Abstract
Background/Aims
Capsule endoscopy (CE) is widely used. However, CE has limitations including incomplete examination, inadequate bowel preparation, and retention. The aim of this study was to estimate the indications for and detection, completion, and retention rates of small intestine CE based on the 10-year data from the Korean Capsule Endoscopy Registry.
Methods
Twenty-four hospitals participated in this study. Clinical information, such as reasons for CE, method and quality of bowel preparation, and incomplete examination and capsule retention rates, was collected and analyzed.
Results
A total of 2,914 CEs were registered. The most common reason for CE was obscure gastrointestinal bleeding (59%). Significant lesions were detected in 66% of cases. Positive CE diagnosis occurred in 63% of cases. The preparation method did not significantly affect the quality of bowel preparation for CE. The overall incomplete rate was 33%, and was high in the elderly and those with poor bowel preparation. Capsule retention was 3% and high in patients with small bowel tumors and Crohn's disease and in children under 10 years of age.
Conclusions
CE is a valuable technique; while the overall detection rate is high, incompletion and retention rates are also relatively high. CE should be carefully considered in the elderly and children less than 10 years of age, as well as in patients with small bowel tumors and Crohn's disease.
Keywords: Capsule endoscopy; Completion; Intestine, small; Preparation; Retention
INTRODUCTION
Capsule endoscopy (CE) is a noninvasive diagnostic tool used to visualize the small intestinal mucosa.1 It is a useful tool for investigating obscure gastrointestinal bleeding (OGIB), small bowel Crohn's disease, polypoid syndrome, small bowel tumors, etc.1,2,3
Although CE has been shown to be superior to other techniques for diagnosing small bowel lesions, complete small bowel examination is limited for various reasons.4 The lifespan of the batteries that power the capsule were previously limited to approximately 8 hours. For various reasons, some capsules cannot pass through the ileocecal valve before battery exhaustion. In these cases, complete examination of the entire small bowel is impossible. However, longer lifespan batteries have recently been developed.
To maximize the diagnostic yield of CE, adequate bowel preparation is important.4,5,6 The presence of impure intestinal juice or air bubbles can cause incomplete visualization of the intestinal mucosa and influence the diagnosis.7
Capsule retention is another complication of CE.8 Although retained capsules are usually asymptomatic, retention can potentially lead to symptomatic small bowel obstruction that often requires surgical or endoscopic intervention to remove the retained capsule.
CE has been performed as a primary diagnostic tool for small bowel disease at most medical centers in Korea. The aim of this study was to assess the indications for and detection, completion, and retention rates of CE based on 10 years of data from the Korean Capsule Endoscopy Registry. We also investigated factors affecting complete examination and capsule retention.
MATERIALS AND METHODS
A total of 2,914 CE examinations were enrolled in the capsule registry from October 2002 to September 2012. CEs were performed at 24 hospitals across Korea. Information including age, gender, reasons for CE, CE findings (small bowel lesions), CE diagnosis, method and quality of bowel preparation, complete examination, and retention was gathered by an Internet web site. Various CE instruments (PillCam SB1 and SB2, Given Imaging, Yokneam, Israel; MiroCam, IntroMedic Co., Ltd., Seoul, Korea; EndoCapsule, Olympus, Tokyo, Japan) were used.
The reasons for CE included OGIB, unexplained abdominal pain, chronic diarrhea, Crohn's disease, small bowel tumor, ulcerative colitis, Behcet's disease, ischemic enteritis, unknown origin of weight loss, cancer, and protein losing enteropathy. OGIB was defined as bleeding of unknown origin that persisted or recurred after an initial upper and lower gastrointestinal endoscopy with negative findings. In addition to cases of melena or hematochezia, persistent iron deficiency anemia or positive stool occult blood with negative findings on the initial endoscopy were also considered OGIB. CE findings and diagnoses were described based upon capsule endoscopy structured terminology.
