Abstract
Purpose
The AT-rich interactive domain 1A (ARID1A) gene encodes BRG1-associated factor 250a, a component of the SWItch/Sucrose NonFermentable chromatin remodeling complex, which is considered a tumor suppressor in many tumors. We aimed to investigate the prognostic significance of ARID1A expression in gastric cancers and explore its relationship with clinicopathologic parameters such as mismatch repair protein expression.
Materials and Methods
Four tissue microarrays were constructed from 191 resected specimens obtained at Soonchunhyang University Cheonan Hospital from 2006 to 2008. Nuclear expression of ARID1A was semiquantitatively assessed and binarized into retained and lost expression.
Results
Loss of ARID1A expression was observed in 62 cases (32.5%). This was associated with more frequent vascular invasion (P=0.019) and location in the upper third of the stomach (P=0.001), and trended toward more poorly differentiated subtypes (P=0.054). ARID1A loss was significantly associated with the mismatch repair-deficient phenotype (P=0.003). ARID1A loss showed a statistically significant correlation with loss of MLH1 (P=0.001) but not MSH2 expression (P=1.000). Kaplan-Meier survival analysis showed no statistically significant difference in overall survival; however, patients with retained ARID1A expression tended to have better overall survival than those with loss of ARID1A expression (P=0.053). In both mismatch repair-deficient and mismatch repair-proficient groups, survival analysis showed no differences related to ARID1A expression status.
Conclusions
Our results demonstrated that loss of ARID1A expression is closely associated with the mismatch repair-deficient phenotype, especially in sporadic microsatellite instability-high gastric cancers.
Keywords: ARID1A, Stomach neoplasms, DNA Mismatch repair, Microsatellite instability, Survival
Introduction
Gastric cancer (GC) is estimated to be the fourth most common cancer and third leading cause of cancer-related death worldwide.1 Outcomes in advanced-stage patients with GC remain poor. Therefore, the identification of molecular markers associated with prognosis is essential for effective application of therapeutic strategies. GC is a heterogeneous disease at the molecular level, with three different representative pathways involved in carcinogenesis: chromosomal instability, microsatellite instability (MSI), and epigenetic instability. MSI, which refers to genetic or epigenetic alteration of the DNA mismatch repair (MMR) system, is characterized by alterations in the number of repeated nucleotides in the microsatellite regions. Defects in the MMR system result in the exaggerated accumulation of mutations, leading to the development of cancers. MSI accounts for about 18% and 11% of GCs in Western and Eastern countries, respectively.2 MSI-high (MSI-H) GCs are associated with a better good prognosis than MSI-low or microsatellite stable (MSS) GCs.2,3
Among the many candidate target genes in GCs, the AT-rich interactive domain 1A (ARID1A) has recently been suggested to encode a novel prognostic marker,4 namely the BRG1-associated factor 250a, a component of the SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeling complex. ARID1A mutations are frequently observed in many solid tumors5 such as endometriosis-associated ovarian carcinomas (clear cell carcinomas and endometrioid carcinomas),6 renal cell carcinomas,7 urothelial carcinomas,8 breast carcinomas,9 and colorectal carcinomas (CRCs).10 The majority of ARID1A mutations are indels and nonsense mutations leading to the production of truncated proteins that are rapidly degraded. Therefore, ARID1A mutations are closely related to the loss of protein expression.11 Although most studies in various organs revealed that ARID1A functions as a tumor suppressor,4 the prognostic implications of ARID1A mutations or loss of ARID1A expression are still controversial in some cancers such as GCs.
