery. This explanation was proposed also by Berman et al. (2012), who found that subjects were not able to increase motor activation using motor imagery when real-time functional MRI feedback was provided to them. In contrast, the same participants were successful in using actual motor execution (finger tapping) to reach a target level of motor activation, both with and without neurofeedback. Berman et al. proposed that the effort to refrain from movement during the imagery task might have lead to an inhibition of M1 activity. EMG recording during the experiment of Berman et al. confirmed that participants kept their hand relaxed during motor imagery. Likewise, in the present study, EMG activity was monitored by the experimenter during the block, and participants were instructed to relax their hand and arm in the event of visibly increased activity.
Other explanations for why the reported imagery was ineffectual in modulating MEP size relate to the type of imagery employed by the participants, the timing of imagery, and the extent to which they maintained the strategy during the block. Regarding imagery type, it has been found that kinaesthetic imagery, that is, imagining oneself actually performing the movement and the sensations associated with it, has a greater effect on excitability than visual imagery. In fact, Stinear et al. (2006) found facilitation of motor excitability for kinaesthetic but not visual imagery. In another study of motor imagery, Neuper et al. (2005) found a different pattern of brain activation for these two types of imagery of an action; activation associated with kinaesthetic imagery seemed to be specific to sensorimotor hand region, whereas no specific spatial pattern of activation was evident for visual imagery. If the imagery used by participants in the present study tended to be visual, rather than kinaesthetic, it might have had little or no further effect on motor excitability than observation alone. Second, it is possible that the timing of imagery relative to the observed movement itself could explain a failure of this strategy to increase resonance. Specifically, it has been shown that imagery during observation enhanced corticospinal excitability, but only when observation and imagery are in phase with each other (Sakamoto et al. 2009). It is possible that imagery of the action was not strictly tied to the timing of what participants were viewing directly, which could have rendered this strategy ineffectual.
Another strategy reported by participants was to focus on a particular part of the stimulus: either the area with the most movement for the increase block, or a part showing little or no movement (the ball or the little finger) for the decrease block. Based on previous work (Bach et al. 2007) showing that spatial cueing during action observation influences motor priming, we would expect this strategy to have an effect on motor responses to action observation. In the experiment of Bach et al., motor priming (reflected in faster responses made with the hand when watching a hand action than a foot action, and vice versa for responses made using the foot) was evident only when participants' attention was cued to the acting effector on the screen. When the participants' attention was drawn to the head of the model in the action stimulus, as opposed to being cued to the acting effector, motor priming did not occur. These results indicate that spatial attention is important for motor activation associated with action observation, so attending to the area of the hand with the most movement would be expected to enhance mirroring relative to attending to a region showing no movement. It is possible that this strategy had no influence in our study because in both cases participants were still viewing the effector. As they were instructed explicitly that they must still watch the stimulus in all conditions, attention across all blocks should have been on the acting effector or the object. Thus perhaps varying spatial attention within an effector does not affect motor processing. Unfortunately, without eye-tracking data to confirm whether and how ocular fixations differed between conditions in the present study, it is not possible to infer the effects of participants' self-reported fixation strategy on motor excitability. However, based on this work showing spatial attention effects on motor priming, this is something that should be explored in future studies.
It is possible, of course, that the imagery and fixation strategies reported by participants were not effective because they were not maintained throughout the blocks. As no feedback was given, and participants were not instructed to persevere with the same strategy, it is possible that the participants tried different methods during the blocks or that they tried only one but did not maintain it during every trial. A related consideration, for imagery, is the timing of this strategy within the block. Specifically, although the participants were told that mirroring was characterized by increased activity during action observation, it is possible that imagery was attempted during fixation trials as well as action trials. Indeed, one participant reported that they imagined the movement when they saw the fixation cross on the screen. However, the fact that there were no significant differences between the blocks even in overall motor excitability (i.e., MEPs elicited during both action and baseline trials) suggests that the participants' strategies had no effect on either overall excitability or mirroring specifically.