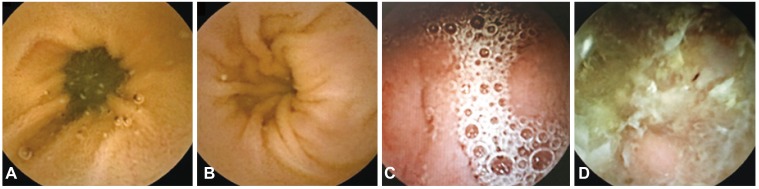
Before the CE study, each patient received bowel preparation according to clinician preference. The various methods of bowel preparation included nothing per os (NPO) for 12 hours or use of purgative agents such as 2 or 4 L sodium phosphate (NaP) or polyethylene glycol (PEG) conducted in each hospital. Independent examiners categorized the quality of bowel preparation for CE. The quality the preparations were categorized as follows: excellent, visualization of ≥90% of the mucosa, no or minimal fluid, debris, and bubbles (Fig. 1A); good, visualization of ≥90% of the mucosa, mild fluid, debris, and bubbles (Fig. 1B); fair, visualization of <90% of the mucosa, moderate fluid, debris, and bubbles (Fig. 1C); poor, visualization of <80% of the mucosa, excessive fluid, debris, and bubbles (Fig. 1D).9,10
Fig. 1. Quality of bowel preparation for capsule endoscopy. (A) Excellent, visualization of ≥90% of the mucosa, no or minimal fluid, debris, and bubbles. (B) Good, visualization of ≥90% of the mucosa, mild fluid, debris, and bubbles. (C) Fair, visualization of <90% of the mucosa, moderate fluid, debris, and bubbles. (D) Poor, visualization of <80% of the mucosa, excessive fluid, debris, and bubbles.
The overall incomplete and retention rates as well as the factors affecting completion and CE retention were investigated. Completion was defined as the capsule reaching the cecum during the recording time. Capsule retention was defined as the capsule remaining in the digestive tract for more than 2 weeks.
Statistical analysis
