Abstract
Purpose
Although several studies report risk factors for anastomotic leakage after gastrectomy for gastric cancer, they have yielded conflicting results. The present retrospective cohort study was performed to identify risk factors that are consistently associated with anastomotic leakage after gastrectomy for stomach cancer.
Materials and Methods
All consecutive patients who underwent gastrectomy at a single gastric surgical unit between May 2003 and December 2012 were identified retrospectively. The associations between anastomotic leakage and 23 variables related to patient history, diagnosis, and surgery were assessed and analyzed with logistic regression.
Results
In total, 3,827 patients were included. The rate of anastomotic leakage was 1.88% (72/3,827). Multiple regression analysis showed that male sex (P=0.001), preoperative/intraoperative transfusion (P<0.001), presence of cardiovascular disease (P=0.023), and tumor location (P<0.001) were predictive of anastomotic leakage. Patients with and without leakage did not differ significantly in terms of their 5-year survival: 97.6 vs. 109.5 months (P=0.076).
Conclusions
Male sex, cardiovascular disease, perioperative transfusion, and tumor location in the upper third of the stomach were associated with an increased risk of anastomotic leakage. Although several studies have reported that an anastomotic complication has a negative impact on long-term survival, this association was not observed in the present study.
Keywords: Stomach neoplasms, Gastrectomy, Complication, Anastomotic leak
Introduction
Stomach cancer is the fourth most common cancer and, the second most common cause of cancer-related death worldwide after lung cancer.1 Although the strategies used to treat stomach cancer depend on its oncological stage, surgical resection is generally considered the first choice of treatment.2 Recently, minimally invasive surgery such as laparoscopy-assisted or robotic-assisted gastrectomy has become a common procedure for stomach cancer.3,4 Numerous studies have assessed the oncological outcomes and complications of both open and laparoscopic gastrectomy.5,6,7,8
Of the various postoperative complications that are associated with gastrectomy, a particularly detrimental one is anastomotic leakage.9,10 This complication not only has immediate clinical consequences and increases postoperative mortality, but it can also affect the long-term outcomes.11,12,13 Anastomotic leakage has been reported to occur in 1% to 6% of patients undergoing gastrectomy.5,10,14,15,16,17 While several studies have identified risk factors for anastomotic leakage, they have yielded inconsistent results. Furthermore, these studies have only examined the risk factors associated with a single gastrectomy method and were focused on the negative impact of anastomotic leakage.
Since identifying risk factors that are associated consistently with anastomotic leakage after gastrectomy would promote the development and implementation of preventive measures, this retrospective cohort study was performed. Its aims were to determine the anastomotic leakage rates in a single gastric surgical unit and to identify the preoperative and intraoperative risk factors.
Materials and Methods
1. Patients
All consecutive patients who underwent gastric resection for cancer between May 2003 and December 2012 at a single, stomach surgical unit at Seoul National University Bundang Hospital, Korea were identified through a retrospective search of the medical database. Patients who underwent palliative gastrostomy or a bypass procedure were excluded from the study. This study was approved by the Seoul National University Bundang Hospital Institutional Review Board (IRB No: B-1411-274-101).
2. Study variables
All patients underwent surgery performed by the same surgical team. Patient demographic characteristics were recorded along with the following clinical, surgical, and pathological characteristics: the operators of the surgical team (A, B, or C), presence of cardiovascular or pulmonary disease, history of diabetes, American Society of Anesthesiologists (ASA) score, Charlson comorbidity score, smoking habit, history of previous laparotomy, preoperative blood tests, tumor location, intraoperative blood loss, surgical approach (open, laparoscopy-assisted, or conversion to open), pre/intraoperative blood transfusion, duration of operation, combined organ resection, type of resection, type of reconstruction, presence of proximal or distal margin involvement, numbers of harvested lymph nodes, TNM stage, and time to first flatus. Patients with and without anastomotic leakage were compared in terms of these clinicopathological and surgical factors.
3. Definition of anastomotic leakage
Clinical signs of anastomotic leakage included abdominal pain, fever, pus or complicated discharge from the abdominal drain catheter, and peritonitis. Clinical suspicion of leakage was documented reoperation or confirmed by a radiographic examination demonstrating contrast leakage from a viscus into a body cavity.
