Abstract
Gastrointestinal endoscopy is effective and safe for the screening, diagnosis, and treatment of gastrointestinal disease. However, issues regarding endoscope-transmitted infections are emerging. Many countries have established and continuously revise guidelines for endoscope reprocessing in order to prevent infections. While there are common processes used in endoscope reprocessing, differences exist among these guidelines. It is important that the reprocessing of gastrointestinal endoscopes be carried out in accordance with the recommendations for each step of the process.
Keywords: Endoscopy, Reprocessing, Guideline
INTRODUCTION
Endoscopy is used worldwide for the screening, diagnosis, and treatment of gastrointestinal (GI) diseases and enables early detection and treatment of malignant GI diseases. Despite its efficacy, there are several reports that raise concerns about infections that are transmitted via endoscopy.1,2,3 Consequently, several countries have revised evidence-based guidelines on endoscope reprocessing in order to reduce the number of infections and improve safety. In March 2015, the Korean Medical Association released its third revision of guidelines for endoscope reprocessing. Herein, we discuss reprocessing protocols for GI endoscopes including recently revised guidelines from medical associations around the world including the Multisociety guidelines, European Society of Gastrointestinal Endoscopy (ESGE) guidelines, European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) guidelines, British Society of Gastroenterology (BSG) guidelines, and World Gastroenterology Organization (WGO) guidelines.
COMMON REPROCESSING PROTOCOL FOR GI ENDOSCOPES
There are seven steps involved in the reprocessing of GI endoscopes: precleaning, cleaning, rinsing, disinfection, rinsing, drying, and storage. These steps are similar across various guidelines.4,5,6,7,8
(1) Precleaning is the first step of reprocessing, and should be conducted at the bedside immediately after completing the endoscopic procedure. Precleaning involves the removal of visible debris by wiping the exterior of the endoscope with an appropriate detergent solution. Air and solution should be passed through the biopsy channel repeatedly. After precleaning, endoscopes need to be transported to another room for the disinfection process. If the room is not adjacent, the container for the endoscopes should be closed.
(2) Cleaning is the next step. Before cleaning the endoscopes, a leak test should be performed, and all detachable parts should be separated. Clean the external surface of the endoscope with detergent using soft cloths, sponges, or brushes. Flush and brush all accessible channels to remove debris and other contaminants. Hard-to-clean areas or accessories should be cleaned with an ultrasonic cleaner. After cleaning, rinse with clean water to remove the detergent on the endoscopes.
(3) Rinsing includes flushing the endoscope, channels, and all accessories with sterile water.
(4) High-level disinfection is recommended after the cleaning and rinsing processes. The endoscope and its components should be completely immersed in a high-level disinfectant solution, ensuring that all channels are well perfused. Selection of high-level disinfectants should be based on US Food and Drug Administration (FDA), Conformité Européene, or Korean Food and Drug Administration approval. The appropriate exposure time and temperature for high-level disinfection are specific to each disinfectant and its concentration. An automated endoscope reprocessor can be used for high-level disinfection.
(5) After disinfection, the endoscope, channels, and all accessories receive a final rinse with sterile water.
(6) The endoscopic channels are dried by flushing with forced air and ethyl or isopropyl alcohol (70% to 90%).
(7) Storage requires keeping the endoscopes in a safe and sterile environment, usually hanging the endoscopes in a vertical position without touching the floor.
KOREAN GUIDELINES FOR ENDOSCOPE REPROCESSING
In 1995, the Korean Society of Gastrointestinal Endoscopy established the first endoscope reprocessing guideline. Since then, the guidelines have been revised in August 2009, August 2012, and March 2015. The most recent 3rd revision included the criteria to assess reprocessing for not only the certification of individual medical facilities but also the quality assessment of national cancer endoscopic screening. The overall process of endoscope reprocessing is the same as the common reprocessing protocol.