Data were represented as mean±standard deviation for continuous variables and number (%) for categorical data. Chi-square tests were used to evaluate the optimal method of bowel preparation. Multiple logistic regression models were used to identify the risk factors for completion and CE retention. Statistics were calculated using SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Demographic characteristics
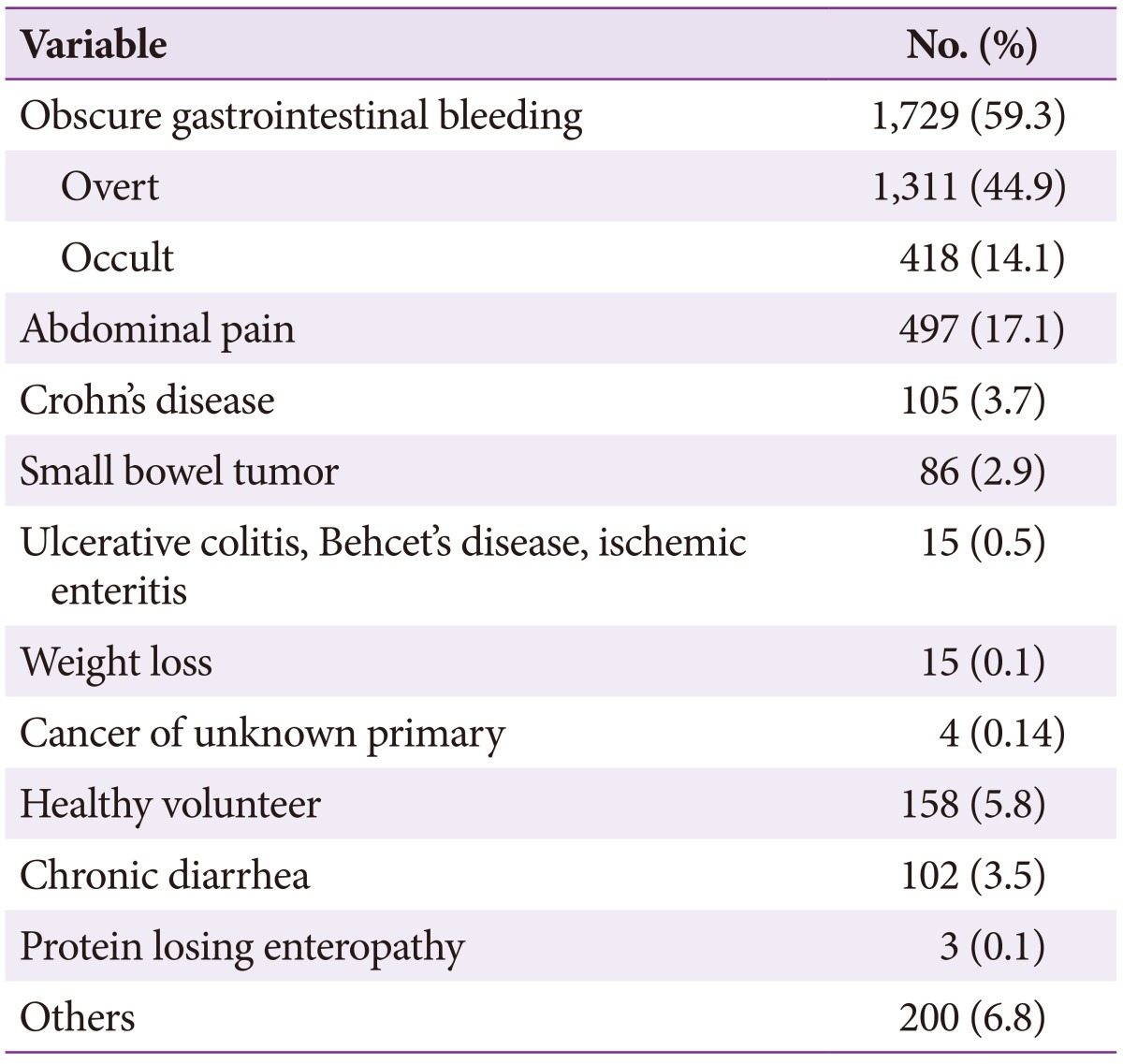
The mean age of the entire study population was 53.0±17.6 years, and male patients were predominant (61%). The reasons for CE are described in Table 1. The common reasons included OGIB (59.3%), abdominal pain (17.1%), healthy volunteer (5.4%), suspected Crohn's disease (3.6%), chronic diarrhea (3.5%), and small bowel tumor (2.9%).
Table 1. Reasons for Capsule Endoscopy.
CE findings and diagnosis
Lesions were detected in 66% of CE examinations, while normal findings were reported in 34% of procedures. Ulcers (20%), erosions (11%), and angiodysplasia (9%) were also common findings.
Positive CE diagnoses were obtained in 63% of examinations. The most common CE diagnosis was small bowel tumor. Lymphangiectasia, lymphoid hyperplasia, lymphangioma, polyp, submucosal tumor, and malignant tumor are all considered small bowel tumors. Vascular lesions, including angiodysplasia, telangiectasia, arteriovenous malformation, and dieulafoy lesions, were the second-most frequently diagnosed entity, followed by nonspecific ulcers (8%), erosions (6%), Crohn's disease (6%), and non-steroidal anti-inflammatory drug (NSAID) enteritis (5.3%) (Table 2).
Table 2. Capsule Endoscopic Diagnosis.