4. Statistical analysis
The association of leakage with independent variables was examined by performing univariate analysis. Some continuous variables were converted into dichotomous variables, namely, age (<60 vs. ≥60 years), body mass index (BMI; <25, ≥25 and <30, ≥30), ASA grade (<3 vs. ≥3), Charlson comorbidity score (<3 vs. ≥3), blood loss (<500 vs. ≥500 ml), and duration of operation (≤300 vs. >300 minutes). Continuous variables were analyzed by using the Mann-Whitney U-test. Categorical variables were analyzed by using the chi-squared test or Kruskal-Wallis test. Survival interval was measured from the date of gastrectomy to the date of death. Survival data were analyzed by using the Kaplan-Meier method, and the log-rank test was used to detect differences between patients with and without anastomotic leakage in terms of cancer-related deaths. All variables with P<0.05 in the univariate analyses were included in the multivariate analysis; P<0.05 was considered to indicate statistical significance. Patient groups were compared in terms of categorical variables by using binary logistical regression. All statistical analyses were performed by using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
In total, 4,219 patients underwent gastrectomy for stomach cancer during the study period. Of these, 392 were excluded because patients underwent palliative gastrostomy (n=152) or a bypass procedure (n=240). The remaining 3,827 patients were included in the study. Table 1 shows the clinicopathological and operative characteristics of these patients.
Table 1. Clinicopathological and operative characteristics of the 3,827 patients who underwent gastrectomy.
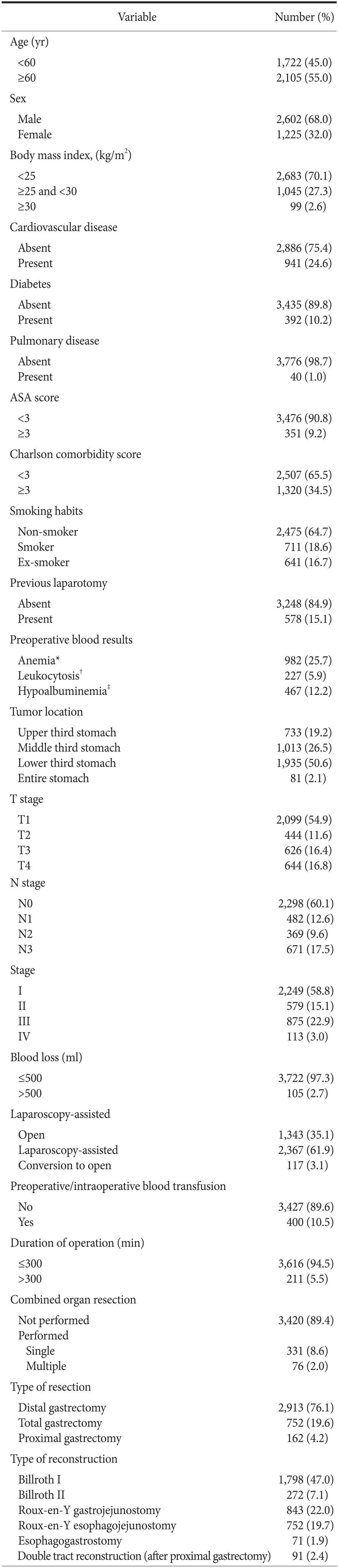
Tumor stages are based on the TNM classification system from the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer. ASA = American Society of Anesthesiologists. *Anemia is defined as blood hemoglobin concentration <12 g/dl in females or <13 g/dl in males. †Leukocytosis is defined as white blood cell >10,000/µl. ‡Hypoalbuminemia is defined as serum albumin <3.5 g/dl.
