Abstract
Many physiologic processes during the early stages of mammalian ontogeny, particularly placental and vascular development, take place in the low oxygen environment of the uterus. Organogenesis is affected by hypoxia inducible factor (HIF) transcription factors that are sensors of hypoxia. In response to hypoxia, HIFs activate downstream target genes – growth and metabolism factors. During hematopoietic system ontogeny, blood cells and hematopoietic progenitor/stem cells are respectively generated from mesodermal precursors, hemangioblasts, and from a specialized subset of endothelial cells that are hemogenic. Since HIFs are known to play a central role in vascular development, and hematopoietic system development occurs in parallel to that of the vascular system, several studies have examined the role of HIFs in hematopoietic development. The response to hypoxia has been examined in early and mid-gestation mouse embryos through genetic deletion of HIF subunits. We review here the data showing that hematopoietic tissues of the embryo are hypoxic and express HIFs and HIF downstream targets, and that HIFs regulate the development and function of hematopoietic progenitor/stem cells.
Keywords: HIF1α, Hypoxia, Hematopoietic stem cells, Aorta, Mouse development
Introduction
The level of oxygen (O2) is a crucial modulator of cell activity and tissue function. The specific oxygen levels within normal mammalian tissues are significantly lower than ambient oxygen levels (21%). Most adult tissues thrive at 2–9% O2 levels (physiological hypoxia). However, some tissues such as the bone marrow (BM) and thymus experience even lower oxygen levels (<1%). Hypoxia is a key regulator of major biological processes such as embryogenesis and stem cell function [1,2]. The term hypoxia in this review refers to physiological hypoxia which is different from pathological hypoxia that is caused by conditions such as high altitude, tissue ischemia, and solid tumors [3,4]. Cells have the molecular machinery for sensing and responding to hypoxia in physiological and pathological conditions. As important background to understand the role of the hypoxic response in the development of the hematopoietic system, this review will first describe the molecular sensing HIFs and the role of the hypoxia response in adult hematopoiesis. Thereafter, the focus is on how HIFs affect hematopoiesis in the embryo.
Molecular basis of oxygen sensing
The heterodimeric transcription factor hypoxia-inducible factor (HIF) is the major regulator of molecular response to hypoxia. HIFs interact via Per–Arnt–Sim (PAS) domains, bind to DNA via N-terminal basic helix–loop–helix (bHLH) domains, and activate transcription with C-terminal transcriptional transactivation domains (TADs). The HIF complex consistsoftwo proteins:anoxygen-sensitive HIFα subunit and an oxygen-insensitive HIFβ subunit (aryl hydrocarbon receptor nuclear translocator (Arnt)). Arnt is expressed constitutively, but the expression and activity of HIFα subunits are regulated by cellular oxygen concentration [1]. Three HIFα subunits have been identified: HIF1α, HIF2α, and HIF3α. Both HIF1α and HIF2α are expressed in a variety of cell types. Oxygen-dependent regulation of HIF activity is mediated by post-translational modification of the oxygen-dependent degradation domain (ODD) in the α subunit. In the absence of hypoxia, prolyl hydroxylase domain proteins (PHD1–3) hydroxylate the two proline residues in the ODD of HIFα (in a reaction requiring oxygen) and enable binding of the von Hippel–Lindau (VHL) tumor suppressor protein, which is the recognition part of an E3 ubiquitin ligase complex. This leads to ubiquitylation and proteasomal degradation of HIFα [5–8]. Moreover, the factor inhibiting HIF (Fih) hydroxylates an aspargine residue in the TAD of HIFα, preventing the binding of the transcriptional coactivator CBP/p300 [9,10]. In hypoxic conditions, HIFα is stabilized, accumulated, and dimerized to HIFβ, followed by translocation to the nucleus and binding to the hypoxia responsive element (HRE) sequence in the promoter region of hypoxia-target genes.
Biological and medical significance throughout development
The high cellular proliferation and oxygen consumption of the rapidly growing embryo lead to physiological hypoxia which is essential for embryo development [1]. HIF expression begins at early stages of embryonic development and plays fundamental roles in tissue formation. In addition, HIF transcription factors regulate the function of stem cells. Hypoxia has been shown to influence the fate of placental trophoblast stem cells [11], to affect the behavior (survival, proliferation, differentiation) of mesenchymal stem cells [12], and to maintain pluripotency of embryonic stem (ES) cells [13]. The activities of important regulators of stem cell function such as Notch, Wnt, and OCT4 are influenced by hypoxia [2].
In pathological conditions, hypoxia and activation of HIFs contribute to aspects of tumor progression including increased genetic instability, cell immortalization, vascularization, glucose metabolism, invasion, and metastasis [14,15]. Hypoxic tumors are aggressive and resistant to therapy [16] and increased levels of HIF1α or HIF2α in solid tumors are associated with poor prognosis in breast, colon, and lung cancers. Cancer stem cells of lymphomas and acute myeloid leukemia (AML) show increased HIF1α activity under normoxia [17]. HIF1α shRNA and HIF inhibitors abolish the CFU activity of such cells. In contrast to other chemotherapeutic drugs, the HIF inhibitor echinomycin, selectively removed cancer stem cells in lymphoma and did not affect normal cells. Also, in a human AML xenotransplantation model, short-term treatment by HIF inhibitor prevented serial transplantation of AML [17]. In another study, HIF1α was shown to be essential for the development of chronic myeloid leukemia (CML) and that HIF1α is required in survival maintenance of leukemia stem cells in CML in a transduced Vav-Cre:HIF1αfl/fl mouse model [18]. Hence, HIF1α plays an important role in regulating cancer stem cells in hematological malignancies. These observations have led researchers to study the HIF inhibitors as therapeutic agents in cancer biology. Altogether, it is evident that hypoxia and its regulatory machinery have crucial physiological and pathological roles, making hypoxia a factor of great interest in fundamental research as well as medical/therapeutic studies.