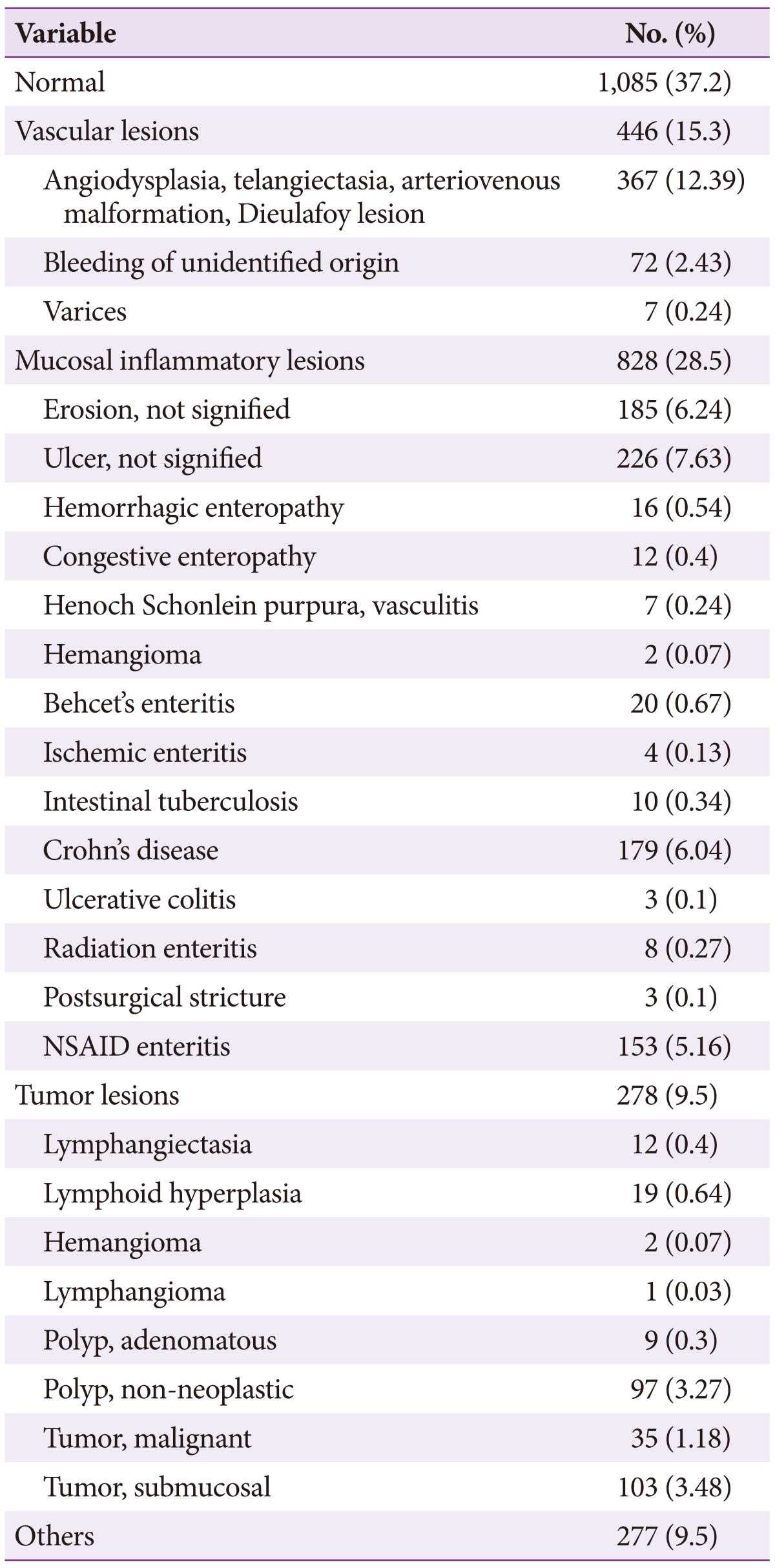
NSAID, non-steroidal anti-inflammatory drug.
Bowel preparation
PEG and NaP laxatives (80%) was more common used regimen for small bowel preparation for CE than NPO alone (20%). The 2 L PEG preparation was the most widely used preparation regimen in Korea for 10 years (52%; 1,123 of 2,143 examinations). With adequate bowel preparation, the mucosa appeared clean for more than 90% of the total examinations, comprised of excellent and good quality and occupied 71.5% (1,532 of 2,143 examinations). The various preparation methods did not significantly affect the quality of bowel preparation for CE (p=0.64) (NPO for 12 hours compared to purgative agents such as NaP and 2 or 4 L PEG) (Table 3).