1. Risk factors for anastomotic leakage after gastrectomy
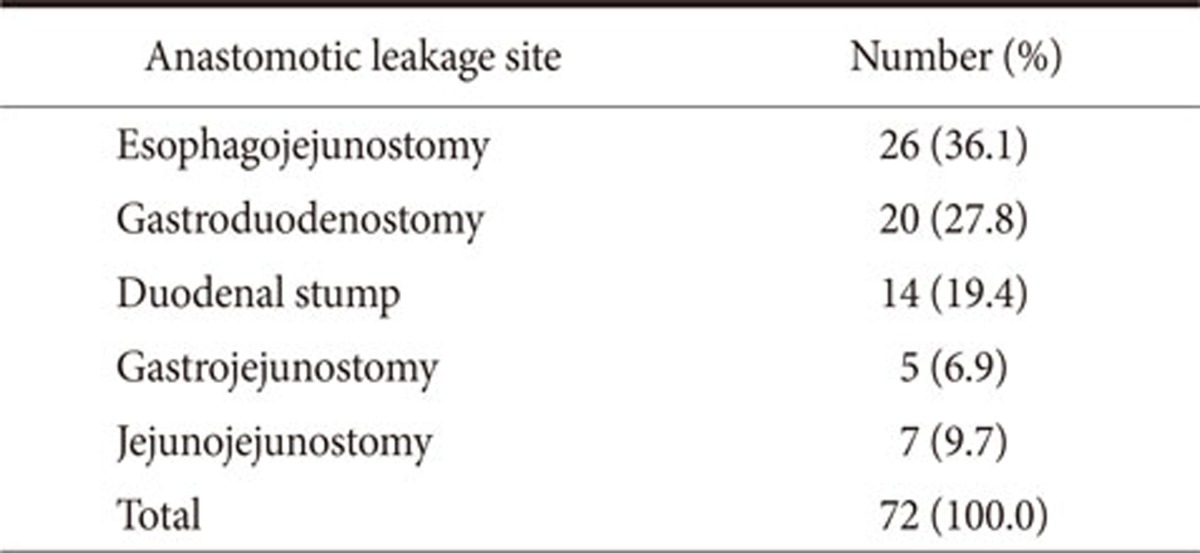
In total, 72 patients had an anastomotic leakage. Thus, the leakage rate was 1.88%. Table 2 shows details of anastomotic leakage sites. Among them, 10 patients underwent reoperation and 62 had percutaneous drainage and conservative management. Mean hospital stay of patients with anastomotic leakage was 15.8 days (9~33 days) and there was no in-hospital mortality. The variables that achieved statistical significance by univariate analyses were sex (P=0.001), presence of cardiovascular disease (P=0.046) and diabetes (P=0.011), history of previous laparotomy (P=0.045), tumor location (P<0.001), time to first flatus (P=0.014), intraoperative blood loss (P=0.001), pre/intraoperative blood transfusion (P<0.001), duration of operation (P=0.04), combined organ resection (P<0.001), type of resection (P<0.001), and type of reconstruction (P=0.003).
Table 2. Anastomotic leakage site.
Multiple regression analysis revealed that sex (P=0.001), presence of cardiovascular disease (P=0.023), tumor location (P=0.001), and pre/intraoperative transfusion (P=0.001) were independent risk factors for the occurrence of anastomotic leakage. Table 3 lists the odds ratios, 95% confidence intervals, and P-values for the variables that achieved statistical significance after being entered into the multivariate logistic regression model.
Table 3. Multivariate analysis to identify clinicopathological and operative variables that are associated with an anastomotic leakage.
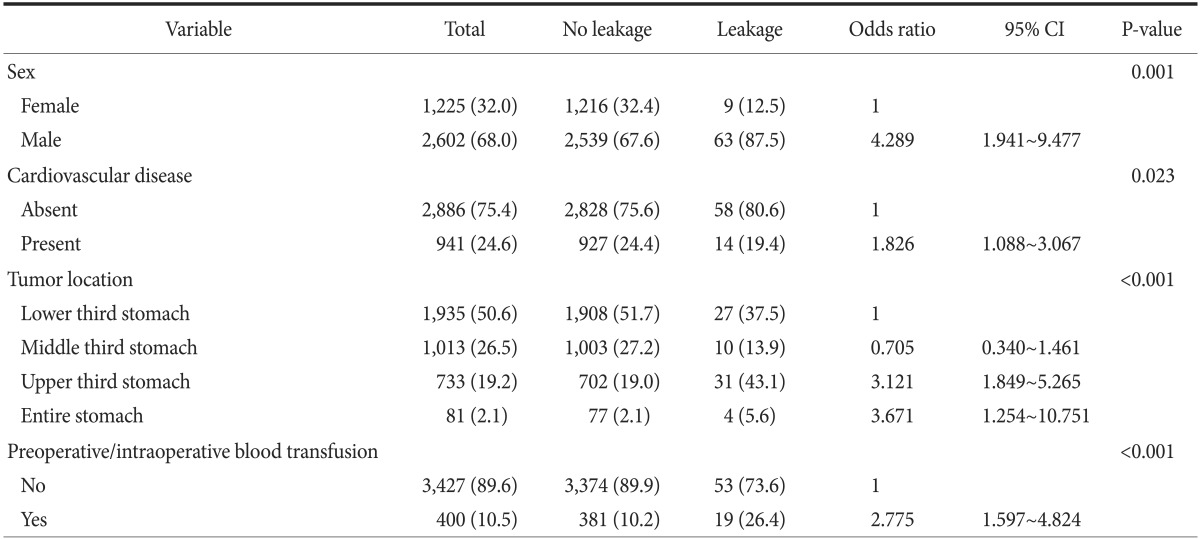
Values are presented as number (%). CI = confidence intervals.