In previous work showing volitional control on physiological activity, participants are given feedback to allow them to learn how to control the responses. In this study, no feedback was given other than the instruction to relax the hand and arm when increased muscle activity was observed by the experimenter. It would be interesting, in future work, to assess changes in mirroring in an experiment in which participants are given feedback about the size of their muscle responses. It is not clear whether such feedback would be effective (or effectively measured) using TMS-evoked MEPs, because this measure assesses activity at such discrete time points. This is in contrast to studies using continuous recording such as EEG to show participant-controlled modulation of activity. Based on the theory that mirroring is automatic but subject to input and output modulation, we might predict that feedback would enable participants to learn to control the mirror response by learning to inhibit motor activity. In other words, the effects of feedback would be on the motor output itself: participants might be able to learn to inhibit or enhance motor activation, but it would not be an effect on the mirror response per se.
To summarize, in the current study we showed that participants were unable to exert any conscious control on motor excitability during action observation, after being given the general instruction to increase or decrease mirroring of the action. Our results suggest that, in the absence of specific instructed strategies or feedback, participants are unable to consciously modulate their mirror responses to observed actions. This pattern of results is consistent with the notion of an automatic mirror response to observed actions. Finally, given that other recent work has demonstrated that preparing an alternate action can modulate motor cortical output during action observation, future work must systematically explore the conditions under which motor cortical output can and cannot be consciously influenced.
GRANTS
This work was supported by a Social Sciences and Humanities Research Council Grant (to S. S. Obhi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.R.N. and S.S.O. conception and design of research; K.R.N. performed experiments; K.R.N. and S.S.O. analyzed data; K.R.N. and S.S.O. interpreted results of experiments; K.R.N. prepared figures; K.R.N. drafted manuscript; K.R.N. and S.S.O. edited and revised manuscript; K.R.N. and S.S.O. approved final version of manuscript.
Footnotes
The change in ground electrode positioning was implemented due to a stimulation artifact that occurred in some individuals tested and in some cases impeded on the MEP window. This artifact resulted in the loss of a few datasets due to not being able to detect or accurately measure MEPs, so we moved the electrode in an attempt to eliminate this artifact. All of the datasets included in the sample, however, showed visible MEPs that were not affected by the stimulation artifact. MEP traces for the included data can be obtained from the authors on request.
REFERENCES
- Bach P, Peatfield NA, Tipper SP. Focusing on body sites: the role of spatial attention in action perception. Exp Brain Res 178: 509–517, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi L, Bundt C, Notebaert W, Brass M. Eliminating mirror responses by instructions. Cortex 70: 128–136, 2015. [DOI] [PubMed] [Google Scholar]
- Belot M, Crawford VP, Heyes C. Players of matching pennies automatically imitate opponents' gestures against strong incentives. Proc Natl Acad Sci USA 110: 2763–2768, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BD, Horovitz SG, Venkataraman G, Hallett M. Self-modulation of primary motor cortex activity with motor and motor imagery tasks using real-time fMRI-based neurofeedback. Neuroimage 59: 917–925, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohschläger A, Prinz W. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn 44: 124–143, 2000. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Riggio L. The mirror neuron system and action recognition. Brain Lang 89: 370–376, 2004. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16: 1905–1910, 2006. [DOI] [PubMed] [Google Scholar]
- Catmur C, Mars RB, Rushworth MF, Heyes C. Making mirrors: premotor cortex stimulation enhances mirror and counter-mirror motor facilitation. J Cogn Neurosci 23: 2352–2362, 2011. [DOI] [PubMed] [Google Scholar]