Table 3. Bowel Preparation Methods for Capsule Endoscopy.
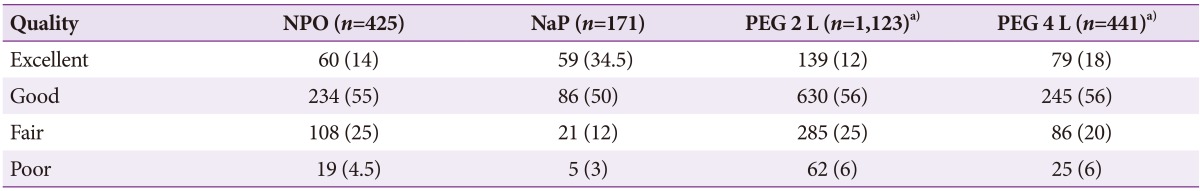
Values are presented as number (%).
NPO, nothing per os; NaP, sodium phosphate; PEG, polyethylene glycol.
a)p=0.64.
Incomplete and retention rates
The overall incomplete rate was 33% (969/2,914). The multiple logistic regression model indicated that completion rates were significantly higher with better bowel preparation and high in patients with OGIB (Table 4). The incomplete rate was significantly higher in elderly patients.
Table 4. Risk Factors for Incompletion Rate (Multiple Logistic Regression Model).

CI, confidence interval; GI, gastrointestinal.