The rate of anastomotic leakage was similar for patients who underwent laparoscopic gastrectomy and those who underwent open gastrectomy (P=0.517).
2. Association between anastomotic leakage and cancer-related survival
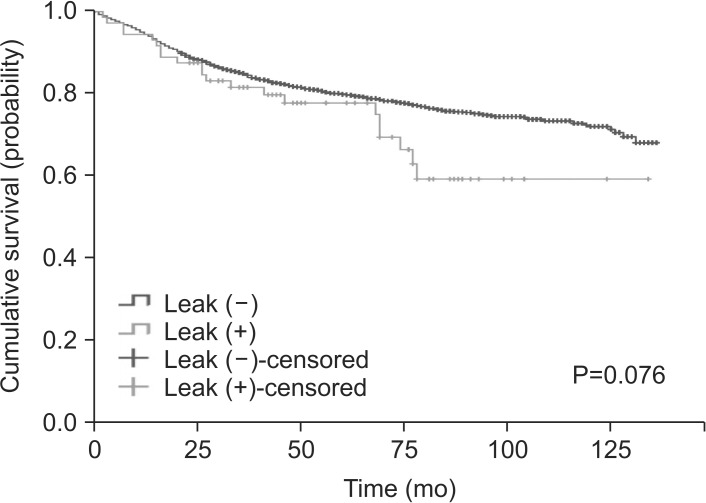
The median follow-up time was 58.5 months (range: 0~136.0 months). In total, 21 patients (29.2%) with anastomotic leakage died, compared with 777 patients (20.7%) without leakage (P=0.082). The patients with and without leakage did not differ significantly in terms of overall mean survival: 97.6 vs. 109.5 months (P=0.076).
Table 4 summarizes the results of the univariate and multivariate survival analyses. The univariate analyses showed that the presence of anastomotic leakage was not associated significantly with cancer-related death (P=0.624). Multivariable analyses revealed that an age ≥60 years, Charlson comorbidity score ≥3, tumor location in the upper third of the stomach, preoperative leukocytosis, preoperative hypoalbuminemia, proximal or distal margin involvement, T stage, and M stage were independent prognostic factors for gastric cancer-related death
Table 4. Univariate and multivariate analyses to identify clinicopathological and operative variables that are associated with overall survival.
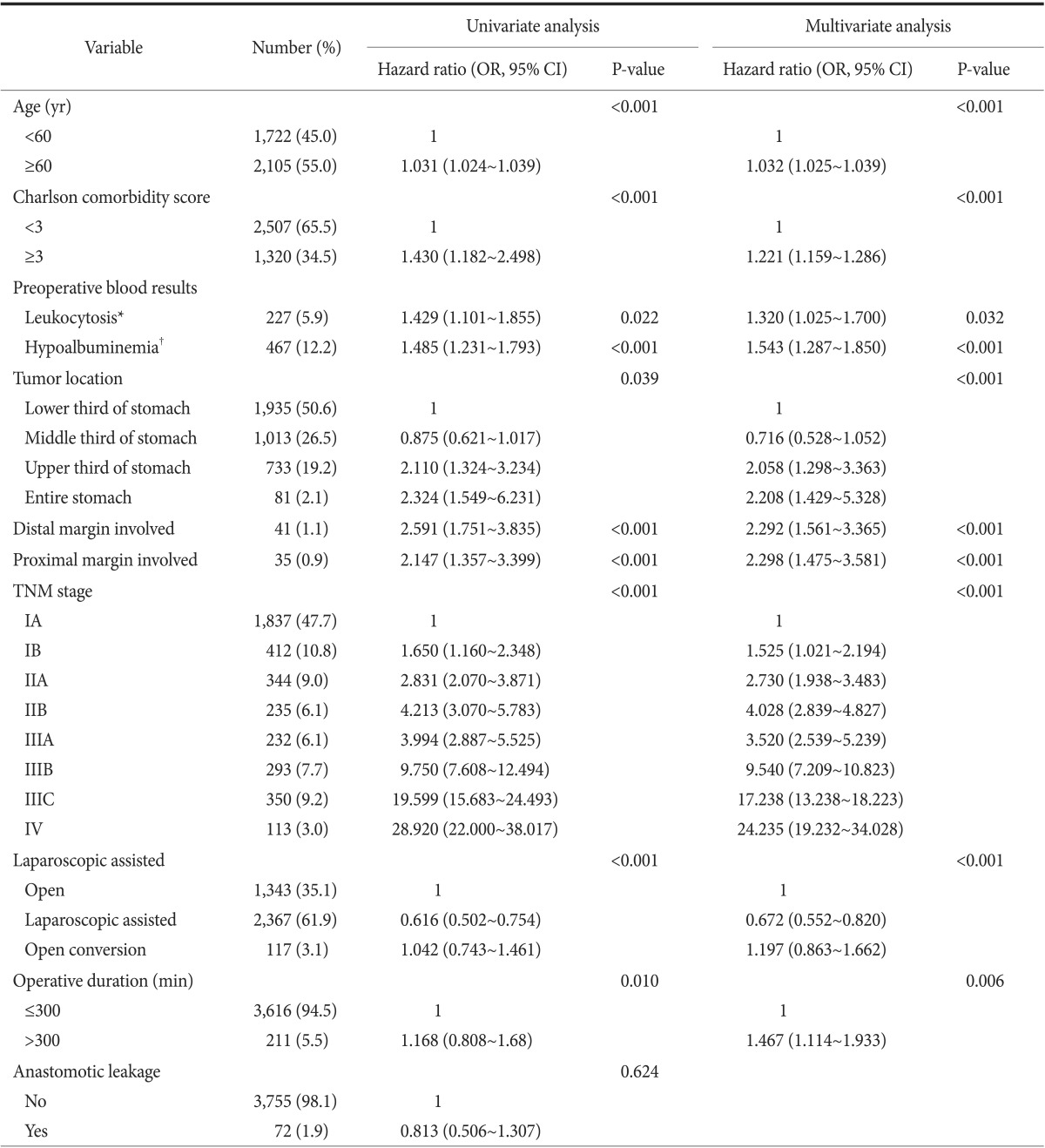
Values are presented as number (%). Tumor stages are based on the TNM classification system from the 7th edition of the Union for International Cancer Control/American Joint Committee on Cancer. Univariate P-values were obtained by Mann-Whitney U-test, the chi-squared test or the Kruskal-Wallis test. OR = odds ratio; CI = confidence interval. *Leukocytosis is defined as white blood cell >10,000/µl. †Hypoalbuminemia is defined as serum albumin <3.5 g/dl.
Discussion
The overall anastomotic leakage rate of 1.88% observed in this study is similar to other reported anastomotic leakage rates ranging from 1.0% to 4.2%.5,10,14,15,16,17
The present study revealed that male sex was a risk factor for anastomotic leakage: men were 4.2 times more likely to develop anastomotic leakage than women (P=0.001). Several studies on colorectal surgery have also shown that men have higher rates of anastomotic leakage than women.18,19,20 Similarly, the study by Kim et al.15 on stomach surgery found that men were more likely to have anastomotic leakage. However, several other studies on anastomotic leakage after gastrectomy have failed to demonstrate statistically significant associations between sex and anastomotic