The main change in the recently updated guidelines addressed the issue of reprocessing endoscopic accessories. It is no longer acceptable to reuse single-use (disposable) forceps or needles after sterilization. However, thus far, the Korean National Health Insurance Service has not provided appropriate reimbursement for single usage of forceps. Therefore, owing to practical limitations, many clinicians have needed to use single-use forceps. As stated above, the Korean Society of Gastrointestinal endoscopy updated and clarified the recommendation regarding endoscopic accessories in their third revision. Thanks to their efforts, the reasonable cost of the biopsy forceps can be recovered.
There are several different details from other guidelines for reprocessing endoscopes. For example, unlike the BSG and ESGE-ESGENA guidelines, there is no recommendation for microbiological surveillance. Also, the water bottle and connectors should undergo high-level disinfection every day and the water should be sterilized.
MULTISOCIETY GUIDELINES
In 2003, the American Society for Gastrointestinal Endoscopy and the Society for Healthcare Epidemiology of America developed an initial set of guidelines for reprocessing GI endoscopes. Updated guidelines were published in 2011 along with additional discussions on evolving issues and the latest literature citations. These guidelines use the Centers for Disease Control and Prevention system to categorize recommendations, from "category IA" to "no recommendation."7
For instance, "category IA" recommendations regarding high-level disinfectants include the following: FDA-approved, high-level disinfectants should be selected. There are six approved disinfectants: glutaraldehyde, orthophthalaldehyde, peracetic acid, hydrogen peroxide, electrolyzed acid water, and a peracetic acid/hydrogen peroxide blend. The guideline mentions that when glutaraldehyde is used, it should be at a 2% concentration level at 25℃ for 20 to 90 minutes. Also, the minimum effective density of the active ingredient should be present in high-level disinfectants and the solution must be checked every day before use. The solution should be discarded when the concentration of the solution is less than the minimal effective density.7,9,10,11
Unlike other guidelines, these guidelines include recommendations that emphasize protection from exposure to hazards and chemicals for those who participate in reprocessing. All participants should wear personal protective equipment and work in a safe environment (category IB and IC). Also, they should be educated properly about hazards (category IC).7,9,10,12
In addition, these guidelines use the Spaulding classification of medical devices, which is used by the FDA, the Centers for Disease Control and Prevention, epidemiologists, and many other international organizations to determine the effectiveness of disinfection or sterilization. Since endoscopes are classified as "semi-critical" devices, they should undergo at least high-level disinfection. High-level disinfection is the destruction of mycobacteria, microorganisms, viruses, fungal spores, and some, but not all, bacterial spores. Biopsy forceps, needles, and cutting instruments such as sphincterotomes, which penetrate the sterile tissue of the vascular structure, are classified as "critical" and should be sterilized.13,14
Two more recommendations were updated in the 2011 guidelines:
(1) Before high-level disinfection, complete cleaning of endoscopes should be more thorough.
(2) Hand hygiene, reprocessing tubing with one-way valves, and endoscopic channels are considered potential sources of infection.
In addition, the following four unresolved issues are discussed:
(1) How long can endoscopes be stored between use? The Association of periOperative Registered Nurses recommends 5 days, while the Association for Professionals in Infection Control and Epidemiology recommends 7 days before reprocessing.
(2) How long can water bottles, tubing for air insufflation, lens wash water, waste vacuum canisters, and suction tubing be used before being replaced? The Association of periOperative Registered Nurses recommends replacing all items after each procedure.
(3) How long can endoscopes be used before replacement? Determining the durability and longevity of endoscopes requires additional research.
(4) Is microbiological surveillance testing required after reprocessing, during storage, or before use of endoscopes? The Gastroenterological Society of Australia and the guidelines of the combined ESGE and the ESGENA committee recommend this measure. However, this has not been advised in the current American standards. The necessity of environmental microbiological testing of endoscopes for quality assurance has not been established but warrants further study.