Hypoxic response in the adult hematopoietic system
In adults, hematopoietic stem cells (HSCs) are maintained in hypoxic niches. The bone marrow (BM) niche is a complex microenvironment composed of different kinds of cells. Among them, endothelial cells and osteoblasts have been demonstrated to regulate hematopoietic stem cell (HSC) function. The balance between the quiescent and proliferative states of HSCs is tightly regulated by intrinsic and extrinsic factors of the surrounding niche. At any time, the majority of long-term repopulating (LTR) HSCs are quiescent (G0), with only a few entering the DNA synthesis and proliferation (S/G2/M) phase [19,20]. Quiescence is a hallmark characteristic of LTR-HSCs and is thought to protect HSCs from DNA damage. The role of the hypoxic response in regulating the quiescence of HSCs in their niche is of great importance, and perhaps an essential remnant characteristic of the hypoxic environment of the embryo in which they were generated.
Oxygen gradients in the HSC supportive BM niche
Several studies suggest that LTR-HSCs are located mainly in the BM endosteal zones [21,22]. Here, the sinusoidal endothelium allows hematopoietic cells to readily pass through the vasculature [23]. The perfusion rate of BM cells in the endosteal zone is limited and the oxygen level is low. It has been suggested that HSCs are located in hypoxic zones where they are maintained in a quiescent state to avoid their exhaustion and differentiation and retain long-term repopulating activity [24–26]. Parmer and colleagues applied Hoechst 33342 staining of BM cells to isolate different hematopoietic subpopulations according to the extent of dye perfusion. HSCs (as shown by in vivo transplantation analyses) are enriched in the lowest dye uptake fraction, i.e. the most hypoxic compartment of BM [26]. Similarly, Takubo et al. performed flow cytometric analysis for different subpopulations of BM mononuclear cells (MNCs) based on the intracellular incorporation of hypoxic marker, Pimonidazole (Pimo). The Pimo positive fraction (30% of BM MNCs) was enriched for LTR-HSCs (68.2%) and quiescent HSCs (Tie2 LSK cells) [27]. In another study, HSCs sorted based on intracellular ROS level showed that ROSlow HSCs had higher self-renewal activity as compared to ROShigh cells. Moreover, ROShigh HSCs were exhausted in serial transplantations [24]. The metabolic properties of HSCs support their adaptation and survival in hypoxic niche. When BM cells were analyzed based on mitochondrial activity, more than 80% of LTR-HSCs were found in the low mitochondrial potential (MP) fraction [28]. These cells express high level of HIF1α, have lower oxygen consumption and ATP content, and rely on glycolysis rather than oxidative respiration to meet their energy demands. Hence, HSCs are primed and adapted to the hypoxic environment.
HIF1α and BM HSC regulation
Since germline deletion of HIF genes affects embryonic growth and development, inducible conditional knock-out (cKO) mice have been a useful resource to investigate the role of HIFs in adult BM HSCs. MX-Cre:HIF1αfl/fl BM HSCs were found to be defective in self-renewal ability. Transplantation recipients of MX-Cre:HIF1αfl/fl BM Lin −Sca1+cKit+ (LSK) cells showed significantly higher peripheral blood cell donor chimerism, as compared to recipients of wild-type (WT) cells [27]. However, fewer donor-derived LSK cells were found in the BM of the cKO recipients at 4 months post-transplantation. Secondary transplantation of the BM LSK cells showed failure of long-term reconstitution ability of cKO HSCs, suggesting that HSC self-renewal was affected [27]. This was supported by data showing increased expression of Ink4a (marker of senescent stem cells) in MX-Cre:HIF1αfl/fl LSK cells of primary recipients. Cell cycle analysis of cKO BM revealed a decrease in the fraction of CD34− LSK cells in G0 phase and an increase of this fraction in G1 phase. Moreover, increased production of ROS was observed in cKO LTR-HSCs. These findings were verified in MX-Cre:VHLfl/fl mice in which HIFα proteins are stabilized in the nucleus [27]. An accumulation of CD34− LSK cells and a significant reduction in Ki67 positive LSK cells were detected in MX-Cre:VHLfl/fl animals. Moreover, LTR HSC content was considerably decreased in the BM of MX-Cre:VHLfl/fl mice. Thus, stabilization of HIFα leads to improved maintenance of HSCs in stress conditions. Interestingly, a recent report on the conditional deletion of HIF2α demonstrates that this HIFα family member does not play a role in the maintenance of HSCs, steady-state hematopoiesis, HSC self-renewal or recovery following hematopoietic injury [29]. Taken together, it appears that HIF1α (and not HIF2α) is a regulator of HSCs, acting to prevent HSCs from over-proliferation and exhaustion in the stress conditions of serial transplantation.
The downstream targets of HIF1α that regulate HSCs are as yet unclear. However, two targets of particular note in adult BM HSCs are vascular endothelial growth factor (VEGF), a well-known angiogenic factor and Cripto/GRP78, a ligand/receptor signaling axis. VEGF has been shown to play a role in the maintenance of HSCs [30], and particularly under hypoxic conditions by deletion of the HRE in the promoter of the VegfA gene in a mouse model. Transplantation experiments showed that the donor-derived chimerism was notably decreased in the recipients of such KO BM cells [31]. Both Cripto and GRP78 are regulated by hypoxia and HIF1α and are highly expressed in LTR-HSCs. GRP78R+ LT-HSCs are located in endosteal region and are quiescent, with high glycolytic activity. HIF1α-deficient mice showed fewer GRP78+ HSCs and less Cripto expression by endosteal cells [32]. Further studies are required to define other HIF targets by which HSC behavior is regulated.