Previous studies suggest that ARID1A alterations are associated with MSI in several types of tumors, including GCs,12 CRCs,10 and endometrial carcinomas.13 Wang et al.14 demonstrated that the somatic mutation rate of ARID1A is higher in MSI-H GCs than in MSS tumors (78% in MSI-H vs. 10% in MSS), with a higher proportion of indel mutations (89%) mostly involving short mononucleotide tracts in MSI-H GCs. In contrast, MSS GCs are mostly associated with single nucleotide variations (59%).14 These results indicate that ARID1A is a driver gene targeted by the MSI pathway. However, a study by Bosse et al.11 showed that loss of ARID1A is associated with sporadic MSI-H endometrial carcinomas with MLH1 promoter hypermethylation, rather than Lynch syndrome in which individuals exhibit germline mutations of MMR genes, which supports a causative role for ARID1A in carcinogenesis in MSI-H tumors
Numerous recent studies, such as The Cancer Genome Atlas (TCGA) project, have reported recurrent mutations and alterations in ARID1A in GCs15; however, their specific roles in gastric carcinogenesis remain elusive. In the current study, we evaluated the expression of ARID1A using immunohistochemistry (IHC) in surgically resected GCs. Additionally, the relationship between ARID1A expression and the expression of the MMR genes MLH1 and MSH2 was examined. The aim of the present study was to investigate the prognostic significance of ARID1A expression in GCs and explore its relationship with clinicopathologic parameters such as MMR protein expression.
Materials and Methods
1. Patients and specimens
We retrospectively analyzed data collected from patients with GCs who underwent radical surgical resection with extended lymph node dissection at Soonchunhyang University Hospital, Cheonan, Korea, from 2006 to 2008. Pathologic diagnosis had been requested in 191 cases. All samples were derived from formalin-fixed paraffin-embedded tissues. Patients' electronic medical records were reviewed for clinicopathologic information such as gender, age, tumor location, TNM stage, tumor depth, lymph node metastasis, tumor differentiation, presence of lymphatic, vascular and perineural invasion, and Lauren and Ming classifications. Patient survival data were obtained by reviewing patient medical records, or from death registry offices. We excluded patients who had undergone neoadjuvant chemotherapy, those with a history of other critical medical problems, or cases for which archival blocks were unavailable. Tumor restaging and histological regrading were performed according to the staging system of the American Joint Committee on Cancer Staging Manual, 7th Edition.
2. Gastric cancer tissue microarray and immunohistochemistry
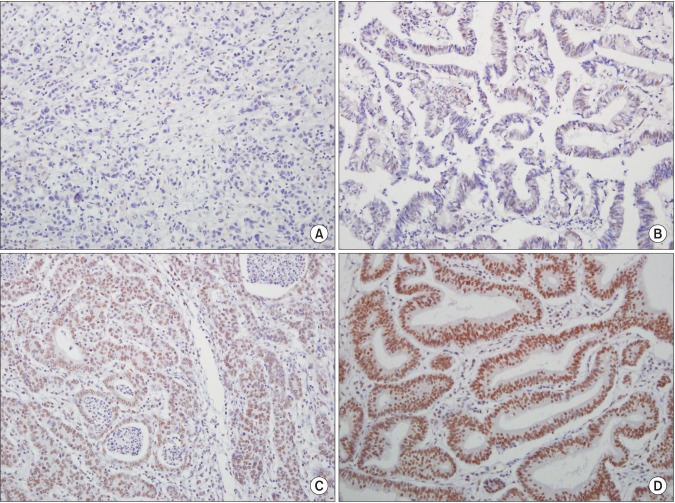
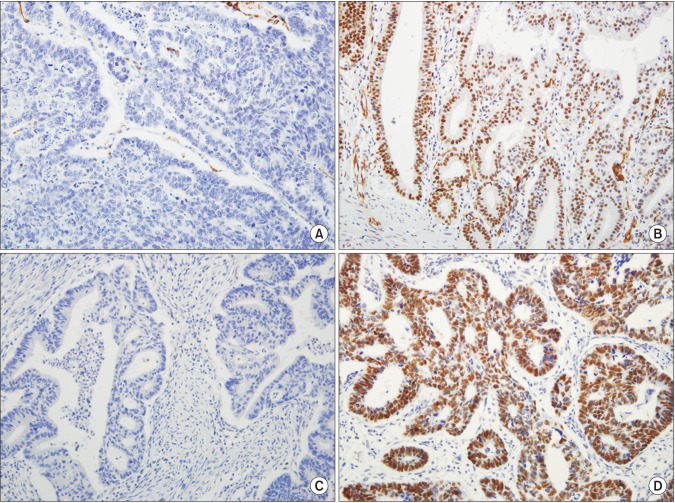
Tissue microarrays (TMAs) were constructed as previously described.16 Briefly, tissue cores (2 mm in diameter) containing representative GC areas were punched from a donor block and transferred to a recipient paraffin block using a trephine apparatus. Four tissue array blocks were prepared from 193 cases. In addition, non-neoplastic gastric mucosal tissue from different locations was included in each microarray block as internal controls. Next, using 4-µm-thick sections from TMAs, immunohistochemical staining for ARID1A, MLH1, and MSH2 was carried out. Immunohistochemical staining for each marker was performed using the Bond Polymer Refine Detection