ESGE-ESGENA GUIDELINES
In 1994, the ESGE-ESGENA Guideline Committee of the ESGE and the ESGENA established guidelines for infection control of GI endoscopes. In 2008, ESGE-ESGENA updated the guidelines for cleaning and disinfection of GI endoscopes.4
Apart from the seven steps of endoscope reprocessing similar to other guidelines, they provided detailed information for three different available reprocessing methods: automated washer-disinfectors, automated disinfection devices, and manual reprocessing followed by precleaning and manual cleaning. Using the automated washer disinfector includes all the processing steps from the leakage test to drying. Using the automated disinfection device includes rinsing and disinfection, along with final rinsing and drying. The ESGE and ESGENA strongly recommend the use of washer-disinfectors including cleaning and disinfection. Compared with manual reprocessing, automated reprocessing provides a standardized and validated reprocessing cycle. In addition, it ensures highly reliable reprocessing and minimal staff hazard and lowers the risk of scope damage.
According to European standard EN 14885, disinfectants are effective at room temperature when they are used manually or in automated disinfection devices.15 They rely on an aldehyde group (glutaraldehyde, formaldehyde, and orthophthalaldehyde) or an oxidizing substance (chlorine dioxide, hypochlorous acid, and peracetic acid and its salts).
Biliopancreatic procedures require the usage of sterile accessories. Reusable devices should be autoclavable. For example, balloons cannot be autoclaved and the use of reprocessed balloons increases the risk of biliopancreatic duct infection. Likewise, injection needles should be used only once, and in no circumstance should be reprocessed, because dismantling of needles is dangerous and the lumen of the needle is inaccessible for cleaning.
They also recommend process validation and microbiological surveillance. Manufacturer's instructions should be followed when performing process validation for washer disinfectors. Regular microbiological surveillance testing, at intervals less than 3 months, is recommended.16 Washer disinfector, endoscopes, and the water used in endoscopy should be tested at the same time. Moreover, regular quality control must include testing of water bottles.6,17
BSG GUIDELINES
The 2008 guidelines were updated in 2014. The process of reprocessing is described in two parts. The first is manual cleaning, which includes precleaning, cleaning, and rinsing. The second is automated disinfection by using endoscope washer disinfectors (EWDs), followed by drying and storage.
Guidelines for the reprocessing of endoscopes recommend almost the same process as other guidelines. However, several details differ from other guidelines. In the manual cleaning process, the use of enzymatic detergents to digest mucus and other biological material from endoscopic channels is no longer recommended, because of reports of occupational asthma and skin reactions.18,19
These guidelines do not recommend any manual disinfection method. The only recommended EWD is the automated disinfection device. Furthermore, they strongly recommend the EWD for the disinfection process after manual cleaning. Glutaraldehyde-based disinfectants, widely used disinfectants, are no longer used in the United Kingdom owing to two reasons: occupational safety concerns, an example of which is asthma, and the potential risk of residual cross-linked protein or prion material. There are four other disinfectants that can be used for reprocessing of endoscopes: orthophthalaldehyde (0.55%), peracetic acid (0.2% to 0.35%), chlorine dioxide, and electrolytically generated hypochlorous acid.20
Accessories that pass through the working channel of endoscopes should be used only once, such as cytology brushes, polypectomy snares, injection needles, biopsy forceps, and most endoscopic retrograde cholangiopancreatography accessories.21,22 Currently, most biopsy forceps are reused but the discovery of variant Creutzfeldt-Jakob disease has caused a shift towards single use. Accessories that are not passed through the working channel can be reused when sterilized, such as bottles and bougies. Also, heater probes can be reused and should be sterilized.21
BSG guidelines also recommend a microbiological surveillance test for assurance. The final rinse water should be sampled from the EWD and tested weekly for its microbiological quality in accordance with the current relevant European Standard, Health Technical Memorandum, or Choice Framework for local Policies and Procedures.23,24,25,26
WGO GUIDELINES
The committee of the WGO is composed of representatives from Europe, America, and Japan. In 2011, they updated the guidelines for reprocessing endoscopes. In order to improve compliance, WGO guidelines introduced the standard procedures with alternatives options that could be used when there are certain external limitations. For example, the cleaning process should be performed with an enzymatic detergent, but it can be performed with a non-enzymatic detergent in case of external limitations. Also, if personnel cannot rinse the endoscopes and valves with filtered water, they can rinse them under running tap water of drinking-water quality. Endoscopic accessories that penetrate the mucosal barrier should be used only once or sterilized between each use. However, single-use is preferred. If disposable accessories are to be reused despite external limitations, they should be perfectly sterilized.9,10,27,28,29,30,31 Microbiological surveillance testing is recommended. Samples of the final rinse water from the automatic reprocessor should be subjected to microbiological testing at least weekly.16,23,24,25,26
CONCLUSIONS
Different organizational guidelines for reprocessing GI endoscopes are similar, but they include subtle differences (Table 1). Reprocessing of GI endoscopes should be carried out according to recommendations for each step of the process, whether using manual or automated methods. Many associations are trying to develop evidence-based guidelines for the reprocessing of GI endoscopes. The evolution of disinfectants and automated machines for reprocessing are required to ensure endoscopic safety. Lastly, guidelines should reflect both new procedures and practical limitations.