Development of the hematopoietic system in the (hypoxic) embryo
The hematopoietic system is rooted in HSCs, the self-renewing cells of the adult hematopoietic cell differentiation hierarchy. Adult BM HSCs originate during embryonic life within a short window of developmental time [33]. As described earlier, the mammalian embryo develops in the relatively hypoxic microenvironment of the uterus. As such, it is interesting to explore the hypoxic response during hematopoietic system development. The hematopoietic system is independently generated in the embryo in three waves (Fig. 1). The first wave of hematopoietic generation occurs in the yolk sac (YS) at mouse embryonic day (E) 7.5. Short-lived primitive erythrocytes are generated and provide oxygen for the rapidly growing embryo. Primitive macrophages and megakaryocytes are also generated [34–37]. The temporal and spatial association of both endothelial and hematopoietic cells in the YS of chick embryos led to the proposition of a common precursor for these two lineages called “hemangioblasts” [38,39]. Hemangioblasts are formed in the primitive streak, migrate to the YS, and differentiate into endothelial, hematopoietic, and vascular smooth muscle cells [40,41]. The second wave of hematopoiesis begins at E8–8.5 when erythro-myeloid progenitors (EMPs) and definitive hematopoietic progenitors are found in the YS, chorion-allantois (which forms the placenta and umbilical cord later) and para-aortic splanchnopleura (pSp) [36,42,43]. Explant cultures of YS and pSp before establishment of circulation indicate that these tissues independently generate hematopoietic progenitors [44]. Moreover, transplantation assays into neonatal recipients with the E9 yolk sac cells from Ncx1−/− mice (which lack the heart beat) showed that the YS de novo generates hematopoietic progenitors with B and T lymphoid potentials [45,46]. Next at E10, spleen colony forming unit (CFU-S) progenitors appear in the YS and intra-embryonic aorta-gonad-mesonephros (AGM) region [47]. The third and most important hematopoietic wave during mammalian embryo development is the generation of the definitive HSCs. The first transplantable HSCs are detected and generated in the AGM at E10.5 [48,49] (Fig. 2A). In addition, HSCs are found in vitelline and umbilical arteries at this stage [50]. Con-focal live-imaging of the dorsal aorta demonstrated the emergence of hematopoietic cells from endothelial cells lining the ventral part of dorsa aorta called “hemogenic endothelium” [51]. Later at E11–11.5, HSCs are detected in the YS, placenta, and fetal liver (FL) [52–54]. Thereafter, FL remains the main tissue for colonization and expansion of HSCs. The spleen starts to be colonized at E12.5, harboring mostly multipotent hematopoietic cells [55,56]. Hematopoietic cells colonize the thymus at E12–13 [56]. At E17, HSCs start to migrate to the BM, the main hematopoietic tissue for the entirety of adult life [57].
Fig. 1.
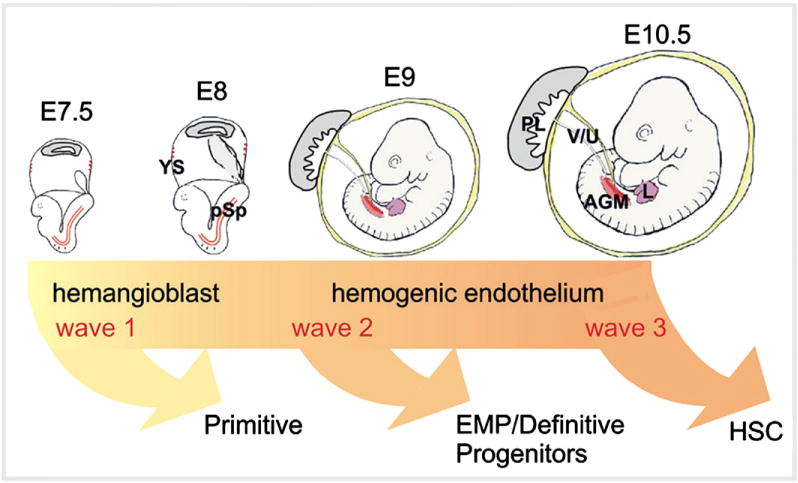
Three independent waves of hematopoietic generation in the developing mouse embryo. Three waves of embryonic hematopoiesis are depicted along a developmental time line. Embryonic day (E) 7.5 to 10.5 mouse embryos are shown. Arrows indicate the onset of hematopoietic cell generation (but do not necessarily indicate the later time point when this generation ceases). The first wave is thought to be derived from hemangioblasts, whereas waves 2 and 3 are derived from hemogenic endothelial cells. The most potent adult-repopulating HSCs are generated at E10.5 inthe AGM. YS = yolk sac; pSp = para-aortic splanchnopleura; AGM = aorta–gonad–mesonephros; V/U = vitelline and umbilical arteries; PL = placenta; EMP = erythro-myeloid progenitors; HSC = hematopoietic stem cell.
Fig. 2.