- Clark S, Tremblay F, Ste-Marie D. Differential modulation of corticospinal excitability during observation, mental imagery and imitation of hand actions. Neuropsychologia 42: 105–112, 2004. [DOI] [PubMed] [Google Scholar]
- Cook R, Bird G, Lünser G, Huck S, Heyes C. Automatic imitation in a strategic context: players of rock-paper-scissors imitate opponents' gestures. Proc Biol Sci 279: 780–786, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCharms RC, Christoff K, Glover GH, Pauly JM, Whitfield S, Gabrieli JD. Learned regulation of spatially localized brain activation using real-time fMRI. Neuroimage 21: 436–443, 2004. [DOI] [PubMed] [Google Scholar]
- Decloe R, Obhi SS. Motor cortical processing is causally involved in object recognition. BMC Neurosci 14: 155, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol 73: 2608–2611, 1995. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage 35: 1674–1684, 2007. [DOI] [PubMed] [Google Scholar]
- Heyes C. Automatic imitation. Psychol Bull 137: 463–483, 2011. [DOI] [PubMed] [Google Scholar]
- Hogeveen J, Obhi SS. Social interaction enhances motor resonance for observed human actions. J Neurosci 32: 5984–5989, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J, Obhi SS. Automatic imitation is automatic, but less so for narcissists. Exp Brain Res 224: 613–621, 2013. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol 3: e79, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman WN. EEG feedback training: enhancement of somatosensory cortical activity. Electroencephalogr Clin Neurophysiol 45: 290–294, 1978. [DOI] [PubMed] [Google Scholar]
- Longo MR, Bertenthal BI. Attention modulates the specificity of automatic imitation to human actors. Exp Brain Res 192: 739–744, 2009. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. Mu rhythm modulation during observation of an object-directed grasp. Brain Res Cogn Brain Res 19: 195–201, 2004. [DOI] [PubMed] [Google Scholar]
- Neuper C, Scherer R, Reiner M, Pfurtscheller G. Imagery of motor actions: differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG. Brain Res Cogn Brain Res 25: 668–677, 2005. [DOI] [PubMed] [Google Scholar]
- Obhi SS, Hogeveen J. The controlled imitation task: a new paradigm for studying self-other control. PeerJ 1: e161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Higashi T, Sugawara K, Ogahara K, Funase K, Kasai T. Excitability changes in the human primary motor cortex during observation with motor imagery of chopstick use. J Phys Ther Sci 23: 703–706, 2011. [Google Scholar]
- Roosink M, Zijdewind I. Corticospinal excitability during observation and imagery of simple and complex hand tasks: implications for motor rehabilitation. Behav Brain Res 213: 35–41, 2010. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex 9: 161–167, 1999. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Muraoka T, Mizuguchi N, Kanosue K. Combining observation and imagery of an action enhances human corticospinal excitability. Neurosci Res 65: 23–27, 2009. [DOI] [PubMed] [Google Scholar]
- Sartori L, Bucchioni G, Castiello U. When emulation becomes reciprocity. Social Cogn Affect Neurosci 8: 662–669, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh H, McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG)-based communication: EEG control vs. system performance in humans. Neurosci Lett 345: 89–92, 2003. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Steyvers M, Levin O, Swinnen SP. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res 168: 157–164, 2006. [DOI] [PubMed] [Google Scholar]
- Tsukazaki I, Uehara K, Morishita T, Ninomiya M, Funase K. Effect of observation combined with motor imagery of a skilled hand-motor task on motor cortical excitability: difference between novice and expert. Neurosci Lett 518: 96–100, 2012. [DOI] [PubMed] [Google Scholar]
- Tzelgov J, Henik A, Berger J. Controlling stroop effects by manipulating expectations for color words. Mem Cognit 20: 727–735, 1992. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Jolesz FA. Functional MRI for neurofeedback: feasibility study on a hand motor task. Neuroreport 13: 1377–1381, 2002. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Fairneny T, Chen NK, Choo SE, Panych LP, Park H, Lee SY, Jolesz FA. Brain-computer interface using fMRI: spatial navigation by thoughts. Neuroreport 15: 1591–1595, 2004. [DOI] [PubMed] [Google Scholar]
- Wright DJ, Williams J, Holmes PS. Combined action observation and imagery facilitates corticospinal excitability. Front Hum Neurosci 8: 951, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