leakage.7,12,13 The reason for this disparity between Kim et al.15's study and our study compared to others is unclear. While it is not apparent why men may be more susceptible to anastomotic leakage, the study by Kunisaki et al.21 suggests that it could relate to the tendency of men to have large visceral fat areas (VFAs). They found that large VFAs are associated with intraoperative and postoperative complications in laparoscopic-assisted distal gastrectomy for gastric cancer. We could not assess this association in our study because the VFAs of our patients were not recorded. Although BMI is related to VFA, we did not find that BMI was associated significantly with anastomotic leakage (P=0.434).
The present study also showed that preoperative or intraoperative transfusion was a risk factor for anastomotic leakage. The decision to provide intraoperative blood transfusion is largely determined by preexisting anemia and by the volume of blood lost during the operation. An association between perioperative transfusion and anastomotic leakage has not been reported previously, although several studies have shown that intraoperative blood loss increases the risk of peritoneal recurrence after curative resection for gastric cancer22 and decreases the long-term survival of patients who undergo surgery for colon cancer.23 Several studies have also revealed that perioperative transfusion is associated with a poor cancer prognosis.24,25,26 The reason for the association between perioperative transfusion and anastomotic leakage that we observed is not clear, but it is likely related to the high intraoperative blood loss in these patients. For the 400 patients who underwent a transfusion in the perioperative period, the average intraoperative blood loss was 262±13.11 ml, whereas that of the remaining 3,427 patients was 110±1.88 ml. This difference was statistically significant (P=0.001). While this difference did not remain significant on multivariate analysis, perioperative transfusion was associated significantly with anastomosis leakage on multivariate analysis (P<0.001): the patients who received a blood transfusion in the perioperative period had a 2.775-fold higher risk of anastomotic leakage than the un-transfused patients. Several studies report that transfusion causes a variety of hematological or immunological complications.25,27 However, it seems unlikely that the association between perioperative transfusion and anastomotic leakage was due to these transfusion-related complications.