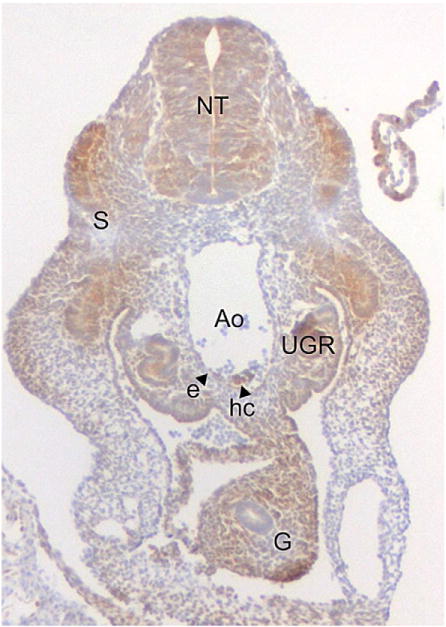
Cellular hypoxia in the E10 mouse embryo. A transverse section through 33 somite pair embryo stained for the hypoxia indicator Pimonidazole (hypoxyprobe) is shown. Hypoxic cells (brown) are found in the neural tube (NT), gut (G), somites (S), aorta (Ao) and urogenital ridges (UGR). Arrow heads indicate hypoxic aortic endothelial cells (e) and hematopoietic cluster cells (hc).
Hypoxia and the expression of hypoxic response molecules in the mouse embryo
Normal embryonic development occurs in a hypoxic environment with oxygen levels ranging from 1 to 5% in the uterus [58]. HIFs are responsible for many aspects of development including placentation, cardiovascular development, and bone formation. To examine what parts of the embryo are hypoxic, Lee and colleagues performed hypoxyprobe immunohistochemistry on mid- and late-gestational stage embryos [59]. At E8.5–9, the hypoxic regions were detected in folding neural tube and neural mesenchyme cells, allantois, and ectoplacental cone and decidua. At E9.5–11.5, the hypoxic regions spread into neural tube and mesenchymal regions of the head. Regarding hematopoietic generation at E10, we have found the AGM (Fig. 2), liver and placenta [60] to be hypoxic. Importantly, aortic hematopoietic cluster cells and some aortic endothelial cells are positive for hypoxyprobe (Fig. 2), suggesting that the emergence of HSCs from the vascular endothelium may require signals from the hypoxic response for HSC generation and/or maintenance.
Also the E10.5 neural tube, somites, gut and lateral mesenchyme were hypoxic. At E14.5 hypoxic sites were detectable in the developing heart, gut, and skeleton [61] and at E16.5, in the olfactory lobe, connective tissues of craniofacial region, and cerebral cortex, liver, kidney, heart, and gastrointestinal tract were partially hypoxic [59]. These data indicate that despite an active circulatory system, the cells of the embryo can still experience hypoxia. Interestingly, HIF1α expression is very high in E8.5 embryos and increases significantly from E9.5 to E18 [62]. Also, the pattern of expression of Vegf was found to colocalize with hypoxic regions in brain, trunk, heart, and intersomitic vessels. Thus, as growth and energy demands of the embryo are great, it is likely that hypoxia stabilizes HIFα, and it activates Vegf expression and henceforth vascular growth to supply blood and nutrients to developing tissue.
Mouse and ES cell germline deletion models examining the hypoxic response in hematopoietic development
Genetic ablation in the mouse model has been used to examine the role of HIFs. A variety of germline HIF-deficient mice have been generated and studies show broad effects in many tissues including the hematopoietic system during early development [1,2]. Some of these studies and effects are summarized in Table 1.
Table 1.
Defects in mouse embryos lacking HIF transcription factors.
| KO mouse model/gene | Lethal by | Phenotypes
|
Reference | |
|---|---|---|---|---|
| In vitro hypoxic cultures | In vivo | |||
| Germline/HIFβ−/− | E10.5 | – No up-regulation of glycolytic enzyme genes in ES cells | – Developmental delay | Maltepe et al., 1997 |
| – No up-regulation of hypoglycemia genes in ES cells | – Defect in YS angiogenesis | |||
| – Lower VEGF level in YS and embryo proper | ||||
| Germline/HIFβ−/− | E9.5–10.5 | – Impaired neural development | [78] | |
| – Small fetal placenta and chorioallantoic plate | ||||
| – Defect in PL vascularization | ||||
| – Fetal placenta labyrinth cavities | ||||
| Germline/HIFβ−/− | Not mentioned | – Abnormal vessel organization | [64] | |
| – Less CD31+ cells throughout the embryo | ||||
| – Defect in chorio–allantoic fusion | ||||
| – Less VEGF expression in embryo | ||||
| – Failure of neural tube closure | ||||
| – Increased number of apoptotic cells in embryo | ||||
| Germline/HIF1α−/− | E11 | – Down-regulation of glycolytic enzyme genes in ES cells | – Developmental delay | [62] |
| – No up-regulation of Vegf | – Pericardial effusion | |||
| – Impaired ES cell proliferation | – Failure of neural tube closure | |||
| – Extensive mesenchymal cell death | ||||
| – Abnormal vascular structure in cephalic region | ||||
| Germline/HIF1α−/− | Not mentioned | – No up-regulation of glycolytic genes and Vegf in ES cells | – Increased level of hypoxia in embryo | Ryan et al., 1998 |
| – Increased number of apoptotic cells in embryo | ||||
| – Defective YS vascularization | ||||
| – Defective cephalic vascularization | ||||
| Conditional/HIF1αfl: Tie2Cre | Not lethal | – Less proliferation of endothelial cells | – Reduction in vessel density | Tang et al., 2004 |
| – Defect in elongation of endothelial cells | – Delayed wound healing | |||
| – Less VEGF level in culture medium | – Smaller, less necrotic, less vascularized tumor | |||
| – No induction of VEGF receptors | ||||
| Germline/HIF2α−/− | E9.5–E12.5 | – Vascular defect in YS and embryo proper | Feng et al., 2011 | |
| Germline/HIF2α−/− | E12.5–E16.5 | – Bradycardia | Tian et al., 1998 | |
| – Reduced level of catecholamine | ||||
| Germline/HIF2α−/− | E13.5 | – Cardiac failure | Compernolle et al., 2002 | |
| Neonatal stage | – Impaired lung maturation | |||
HIF1β/Arnt
One of the first HIF genes to be deleted in mouse ES cells was HIF1β (Arnt). In hematopoietic differentiation cultures, Arnt−/− ES cells were found to be significantly reduced in hematopoietic progenitor activity and no hypoxic induction of progenitor activity was observed [63]. Similarly, YS of Arnt−/− embryos showed considerably fewer hematopoietic progenitors at both E8.5 and E9.5 than WT embryos. Vitelline vessels lacked blood suggesting a lack in erythro/hematopoiesis. Molecular analyses showed that Arnt−/− EB cells express less Vegf and Epo, which are targets of HIF1α. Since the hematopoietic progenitor activity of Arnt−/− EB cells could be rescued with addition of VEGF, it was concluded that hypoxia-induced VEGF production is responsible for the survival, and expansion of hematopoietic progenitors.