Kit (Leica Microsystems GmbH, Wetzlar, Germany) with the following antibodies: anti-ARID1A (ab171870, 1:50; Abcam, Cambridge, MA, USA), anti-MLH1 (G168728, 1:50; Cell Marque, Rocklin, CA, USA), and anti-MSH2 (FE11, 1:200; Invitrogen, Camarillo, CA, USA). Immunoreactivity for the ARID1A protein was semiquantitatively assessed based on the intensity and extent of nuclear staining. The intensity was initially scored and categorized into four groups: completely negative staining (0), faint positive (1+), intermediately positive (2+), and strong positive (3+) (Fig. 1). If 10% or more of the tumor area in a core showed 2+ or 3+ nuclear intensity, it was considered to exhibit retention of ARID1A expression, and the remainder was regarded to show loss of ARID1A expression. The expression of the MMR genes MLH1 and MSH2 was assessed as lost (negative) and retained (positive) expression (Fig. 2). Expression was considered to have been retained when nuclear staining was observed, regardless of the extent of positive area, and lost when all tumor cells were completely negative in the presence of clear positive staining of internal control cells (i.e., fibroblasts of lymphocytes).
Fig. 1. (A) Representative images of ARID1A immunohistochemical staining (IHC) of gastric cancers (GCs) (×200) exhibiting score 0. (B) Representative images of ARID1A IHC of GCs (×200) exhibiting score 1. (C) Representative images of ARID1A IHC of GCs (×200) exhibiting score 2. (D) Representative images of ARID1A IHC of GCs (×200) exhibiting score 3.
Fig. 2. (A) Representative images of MLH1 immunohistochemical staining (IHC) of gastric cancers (GCs) (×200) with loss of expression. (B) Representative images of MLH1 IHC of GCs (×200) with retained expression. (C) Representative images of MSH2 IHC of GCs (×200) with loss of expression. (D) Representative images of MSH2 IHC of GCs (×200) with retained expression.
3. Statistical analysis
Correlation between clinicopathologic parameters and ARID1A expression was analyzed using Pearson's chi-square test or Fisher's exact test (for cases with an n value of <10). Overall survival (OS) was defined as the duration from the date of surgery to the date of death or the last follow-up visit. Recurrencefree survival (RFS) was defined as the duration in months from the date of surgery to the date of death, tumor recurrence, or last follow-up. The Kaplan-Meier method with the log-rank test was performed to compare OS and RFS between two groups according to ARID1A expression. All statistical analyses were performed using the IBM SPSS software program ver. 20 (IBM Co., Armonk, NY, USA) and statistical significance was determined at P<0.05.
Results
1. Association between ARID1A expression and clinicopathologic features
Loss of ARID1A expression, observed in 62 patients (32.5%) with GC, was associated with more frequent vascular invasion (P=0.019) and a tendency for GCs to be located in the upper third of the stomach (P=0.001). Additionally, GCs with loss of ARID1A expression showed more poorly differentiated subtypes (P=0.054); however, statistical significance was not observed for this tendency. No significant difference was observed with respect to other parameters such as gender, age, stage, or Lauren and Ming classifications. Table 1 summarizes ARID1A protein expression and clinicopathologic characteristics.
Table 1. Association between ARID1A expression and clinicopathologic factors in gastric cancer.