The present study also revealed that tumor location in the upper third of the stomach was a risk factor for anastomotic leakage. This may reflect the fact that esophagojejunostomy leakage was the most common leakage in the study; it accounted for 24.1% of the leakages. When the tumor is located in the upper third of the stomach, resection of the tumor must be followed by esophagojejunostomy, regardless of whether open surgery or a laparoscopic procedure is being performed. Several studies have shown that esophagojejunostomy is a technically difficult and complex procedure and new techniques to prevent esophagojejunostomy leakage have been suggested.17,28,29,30,31 Thus, the association between anastomotic leakage and tumor location probably relates to the difficulty of the esophagojejunostomy procedure.
Several investigators have discussed how to prevent and manage esophagojejunostomy leakage.32,33,34
In our multivariate analysis, the presence of cardiovascular disease (hypertension or coronary artery disease) remained as an independent risk factor for anastomotic leakage (odds ratio 1.826, P=0.023). Similarly, Jeong et al.35 reported that patients with heart or liver disease have higher morbidity rates after gastric surgery than patients without these comorbidities. Kim et al.36 also reported that comorbidity has a negative impact on the surgical outcomes of laparoscopy-assisted distal gastrectomy. In our study, although we assessed the association between anastomotic leakage and several comorbidities and the Charlson comorbidity index, none showed a statistically significant association except for cardiovascular disease. This association may reflect the importance of adequate microcirculation to the anastomotic site for healing and the fact that patients with risk factors such as cardiovascular disease have insufficient microcirculation.37
Several other reported risk factors for anastomotic leakage include prolonged operating time, pulmonary insufficiency, chronic renal failure, and procedure type.17,30 However, the present study did not find that these variables were statistically significant risk factors. Similarly, although several studies found that obese patients have higher risks of anastomotic leakage,38,39 a statistically significant association between anastomotic leakage and BMI was not detected in our study. Nevertheless, it cannot be concluded as yet that these variables are not true risk factors of anastomotic leakage.
Nagasako et al.40 reported that anastomotic complications have a negative impact on long-term survival: the hazard ratio for anastomotic complication regarding overall survival was 2.45 (P=0.009). Similarly, Sierzega et al.11 and Yoo et al.12 reported that anastomotic leakage is an independent risk factor for a worse survival rate; they reported hazard ratios of 3.47 and 3.58, respectively. However, in our study, anastomotic leakage was not associated significantly with decreased survival, even when we stratified patients according to their pathological stage and performed multivariate analysis (Fig. 1).
Fig. 1. Kaplan-Meier curve presenting the overall survival rate.
Being a retrospective analysis, the present study has several limitations. First, although most of our data was originally collected at the time of the patient's initial treatment, several characteristics were examined retrospectively at the time of this study. Second, the relatively low anastomotic leakage rate made the statistical analysis very sensitive. Indeed, when we used the random sampling method, different results were obtained. Third, we could not analyze immeasurable factors such as tension on the suture line, blood supply, and technical error that can be associated with anastomotic leakage. Furthermore, the definition of cardiovascular disease in our study was too broad, and intake history of anticoagulant was not analyzed. These limitations could yield biased results.