Table 1. Comparison of Different Details among Guidelines.
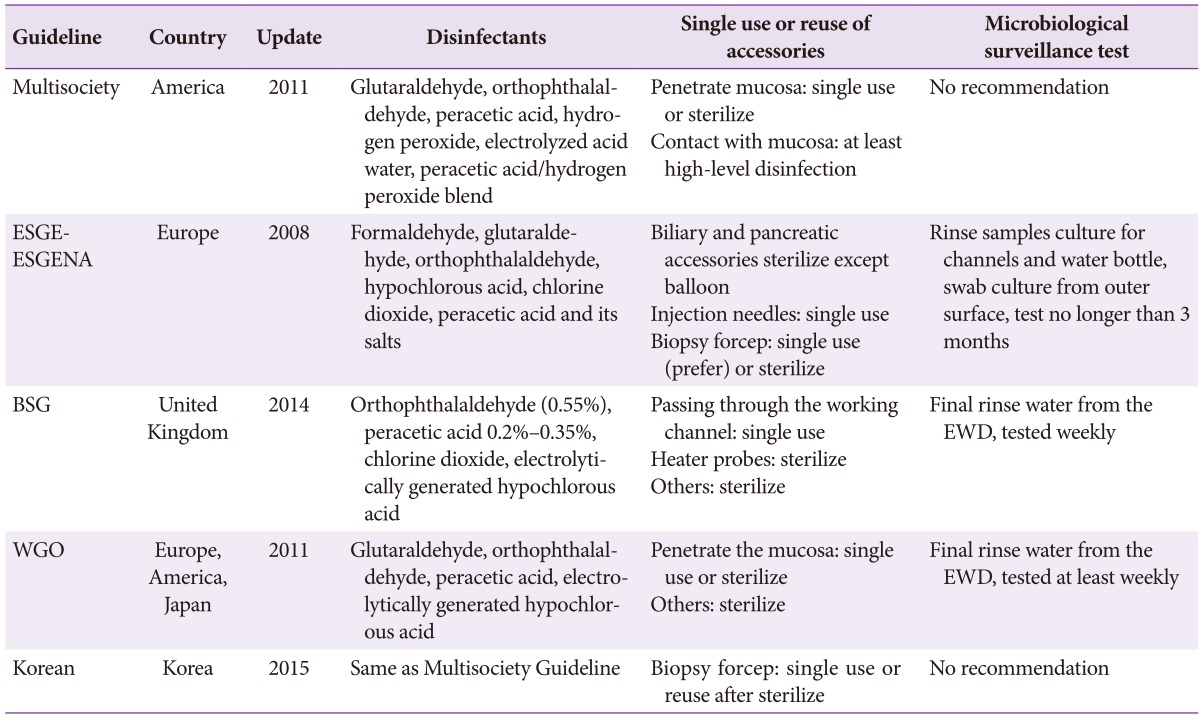
ESGE, European Society of Gastrointestinal Endoscopy; ESGENA, European Society of Gastroenterology and Endoscopy Nurses and Associates; BSG, British Society of Gastroenterology; EWD, endoscope washer disinfector; WGO, World Gastroenterology Organization.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Gorse GJ, Messner RL. Infection control practices in gastrointestinal endoscopy in the United States: a national survey. Gastroenterol Nurs. 1991;14:72–79. doi: 10.1097/00001610-199110000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Nelson DB. Infectious disease complications of GI endoscopy: part II, exogenous infections. Gastrointest Endosc. 2003;57:695–711. doi: 10.1067/mge.2003.202. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DB. Recent advances in epidemiology and prevention of gastrointestinal endoscopy related infections. Curr Opin Infect Dis. 2005;18:326–330. doi: 10.1097/01.qco.0000171925.47452.8f. [DOI] [PubMed] [Google Scholar]
- 4.Beilenhoff U, Neumann CS, Rey JF, et al. ESGE-ESGENA Guideline: cleaning and disinfection in gastrointestinal endoscopy. Endoscopy. 2008;40:939–957. doi: 10.1055/s-2008-1077722. [DOI] [PubMed] [Google Scholar]
- 5.ASGE Quality Assurance In Endoscopy Committee. Petersen BT, Chennat J, et al. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Gastrointest Endosc. 2011;73:1075–1084. doi: 10.1016/j.gie.2011.03.1183. [DOI] [PubMed] [Google Scholar]
- 6.Loveday HP, Wilson JA, Pratt RJ, et al. Epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86(Suppl 1):S1–S70. doi: 10.1016/S0195-6701(13)60012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutala WA, Weber DJ Centers for Disease Control (US. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Washington, DC: Centers for Disease Control (U.S.); 2008. [Google Scholar]
- 8.Rey J, Bjorkman D, Nelson N, et al. Endoscope disinfection: a resource-sensitive approach [Internet] Milwaukee: World Gastroenterology Organisation; c2011. [cited 2015 Sep 10]. Available from: http://www.worldendo.org/assets/downloads/pdf/guidelines/wgo_weo_endoscope_disinfection.pdf. [Google Scholar]
- 9.Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am J Infect Control. 2000;28:138–155. [PubMed] [Google Scholar]