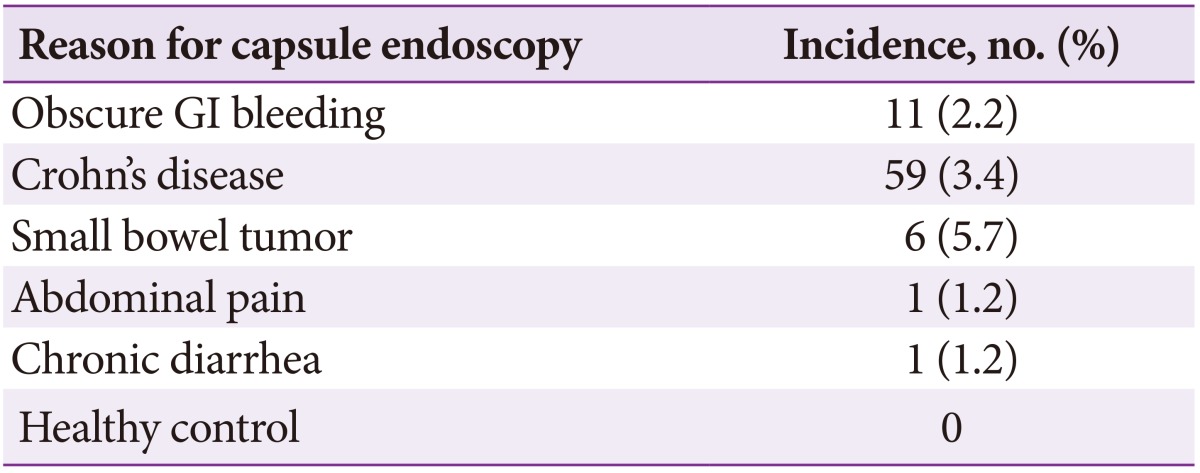
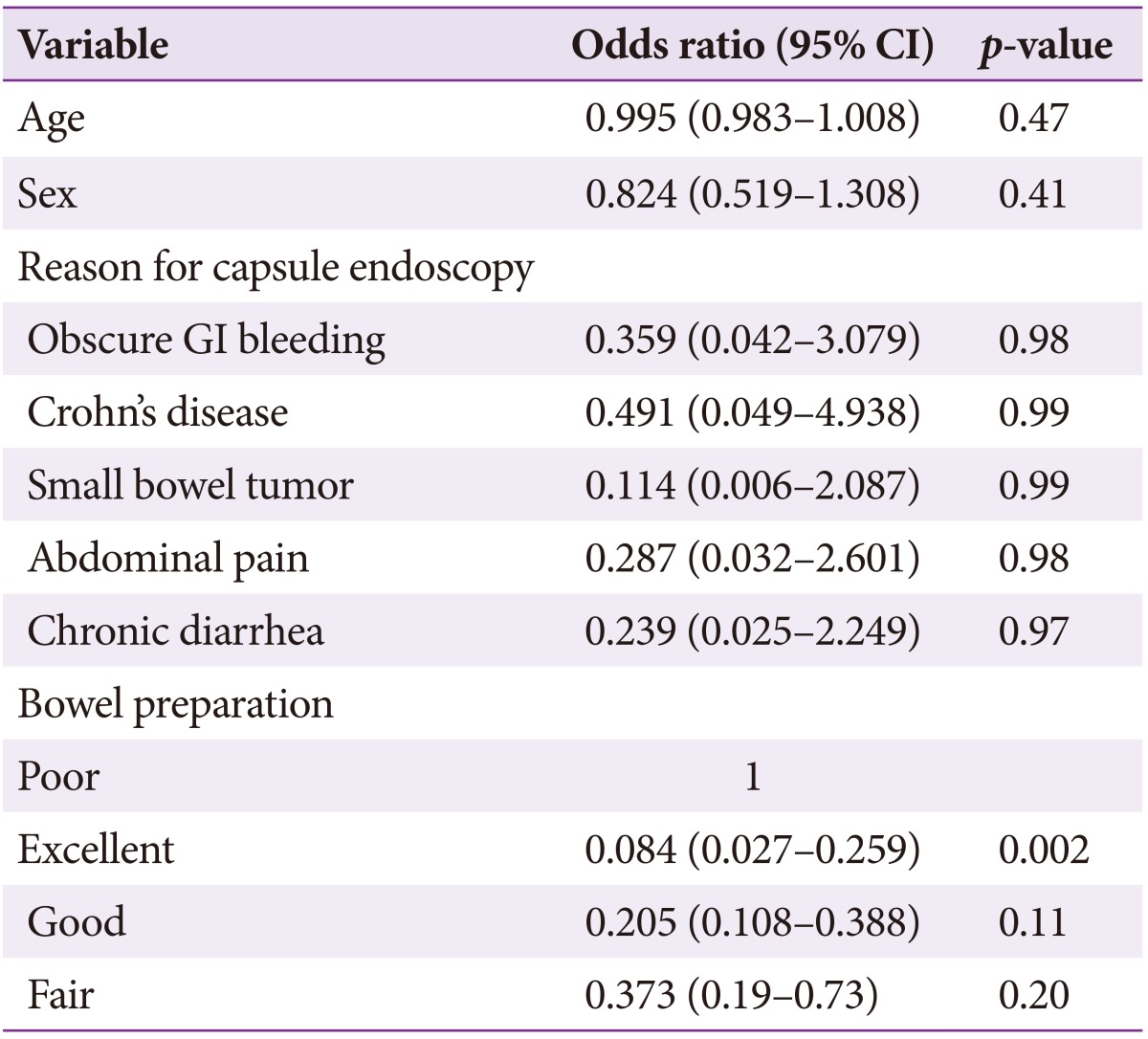
The overall capsule retention rate was 3% (90/2,914). The rate was high in patients with small bowel tumors (5.7%) and Crohn's disease (3.4%) (Table 5). The retention rate in children under 10 years was very high (8.3%). Capsule retention was significantly lower in cases with excellent bowel preparation (Table 6).
Table 5. Capsule Retention according to Reason for Capsule Endoscopy.
GI, gastrointestinal.
Table 6. Risk Factor Analysis for Capsule Retention (Multiple Logistic Regression Model).
CI, confidence interval; GI, gastrointestinal.