In conclusion, the identification of risk factors for anastomotic leakage may help to change techniques and preoperative management. Although the exact mechanism by which anastomotic leakage occurs is unknown, it is important to understand the clinicopathological and operative factors that may promote the development of this complication. To determine which variables consistently act as risk factors for anastomosis leakage after resection for gastric cancer, further studies should be conducted.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin North Am. 2013;42:219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Russell MC, Mansfield PF. Surgical approaches to gastric cancer. J Surg Oncol. 2013;107:250–258. doi: 10.1002/jso.23180. [DOI] [PubMed] [Google Scholar]
- 3.Hur H, Xuan Y, Ahn CW, Cho YK, Han SU. Trends and outcomes of minimally invasive surgery for gastric cancer: 750 consecutive cases in seven years at a single center. Am J Surg. 2013;205:45–51. doi: 10.1016/j.amjsurg.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Shehzad K, Mohiuddin K, Nizami S, Sharma H, Khan IM, Memon B, et al. Current status of minimal access surgery for gastric cancer. Surg Oncol. 2007;16:85–98. doi: 10.1016/j.suronc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99:1681–1687. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 6.Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, et al. Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol. 2014;21:891–898. doi: 10.1245/s10434-013-3384-9. [DOI] [PubMed] [Google Scholar]
- 7.Lee MS, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc. 2013;27:2598–2605. doi: 10.1007/s00464-013-2796-8. [DOI] [PubMed] [Google Scholar]
- 8.Xiong JJ, Nunes QM, Huang W, Tan CL, Ke NW, Xie SM, et al. Laparoscopic vs open total gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:8114–8132. doi: 10.3748/wjg.v19.i44.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo CH, Chen JH, Wu CW, Lo SS, Hsieh MC, Lui WY. Risk factors and management of intra-abdominal infection after extended radical gastrectomy. Am J Surg. 2008;196:741–745. doi: 10.1016/j.amjsurg.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH Yonsei Gastric Cancer Clinic. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastrointest Surg. 2009;13:239–245. doi: 10.1007/s11605-008-0716-3. [DOI] [PubMed] [Google Scholar]
- 11.Sierzega M, Kolodziejczyk P, Kulig J Polish Gastric Cancer Study Group. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg. 2010;97:1035–1042. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
- 12.Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol. 2011;104:734–740. doi: 10.1002/jso.22045. [DOI] [PubMed] [Google Scholar]
- 13.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–1583. doi: 10.1245/s10434-012-2720-9. [DOI] [PubMed] [Google Scholar]
- 14.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a large-scale korean multicenter study. Ann Surg Oncol. 2008;15:2692–2700. doi: 10.1245/s10434-008-0075-z. [DOI] [PubMed] [Google Scholar]
- 16.Park JM, Jin SH, Lee SR, Kim H, Jung IH, Cho YK, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc. 2008;22:2133–2139. doi: 10.1007/s00464-008-9962-4. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi Y, Fukagawa T, Morita S, Ohashi M, Saka M, Katai H. Identification of risk factors for esophagojejunal anastomotic leakage after gastric surgery. World J Surg. 2012;36:1617–1622. doi: 10.1007/s00268-012-1559-3. [DOI] [PubMed] [Google Scholar]
- 18.Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. Anastomotic leakage after low anterior resection for rectal cancer is different between minimally invasive surgery and open surgery. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001157. [in print] [DOI] [PubMed] [Google Scholar]
- 19.Lipska MA, Bissett IP, Parry BR, Merrie AE. Anastomotic leakage after lower gastrointestinal anastomosis: men are at a higher risk. ANZ J Surg. 2006;76:579–585. doi: 10.1111/j.1445-2197.2006.03780.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertelsen CA, Andreasen AH, Jørgensen T, Harling H Danish Colorectal Cancer Group. Anastomotic leakage after anterior resection for rectal cancer: risk factors. Colorectal Dis. 2010;12:37–43. doi: 10.1111/j.1463-1318.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 21.Kunisaki C, Makino H, Takagawa R, Sato K, Kawamata M, Kanazawa A, et al. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2085–2093. doi: 10.1007/s00464-008-0247-8. [DOI] [PubMed] [Google Scholar]
- 22.Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. 2009;33:1240–1246. doi: 10.1007/s00268-009-9979-4. [DOI] [PubMed] [Google Scholar]