Another study showed that the vessels in E9.5 Arnt−/− embryos are disorganized, especially in the pSp/AGM region where the first definitive hematopoietic cells arise [64]. The vessels express lower levels of PECAM (CD31) and fewer CD34+ cells were found in the dorsal aorta and throughout the embryo, suggesting a role for hypoxic response in vessel formation. To avoid the complications of a general developmental delay, the pSp region from E9.5 embryos was cultured ex vivo. Arnt−/− pSp explants, in contrast to WT, formed few or no vascular beds, a much lower frequency of CD45+ hematopoietic cells and decreased CFU-C activity, demonstrating that the defect extends into the hematopoietic system. Surprisingly, the addition of WT Sca-1+ BM cells to Arnt−/− pSp cultures rescued the vessel and hematopoietic defect, despite the fact that WT Sca-1+ cells showed no contribution into vessel development. Importantly, VEGF treatment could partially rescue the vessel and hematopoietic defect in Arnt−/− embryos [64]. These data indicate that HIF activity is essential for the early development of the hematovascular system through mechanisms that include the production of VEGF and perhaps other growth factors.
HIF2α
Like the germline deletion of Arnt, HIF2α−/− embryos suffer from developmental delays, many tissue defects and lethality before birth. In some rare cases HIF2α−/− animals survived to adulthood, and hypocellularity was observed in the BM and the number of red and white blood cells was decreased [65]. In vivo transplantation assay showed that there was no difference in the repopulating activity of WT and HIF2α−/− BM cells. This was confirmed recently in transplantations with conditional (Vav-Cre) HIF2α deleted BM [29]. However, in reverse transplantations (transplantation of WT and cKO donor BM into HIF2α−/− recipients), both WT and HIF2α−/− BM cells resulted in differences in hematocrit values and peripheral blood cell counts, suggesting that the lack of HIF2α− affects the BM niche. HIF2α is expressed in the BM (stroma, vascular, and bone lining cells) of adult mice. Whereas some small changes in vascular cell adhesion molecule (VCAM) expression and vascular endothelial cadherin (VECAD) expression were observed, expression of VEGF and its receptors was not altered, suggesting that HIF2α is not involved in the regulation of Vegf in BM. Hence, HIF2α appears to play onlyan indirect roleinhematopoiesis through small changes in the microenvironment [65].
HIF1α
Specific examination of erythroid lineage development in E9.5 HIF1α−/− YS was performed by Yoon and colleagues [66]. Erythropoiesis in the YS is normally induced through EPO/EPOR and VEGF/VEGFR signaling pathways. A decrease in BFU-E colonies was detected and the ery-throid cells were not fully hemoglobinized. Epo, EpoR and VegfR1 mRNA levels were found to be significantly decreased in HIF1α−/− embryos and YS, as compared to WTs. Furthermore, the transferrin receptor (Tfr) protein levels (provide the iron required for YS erythropoiesis) were lower in KO embryos and YS. The authors concluded that HIF1α is not necessary for the formation of erythroid hematopoietic progenitors, but has a role in their expansion and differentiation [66].
Mouse conditional deletion model to study the role of HIF1α in embryonic hematopoiesis
The ubiquitous function of HIF transcription factors and the complication of multi-tissue developmental deficiencies in germline KO mice preclude the examination of the precise role of HIFs specifically in the development of the hematopoietic compartment. To overcome this difficulty, a conditional gene deletion approach using Cre/loxp strategy has been used to specifically remove HIF1α from vascular endothelial-cadherin (VEC) expressing endothelial cells, the immediate cell precursors to definitive hematopoietic progenitors and HSCs [60]. Despite no visible abnormalities, E10 VEC-Cre:HIF1αfl/fl embryos exhibited significant decreases in hematopoietic progenitor activity in the AGM and placenta (3 and 2.1 fold, respectively). This decrease was significant for CFU-MIX colonies (4.8 and 5.1 fold, respectively), the most immature of hematopoietic progenitor cells. At E11, progenitor decreases in VEC-Cre:HIF1αfl/fl embryos were also observed in the FL (1.6 fold). These decreases, along with high recombination efficiency in single CFU-Cs of all hematopoietic tissues suggest that HIF1α deficiency affects the generation and/or expansion of hematopoietic progenitors and not their activity. The phenotypic HP/SCs, which are enriched in the cKit+ [67,68] and CD41+ fractions [69], were significantly decreased in AGM (4.7 and 1.4 fold, respectively) and placenta (3.6 and 3.4 fold, respectively) of E10 cKO embryos. Moreover, confocal whole-mount imaging of E10 VEC-Cre:HIF1αfl/fl embryos showed a significant decrease in the number of cKit+ cells and hematopoietic clusters (1.4 and 1.6 fold) compared to their WT counterparts. These decreases in HP/SC generation were confirmed in in vivo transplantations of HIF1α-deficient AGM (and placenta) cells. AGM recipients showed only very low (1.2–7.4%) or no chimerism compared to 90–95% obtained from WT cells. Similar results were observed for the placenta. These results are summarized in Fig. 3. Altogether, either HP/SC formation from HIF1α-deficient hemogenic endothelium is impaired and/or the HIF1α-deficient HP/SCs fail to expand normally after emergence.