DISCUSSION
Since its first use 10 years ago, CE has been a first-line diagnostic method for evaluation of the small bowel. The most common indications for CE include OGIB, suspected Crohn's disease, small bowel tumor, and polyposis syndrome. However, healthy volunteers, unexplained abdominal pain, chronic diarrhea, weight loss, and unknown primary cancer were also common reasons for recommending CE in Korea during the 10-year period. CE is a useful diagnostic modality: lesions are detected in 66% of cases, with a positive diagnostic rate of 63%. However, CE has some limitations, such as inadequate bowel preparation and incompletion. To increase diagnosis yield, adequate bowel preparation and complete examination of the entire small bowel are important. Adequate bowel preparation is often difficult because lumen visualization is impaired by bubbles, bile, and debris.9,11 Previous recommendations for CE preparation included a 12-hour fast after 24 hours of clear liquid intake. However, fasting alone did not result in satisfactory bowel preparation. Current evidence suggests that laxatives containing PEG or NaP are more effective than fasting alone for improving visualization of small bowel mucosa.6,7 However, purgatives do not decrease gastric emptying or small bowel transit time.11 In the present study, there were no significant differences in the quality of bowel preparation between patients who used purgatives such as NaP and PEG and those who were NPO for 12 hours. The present study showed that poor bowel preparation was significantly associated with increased incompletion and capsule retention rates. The method, dose, and time of administration for attaining the best quality small bowel images remain to be determined.
In the present study, the incomplete small bowel examination rate was higher (33%) than in previous studies (20% to 30%).12 This discrepancy may be due to subject traits such as age, underlying disease, delayed gastric emptying, and bowel preparation. Battery exhaustion can easily occur in patients with delayed gastric emptying, namely due to the capsule failing to enter the duodenum or remaining in the stomach for more than 1.5 hours. However, incompletion rates are expected to fall with development of longer-lived batteries (up to 13 to 16 hours). The MiroCam has battery that permits 9 to 11 hours of transmission while the PillCam SB1, SB2, and EndoCapsule offer 7 to 8 hours of transmission.13 In these study, incompletion rate did not differ according to device manufacturer. Older age, male gender, Crohn's disease, and tumors are factors known to affect incomplete CE examination rates.14 The results of the present study indicate that older age and poor bowel preparation may affect incomplete examination rates. One limitation of the present study was the lack of data regarding gastric transit time, which is a crucial factor in incomplete examinations.
Retention rates reported in previous studies ranged from 1.3% to 2.5%.12 The risk of retention is high in patients with prolonged NSAID use, abdominal radiation injury, extensive Crohn's disease, and previous major abdominal surgery or small-bowel resection.12,15 A recent large study reported a retention rate of 1.4%, finding NSAIDs enteropathy to be the main reason for retention.16 However, in the present study, the capsule retention rate was 3% (90/2,914) and often occurred in cases of small bowel tumor (5.7%), Crohn's disease (3.4%) and in children under 10 years of age (8.3%).
Both the incomplete examination (33%) and capsule retention (3%) rates were relatively higher than those reported in previous studies.16,17,18,19 One possible explanation could be patient age and disease distribution. In a large multicenter study, small bowel tumors were associated with capsule retention (9.8%).19 In the present study, small bowel tumors were identified as high-risk factors for capsule retention (5.7%).