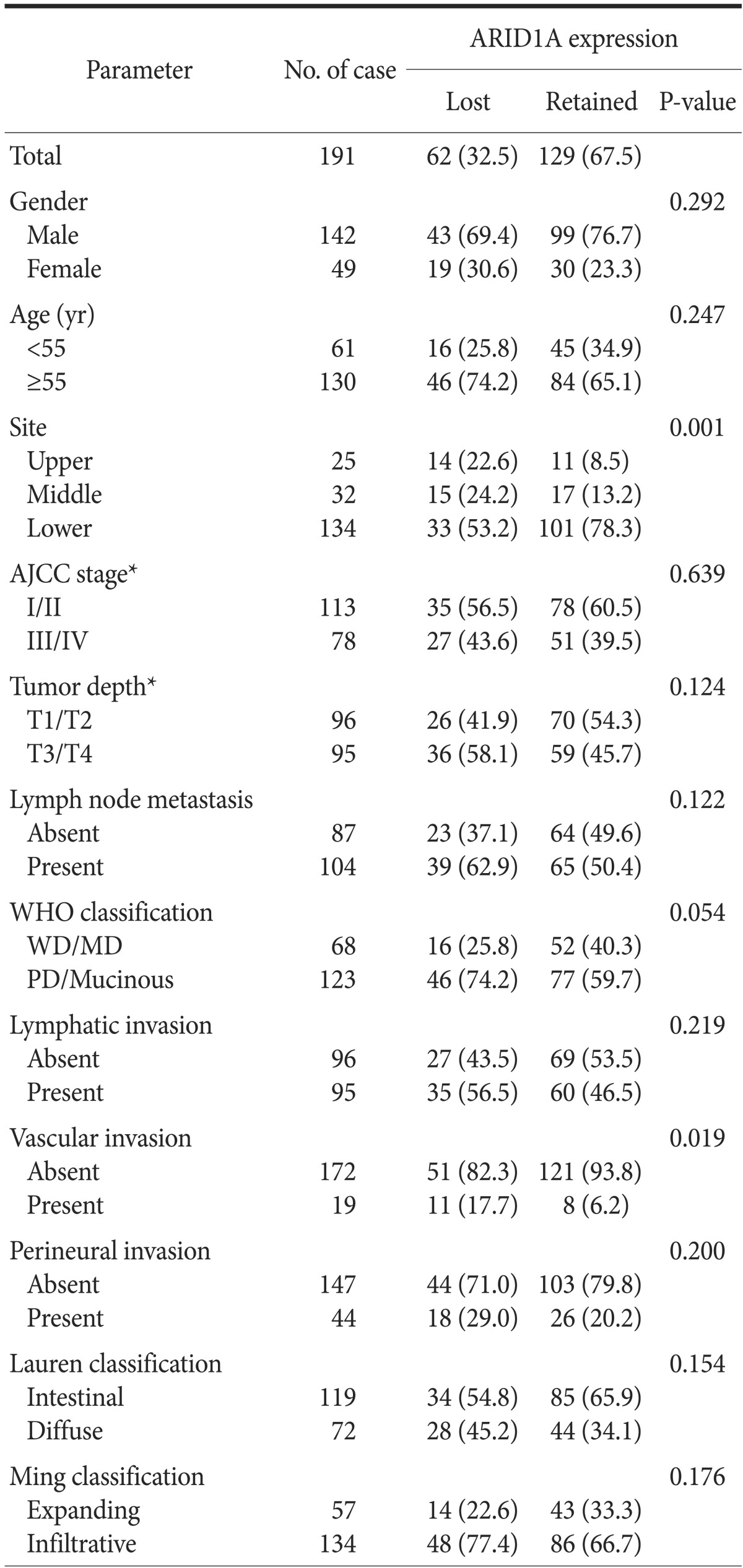
Values are presented as number only or number (%). AJCC = American Joint Committee on Cancer; WHO = World Health Organization; WD = well differentiated adenocarcinoma; MD = moderately differentiated adenocarcinoma; PD = poorly differentiated adenocarcinoma. *Classification according to the 7th edition of the AJCC staging system.