Fig.3.
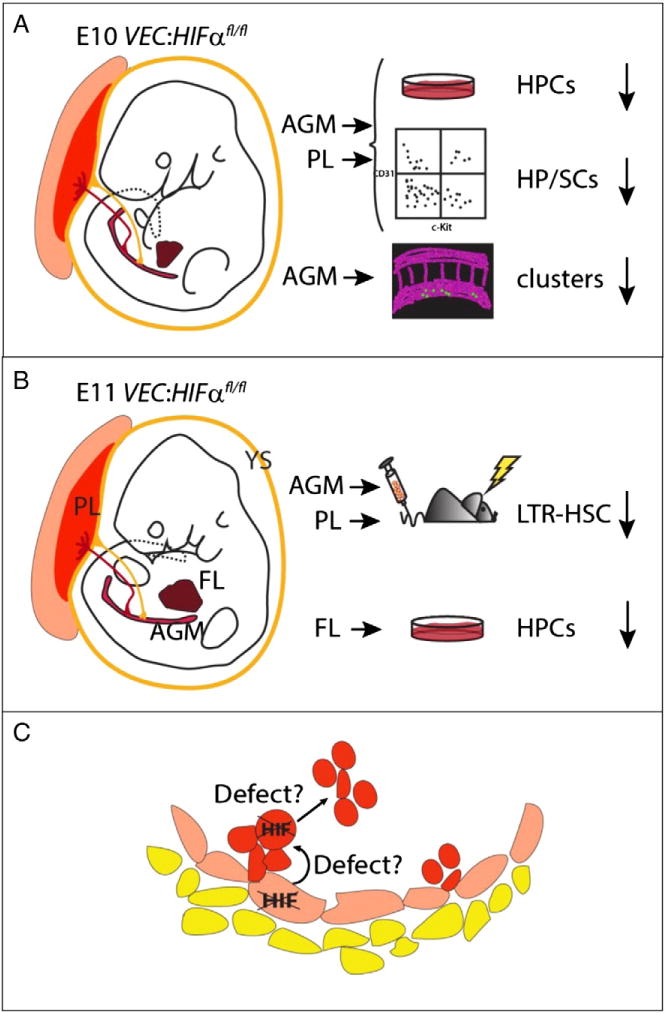
Hematopoiesis defects in VEC-Cre/+:HIF1αfl/fl embryos.A) E10 and B) E11 mouse VEC-Cre/+:HIF1αfl/fl embryos are depicted at the time when the first hematopoietic progenitor and stem cells (HPSCs) are generated in the aorta. Tissues where hematopoietic cells appear in the embryo are shown: The extraembryonic yolk sac (YS) and placenta (PL), the intraembryonic aorta–gonad–mesonephros (AGM) and fetal liver (FL). The results of CFU-C progenitor (HPC), phenotypic (FACS) analysis for HP/SC, 3-D imaging for aortic hematopoietic clusters and long-term in vivo reconstitution assays are shown. C) Schematic close-up of the ventral wall of the aorta showing hematopoietic cluster emergence from hemogenic endothelium in VEC-Cre/+:HIF1αfl/fl embryos. Defects in HPSCs detected by different hematopoietic assays are proposed in the hemogenic endothelium and/or in hematopoietic cell emergence/expansion/maintenance [60].
Impact of oxygen level and HIFs on placental development
Derived from extraembryonic cells of the conceptus, the placenta forms at E8/8.5 in the mouse embryo and is a highly vascularized tissue that harbors a large pool of hematopoietic progenitors and HSCs [52,54,70,71]. The mature placenta consists of three different fetal layers [1]: Proximal to the embryo is the labyrinth, where the fetal vasculature and maternal blood sinuses are brought into proximity to allow the nutrient and oxygen exchange. Next to the labyrinth is the spongiotrophoblast layer that provides structural support for the developing labyrinth layer and is a source of trophoblast cells. The trophoblast giant cell (TGC) layer borders the decidua. These cells facilitate the embryo implantation and decidua invasion, and produce the pregnancy hormones.
The placenta develops in a hypoxic environment and its development is under the influence of oxygen levels. Between E6.5 and E14.5 in the mouse embryo, hypoxic cells and HIF expressing cells are detected in decidua and placenta [72–74]. We have found hypoxic cells in some endothelial cells in the fetal part of the E10 placentas [60]. Germline deletion of HIF1α, HIF2α, and HIFβ in mouse leads to failure in placenta formation and embryo lethality [11,75–78]. Defects in HIF1α−/− or HIF2α−/− placenta were less severe compared to double HIFα-deleted and Arnt−/− placentas. These results show that hypoxia regulates placenta development both through HIF1α/Arnt and HIF2α/Arnt [76].