There is still no accepted method to prevent capsule retention. The Agile patency capsule (Given Imaging) can dramatically reduce retention rates when pretest suspicion is high.8
The strength of this study is that it is the largest study to estimate the indications for and detection, completion, and retention rates of small bowel CE in Korea over 10 years. However, this study has some limitations. First, this is a retrospective analysis. Second, there might be differences in interpretation of CE findings between institutions. Third, because data were selected from the registry, selection bias is possible. Although both incomplete examination and capsule retention rates (33% and 3%, respectively) were relatively high, CE is a useful diagnostic tool with a high detection rate. Poor bowel preparation and old age were significantly associated with incompletion. To reduce capsule retention rates, CE examination should be carefully considered in patients suspected to have small bowel tumors and Crohn's disease, and in children less than 10 years of age.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Gut Image Study Group. Lim YJ, Moon JS, et al. Korean Society of Gastrointestinal Endoscopy (KSGE) guidelines for credentialing and granting previleges for capsule endoscopy. Korean J Gastrointest Endosc. 2008;37:393–402. [Google Scholar]
- 2.Shim KN, Moon JS, Chang DK, et al. Guideline for capsule endoscopy: obscure gastrointestinal bleeding. Clin Endosc. 2013;46:45–53. doi: 10.5946/ce.2013.46.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685–689. doi: 10.1055/s-2002-33446. [DOI] [PubMed] [Google Scholar]
- 4.Höög CM, Bark LÅ, Arkani J, Gorsetman J, Broström O, Sjöqvist U. Capsule retentions and incomplete capsule endoscopy examinations: an analysis of 2300 examinations. Gastroenterol Res Pract. 2012;2012:518718. doi: 10.1155/2012/518718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Tuyl SA, den Ouden H, Stolk MF, Kuipers EJ. Optimal preparation for video capsule endoscopy: a prospective, randomized, single-blind study. Endoscopy. 2007;39:1037–1040. doi: 10.1055/s-2007-966988. [DOI] [PubMed] [Google Scholar]
- 6.Dai N, Gubler C, Hengstler P, Meyenberger C, Bauerfeind P. Improved capsule endoscopy after bowel preparation. Gastrointest Endosc. 2005;61:28–31. doi: 10.1016/s0016-5107(04)02444-7. [DOI] [PubMed] [Google Scholar]
- 7.Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy? A meta-analysis. Am J Gastroenterol. 2009;104:219–227. doi: 10.1038/ajg.2008.63. [DOI] [PubMed] [Google Scholar]
- 8.Delvaux M, Ben Soussan E, Laurent V, Lerebours E, Gay G. Clinical evaluation of the use of the M2A patency capsule system before a capsule endoscopy procedure, in patients with known or suspected intestinal stenosis. Endoscopy. 2005;37:801–807. doi: 10.1055/s-2005-870241. [DOI] [PubMed] [Google Scholar]
- 9.Lapalus MG, Ben Soussan E, Saurin JC, et al. Capsule endoscopy and bowel preparation with oral sodium phosphate: a prospective randomized controlled trial. Gastrointest Endosc. 2008;67:1091–1096. doi: 10.1016/j.gie.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Brotz C, Nandi N, Conn M, et al. A validation study of 3 grading systems to evaluate small-bowel cleansing for wireless capsule endoscopy: a quantitative index, a qualitative evaluation, and an overall adequacy assessment. Gastrointest Endosc. 2009;69:262–270.e1. doi: 10.1016/j.gie.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Kalantzis C, Triantafyllou K, Papadopoulos AA, et al. Effect of three bowel preparations on video-capsule endoscopy gastric and small-bowel transit time and completeness of the examination. Scand J Gastroenterol. 2007;42:1120–1126. doi: 10.1080/00365520701251601. [DOI] [PubMed] [Google Scholar]
- 12.Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Eliakim R. Capsule endoscopy: where are we at 2011 and where are we headed. Intest Res. 2012;10:235–243. [Google Scholar]
- 14.Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc. 2009;69:74–80. doi: 10.1016/j.gie.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Lim YJ, Yang CH. Non-steroidal anti-inflammatory drug-induced enteropathy. Clin Endosc. 2012;45:138–144. doi: 10.5946/ce.2012.45.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Gurudu SR, De Petris G, et al. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc. 2008;68:174–180. doi: 10.1016/j.gie.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 17.Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488–495. doi: 10.1055/s-2007-995783. [DOI] [PubMed] [Google Scholar]
- 18.Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol. 2013;19:3726–3746. doi: 10.3748/wjg.v19.i24.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712–716. doi: 10.1016/j.gie.2005.05.002. [DOI] [PubMed] [Google Scholar]