- 10.ASGE Standards of Practice Committee. Banerjee S, Shen B, et al. Infection control during GI endoscopy. Gastrointest Endosc. 2008;67:781–790. doi: 10.1016/j.gie.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. FDA-cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices: March 2009 [Internet] Silver Spring: U.S. Food and Drug Administration; 2015. [updated 2015 Mar 12]. [cited 2015 Sep 10]. Available from: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofSingle-UseDevices/ucm133514.htm. [Google Scholar]
- 12.Daniel G, Hoffman W, McDonald B. OSHA compliance: issues ethylene oxide in a commercial sterilization operation. J Occup Environ Hyg. 2004;1:D121–D125. doi: 10.1080/15459620490513501. [DOI] [PubMed] [Google Scholar]
- 13.Rutala WA, Clontz EP, Weber DJ, Hoffmann KK. Disinfection practices for endoscopes and other semicritical items. Infect Control Hosp Epidemiol. 1991;12:282–288. doi: 10.1086/646340. [DOI] [PubMed] [Google Scholar]
- 14.Rutala WA, Weber DJ. Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis. 2004;39:702–709. doi: 10.1086/423182. [DOI] [PubMed] [Google Scholar]
- 15.Gebel J, Exner M, French G, et al. The role of surface disinfection in infection prevention. GMS Hyg Infect Control. 2013;8:Doc10. doi: 10.3205/dgkh000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beilenhoff U, Neumann CS, Rey JF, et al. ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy. 2007;39:175–181. doi: 10.1055/s-2006-945181. [DOI] [PubMed] [Google Scholar]
- 17.Bader L, Blumenstock G, Birkner B, et al. HYGEA (Hygiene in gastroenterology: endoscope reprocessing): study on quality of reprocessing flexible endoscopes in hospitals and in the practice setting. Z Gastroenterol. 2002;40:157–170. doi: 10.1055/s-2002-22326. [DOI] [PubMed] [Google Scholar]
- 18.Cobbold A, Lord S. A critical guideline appraisal overview: Choice Framework for local Policy and Procedures (CFPP) 01-06 Decontamination of flexible endoscopes 2013. Part 1: Policy and management and Part 3: Operational management manual. J Perioper Pract. 2014;24:79–83. doi: 10.1177/175045891602400404. [DOI] [PubMed] [Google Scholar]
- 19.Heederik D, Houba R, Liss GM, Millerick-May M. Protecting the worker and modifying the work environment. In: Malo JL, Chan-Yeung M, Bernstein DI, editors. Asthma in the Workplace. 4th ed. Boca Raton: CRC Press; 2013. pp. 138–149. [Google Scholar]
- 20.Niven K. An evaluation of chemical disinfecting agents used in endoscopy suites in the NHS [Internet] Liverpool: Health and Safety Executive Research; 2007. [cited 2015 Sep 10]. Available from: http://www.hse.gov.uk/research/rrpdf/rr445.pdf. [Google Scholar]