The main placenta defects are: incomplete labyrinth development, limited vascularization of placenta, failure in chorion–allantois interactions, defect in trophoblast differentiation, and the higher number of TGCs and significant decrease of spongiotrophoblast layer. Also vascularization of placenta depends on HIF activity, through the key angiogenic HIF targets Vegf, Flk1, Ang1 and Tie2 [1]. Germline deletion of HIFs precludes the study of the hematopoietic compartment of the placenta. However, conditional deletion of HIF1α in the endothelial compartment revealed clear defects in HPCs and HSCs when VEC-Cre:HIF1αfl/fl placentas were examined [60]. Thus, in the context of normal placenta development and vascularization, hematopoietic development (emergence or expansion of HP/SCs) is significantly affected. Taken together, hypoxia and the hypoxic response not only control placenta morphogenesis but they also regulate hematopoiesis.
Vegf: a HIF1α target for HSC generation and regulation
The underlying mechanism by which HIF1α regulates HP/SC emergence/expansion in the developing embryo is unclear. Vegf is a downstream target activated by HIFs, and VEGFA has been shown to be important in hematopoiesis. The two major receptors of VEGF, VEGFR1 (Flt1) and VEGFR2 (Flk1 or KDR), are expressed on endothelial as well as hematopoietic cells [79,80]. Complete or haploinsufficient VegfA-deficient animals die early during development because of failure in hematopoietic and vascular development [81,82]. Interestingly, in a conditional KO mouse model, VegfA-deficient HSCs fail to reconstitute the hematopoietic system of irradiated mice despite the presence of VEGFA in the microenvironment, thus demonstrating a cell-intrinsic role for VEGF in HSCs [30]. In addition, VEGF regulates HSC function indirectly via the establishment of the osteoblastic niche which depends on VEGFA availability [83]. The BM of irradiated Flk1-deficient recipients is not successfully reconstituted by transplanted WT HSCs due to the failure in regeneration of the BM sinusoidal vasculature [84].
In the developing mouse embryo, Flk1 has been shown to be expressed on endothelial cells of the vasculature, as well as emerging aortic hematopoietic cluster cells [67,68]. Together with the fact that the deletion of the HRE of VegfA affects HSC repopulating activity [31] and deletion of HIF1α in vascular endothelial cells in the embryo affects emergence of vascular hematopoietic clusters and HS/PC function [60], we suggest that VEGF/FLK1 signaling axis is important already at the earliest stages of HSC development. The VEGF/FLK1 signaling pathway may regulate endothelial-to-hematopoietic transition in the endothelial cells of the major vasculature of the midgestation mouse embryo — the aorta, vitelline and umbilical arteries. Some of the endothelial cells, presumably hemogenic endothelium in these vessels have been shown to be hypoxic [60]. Alternatively or in addition, the production of VEGF by hematopoietic cells themselves (those produced in waves 1 and/or 2 of hematopoietic development; Fig. 1) may play a role in their expansion and function. The influence of the earlier wave of EMPs (wave 2) on HSC development (wave 3) was implied by Cbfb transcription factor rescue experiments [85]. Hence, the regulation of hematopoietic cell development in the hypoxic environment of the embryo is complex, involving many interacting cell types and, thus, highlights the need for further, more specific conditional deletions of HIF genes and downstream HIF target genes. Through a detailed understanding of the direct and indirect actions of hypoxia and the molecular response to hypoxia, we will gain insight into aspects of HSC development that should allow us to manipulate HSCs and/or their niche to facilitate the in vitro growth and expansion of these cells for clinical interventions, and uncover a new arsenal of drugs to treat hematological malignancies.
Acknowledgments
The authors thank all members of the lab, especially Parham Solaimani and Mihaela Crisan for critical discussions, Dorota Kurek for providing the hypoxyprobe transverse section image and Emma de Pater for critical comments on the manuscript. We acknowledge the support from the Landsteiner Society for Blood Research (LSBR 1109), ZonMW (Dutch Medical Research Council) (911-09-036 and 016.126.088), FES NIRM (Dutch Innovation Grant) and NIH (RO37 DK54077).
Footnotes
Conflict of interest
The authors declare no competing financial or personal interests.