- 23.Mörner ME, Gunnarsson U, Jestin P, Svanfeldt M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg. 2012;255:1126–1128. doi: 10.1097/SLA.0b013e3182512df0. [DOI] [PubMed] [Google Scholar]
- 24.Ross A, Mohammed S, Vanburen G, Silberfein EJ, Artinyan A, Hodges SE, et al. An assessment of the necessity of transfusion during pancreatoduodenectomy. Surgery. 2013;154:504–511. doi: 10.1016/j.surg.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Wu WC, Trivedi A, Friedmann PD, Henderson WG, Smith TS, Poses RM, et al. Association between hospital intraoperative blood transfusion practices for surgical blood loss and hospital surgical mortality rates. Ann Surg. 2012;255:708–714. doi: 10.1097/SLA.0b013e31824a55b9. [DOI] [PubMed] [Google Scholar]
- 26.Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, et al. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion. 1987;27:375–377. doi: 10.1046/j.1537-2995.1987.27587320526.x. [DOI] [PubMed] [Google Scholar]
- 27.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108:759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 28.El Halabi HM, Lawrence W., Jr Clinical results of various reconstructions employed after total gastrectomy. J Surg Oncol. 2008;97:186–192. doi: 10.1002/jso.20928. [DOI] [PubMed] [Google Scholar]
- 29.Hyodo M, Hosoya Y, Hirashima Y, Haruta H, Kurashina K, Saito S, et al. Minimum leakage rate (0.5%) of stapled esophagojejunostomy with sacrifice of a small part of the jejunum after total gastrectomy in 390 consecutive patients. Dig Surg. 2007;24:169–172. doi: 10.1159/000102100. [DOI] [PubMed] [Google Scholar]
- 30.Migita K, Takayama T, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, et al. Risk factors for esophagojejunal anastomotic leakage after elective gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1659–1665. doi: 10.1007/s11605-012-1932-4. [DOI] [PubMed] [Google Scholar]
- 31.Nunobe S, Hiki N, Tanimura S, Kubota T, Kumagai K, Sano T, et al. Three-step esophagojejunal anastomosis with atraumatic anvil insertion technique after laparoscopic total gastrectomy. J Gastrointest Surg. 2011;15:1520–1525. doi: 10.1007/s11605-011-1489-7. [DOI] [PubMed] [Google Scholar]
- 32.Schietroma M, Cecilia EM, Carlei F, Sista F, De Santis G, Piccione F, et al. Prevention of anastomotic leakage after total gastrectomy with perioperative supplemental oxygen administration: a prospective randomized, double-blind, controlled, single-center trial. Ann Surg Oncol. 2013;20:1584–1590. doi: 10.1245/s10434-012-2714-7. [DOI] [PubMed] [Google Scholar]
- 33.Meyer L, Meyer F, Dralle H, Ernst M, Lippert H, Gastinger I East German Study Group for Quality Control in Operative Medicine and Regional Development in Surgery. Insufficiency risk of esophagojejunal anastomosis after total abdominal gastrectomy for gastric carcinoma. Langenbecks Arch Surg. 2005;390:510–516. doi: 10.1007/s00423-005-0575-2. [DOI] [PubMed] [Google Scholar]
- 34.Raimondo D, Sinagra E, Facella T, Rossi F, Messina M, Spada M, et al. Self-expandable metal stent placement for closure of a leak after total gastrectomy for gastric cancer: report on three cases and review of the literature. Case Rep Gastrointest Med. 2014;2014:409283. doi: 10.1155/2014/409283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong SH, Ahn HS, Yoo MW, Cho JJ, Lee HJ, Kim HH, et al. Increased morbidity rates in patients with heart disease or chronic liver disease following radical gastric surgery. J Surg Oncol. 2010;101:200–204. doi: 10.1002/jso.21467. [DOI] [PubMed] [Google Scholar]
- 36.Kim W, Song KY, Lee HJ, Han SU, Hyung WJ, Cho GS. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg. 2008;248:793–799. doi: 10.1097/SLA.0b013e3181887516. [DOI] [PubMed] [Google Scholar]
- 37.Fawcett A, Shembekar M, Church JS, Vashisht R, Springall RG, Nott DM. Smoking, hypertension, and colonic anastomotic healing; a combined clinical and histopathological study. Gut. 1996;38:714–718. doi: 10.1136/gut.38.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pata G, Solaini L, Roncali S, Pasini M, Ragni F. Impact of obesity on early surgical and oncologic outcomes after total gastrectomy with "over-D1" lymphadenectomy for gastric cancer. World J Surg. 2013;37:1072–1081. doi: 10.1007/s00268-013-1942-8. [DOI] [PubMed] [Google Scholar]
- 39.Bickenbach KA, Denton B, Gonen M, Brennan MF, Coit DG, Strong VE. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol. 2013;20:780–787. doi: 10.1245/s10434-012-2653-3. [DOI] [PubMed] [Google Scholar]
- 40.Nagasako Y, Satoh S, Isogaki J, Inaba K, Taniguchi K, Uyama I. Impact of anastomotic complications on outcome after laparoscopic gastrectomy for early gastric cancer. Br J Surg. 2012;99:849–854. doi: 10.1002/bjs.8730. [DOI] [PubMed] [Google Scholar]