- 21.Bramble MG, Ironside JW. Creutzfeldt-Jakob disease: implications for gastroenterology. Gut. 2002;50:888–890. doi: 10.1136/gut.50.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaulding E, Groschel D. Hospital disinfectants and antiseptics. In: Lennette EH, Spaulding EH, Truant JP, editors. Manual of Clinical Microbiology. 2nd ed. Washington, DC: American Society for Microbiology; 1974. pp. 852–857. [Google Scholar]
- 23.Leung J, Vallero R, Wilson R. Surveillance cultures to monitor quality of gastrointestinal endoscope reprocessing. Am J Gastroenterol. 2003;98:3–5. doi: 10.1111/j.1572-0241.2003.07171.x. [DOI] [PubMed] [Google Scholar]
- 24.Moses FM, Lee J. Surveillance cultures to monitor quality of gastrointestinal endoscope reprocessing. Am J Gastroenterol. 2003;98:77–81. doi: 10.1111/j.1572-0241.2003.07165.x. [DOI] [PubMed] [Google Scholar]
- 25.Moses FM, Lee JS. Current GI endoscope disinfection and QA practices. Dig Dis Sci. 2004;49:1791–1797. doi: 10.1007/s10620-004-9572-5. [DOI] [PubMed] [Google Scholar]
- 26.Tunuguntla A, Sullivan MJ. Monitoring quality of flexible endoscope disinfection by microbiologic surveillance cultures. Tenn Med. 2004;97:453–456. [PubMed] [Google Scholar]
- 27.SGNA Practice Committee. Reprocessing of endoscopic accessories and valves. Gastroenterol Nurs. 2006;29:394–395. doi: 10.1097/00001610-200609000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Bronowicki JP, Venard V, Botté C, et al. Patient-to-patient transmission of hepatitis C virus during colonoscopy. N Engl J Med. 1997;337:237–240. doi: 10.1056/NEJM199707243370404. [DOI] [PubMed] [Google Scholar]
- 29.BSG Endoscopy Committee Working Party. Cleaning and disinfection of equipment for gastrointestinal endoscopy. Report of a Working Party of the British Society of Gastroenterology Endoscopy Committee. Gut. 1998;42:585–593. [PMC free article] [PubMed] [Google Scholar]
- 30.Association of periOperative Registered Nurses. Recommended practices for cleaning and processing endoscopes and endoscope accessories. AORN J. 2003;77:434–438. 441–442. doi: 10.1016/s0001-2092(06)61212-x. [DOI] [PubMed] [Google Scholar]
- 31.Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Med. 1993;118:117–128. doi: 10.7326/0003-4819-118-2-199301150-00008. [DOI] [PubMed] [Google Scholar]