References
- 1.Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 7.Ruas JL, Poellinger L. Hypoxia-dependent activation of HIF into a transcriptional regulator. Semin Cell Dev Biol. 2005;16:514–522. doi: 10.1016/j.semcdb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Schofield CJ, Ratcliffe PJ. Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun. 2005;338:617–626. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- 9.Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008;15:642–649. doi: 10.1038/sj.cdd.4402315. [DOI] [PubMed] [Google Scholar]
- 10.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 13.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh MY, Powis G. Passing the baton: the HIF switch. Trends Biochem Sci. 2012;37:364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Liu Y, Malek SN, Zheng P. Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Li H, Xi HS, Li S. HIF1alpha is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595–2607. doi: 10.1182/blood-2011-10-387381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 21.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 23.Draenert K, Draenert Y. The vascular system of bone marrow. Scan Electron Microsc. 1980:113–122. [PubMed] [Google Scholar]
- 24.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun. 2008;366:335–339. doi: 10.1016/j.bbrc.2007.11.086. [DOI] [PubMed] [Google Scholar]
- 26.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guitart AV, Subramani C, Armesilla-Diaz A, Smith G, Sepulveda C, Gezer D, Vukovic M, Dunn K, Pollard P, Holyoake TL, et al. Hif-2alpha is not essential for cell-autonomous hematopoietic stem cell maintenance. Blood. 2013;122:1741–1745. doi: 10.1182/blood-2013-02-484923. [DOI] [PubMed] [Google Scholar]
- 30.Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 31.Rehn M, Olsson A, Reckzeh K, Diffner E, Carmeliet P, Landberg G, Cammenga J. Hypoxic induction of vascular endothelial growth factor regulates murine hematopoietic stem cell function in the low-oxygenic niche. Blood. 2011;118:1534–1543. doi: 10.1182/blood-2011-01-332890. [DOI] [PubMed] [Google Scholar]
- 32.Miharada K, Karlsson G, Rehn M, Rorby E, Siva K, Cammenga J, Karlsson S. Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. 2011;9:330–344. doi: 10.1016/j.stem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 36.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 37.Xu MJ, Matsuoka S, Yang FC, Ebihara Y, Manabe A, Tanaka R, Eguchi M, Asano S, Nakahata T, Tsuji K. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97:2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- 38.Murray P. The development in vitro of the blood of the early chick embryo. Proc R Soc Lond. 1932;111:497–521. [Google Scholar]
- 39.Sabin F. Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Contrib Epidemiol. 1920;9:213–262. [Google Scholar]
- 40.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 41.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH, Yoder MC. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medvinsky AL, Samoylina NL, Muller AM, Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 48.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 49.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity inthe mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 50.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 52.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta–gonad–mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 54.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Delassus S, Cumano A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity. 1996;4:97–106. doi: 10.1016/s1074-7613(00)80302-7. [DOI] [PubMed] [Google Scholar]
- 56.Godin I, Dieterlen-Lievre F, Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci U S A. 1995;92:773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okazaki K, Maltepe E. Oxygen, epigenetics and stem cell fate. Regen Med. 2006;1:71–83. doi: 10.2217/17460751.1.1.71. [DOI] [PubMed] [Google Scholar]
- 59.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic regionby hypoxia markerindeveloping mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 60.Imanirad P, Solaimani-Kartalaei P, Crisan M, Vink C, Yamada-Inagawa T, de Pater E, van der Linden R, Speck N, Dzierzak E. HIF1α is a regulator of hematopoietic progenitor and stem cell development. doi: 10.1016/j.scr.2013.09.006. (In revision) (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ream M, Ray AM, Chandra R, Chikaraishi DM. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;295:R583–R595. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramirez-Bergeron DL, Runge A, Adelman DM, Gohil M, Simon MC. HIF-dependent hematopoietic factors regulate the development of the embryonic vasculature. Dev Cell. 2006;11:81–92. doi: 10.1016/j.devcel.2006.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 66.Yoon D, Pastore YD, Divoky V, Liu E, Mlodnicka AE, Rainey K, Ponka P, Semenza GL, Schumacher A, Prchal JT. Hypoxia-inducible factor-1 deficiency results in dysregulated erythropoiesis signaling and iron homeostasis in mouse development. J Biol Chem. 2006;281:25703–25711. doi: 10.1074/jbc.M602329200. [DOI] [PubMed] [Google Scholar]
- 67.Prager GW, Lackner EM, Krauth MT, Unseld M, Poettler M, Laffer S, Cerny-Reiterer S, Lamm W, Kornek GV, Binder BR, et al. Targeting of VEGF-dependent transendothelial migration of cancer cells by bevacizumab. Mol Oncol. 2010;4:150–160. doi: 10.1016/j.molonc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robin C, Ottersbach K, Boisset JC, Oziemlak A, Dzierzak E. CD41 is developmentally regulated and differentially expressed on mouse hematopoietic stem cells. Blood. 2011;117:5088–5091. doi: 10.1182/blood-2011-01-329516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dieterlen-Lievre F, Corbel C, Salaun J. Allantois and placenta as developmental sources of hematopoietic stem cells. Int J Dev Biol. 2010;54:1079–1087. doi: 10.1387/ijdb.093047fd. [DOI] [PubMed] [Google Scholar]
- 71.Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio–allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- 72.Pringle KG, Kind KL, Thompson JG, Roberts CT. Complex interactions between hypoxia inducible factors, insulin-like growth factor-II and oxygen in early murine trophoblasts. Placenta. 2007;28:1147–1157. doi: 10.1016/j.placenta.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Schaffer L, Vogel J, Breymann C, Gassmann M, Marti HH. Preserved placental oxygenation and development during severe systemic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2006;290:R844–R851. doi: 10.1152/ajpregu.00237.2005. [DOI] [PubMed] [Google Scholar]
- 74.Withington SL, Scott AN, Saunders DN, Lopes Floro K, Preis JI, Michalicek J, Maclean K, Sparrow DB, Barbera JP, Dunwoodie SL. Loss of Cited2 affects tropho-blast formation and vascularization of the mouse placenta. Dev Biol. 2006;294:67–82. doi: 10.1016/j.ydbio.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 75.Abbott BD, Buckalew AR. Placental defects in ARNT-knockout conceptus correlate with localized decreases in VEGF-R2, Ang-1, and Tie-2. Dev Dyn. 2000;219:526–538. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1080>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 76.Cowden Dahl KD, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM, Carmeliet P, Simon MC. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell. 2005;16:1901–1912. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kozak KR, Abbott B, Hankinson O. ARNT-deficient mice and placental differentiation. Dev Biol. 1997;191:297–305. doi: 10.1006/dbio.1997.8758. [DOI] [PubMed] [Google Scholar]
- 79.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kabrun N, Buhring HJ, Choi K, Ullrich A, Risau W, Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- 81.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 82.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 83.Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