2. ARID1A expression and status of mismatch repair protein expression
Table 2 summarizes the association between ARID1A expression and MMR protein expression status. Among the 186 cases studied, 33 cases (17.7%) and 6 cases (3.2%) showed loss of MLH1 expression or MSH2 expression, respectively. Two cases (1.1%) showed concurrent loss of MLH1 and MSH2 protein expression. The remaining 149 cases retained expression of both proteins. Overall, 37 cases (19.9%) were regarded as being MMR-deficient type. ARID1A loss is significantly associated with MMR-deficient GCs (P=0.003). In particular, in MMR proteins, ARID1A loss was found to show a statistically significant correlation with loss of MLH1 expression (P=0.001) but not with loss of MSH2 expression (P=1.000).
Table 2. Association between ARID1A expression and mismatch repair (MMR) proteins.
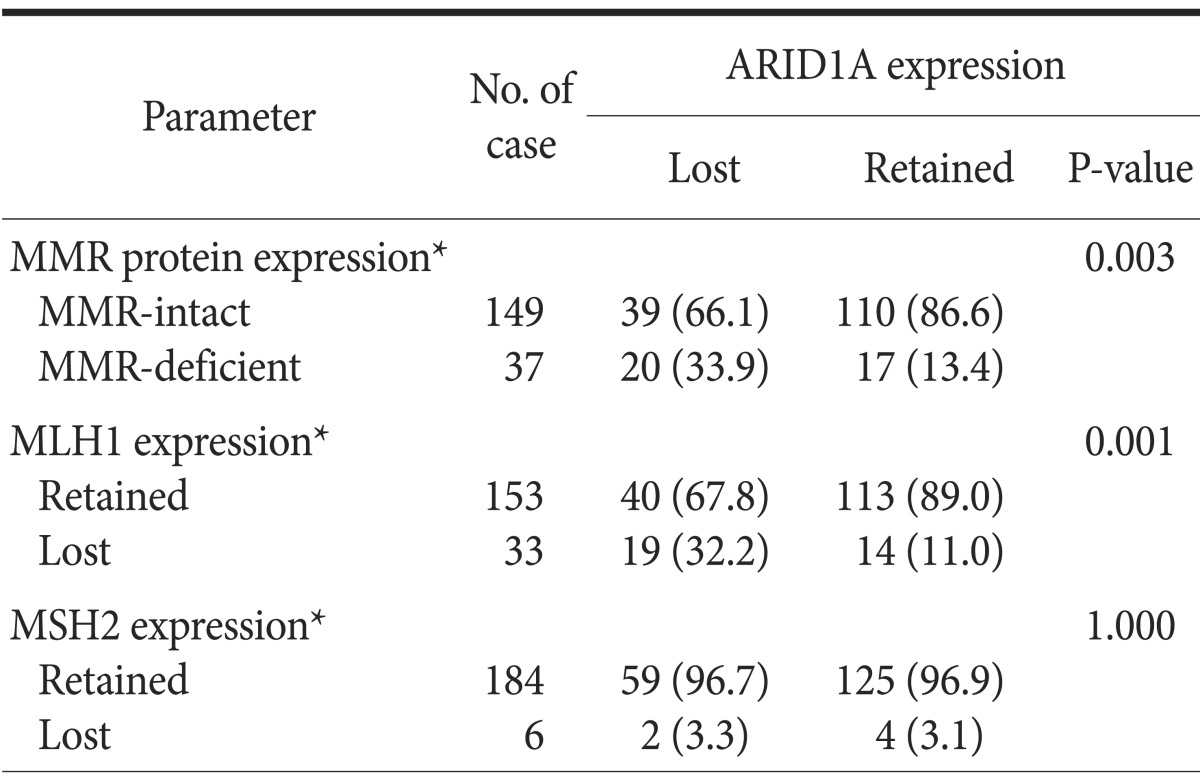
Values are presented as number only or number (%). *Included only for patients with available tissue microarray data.
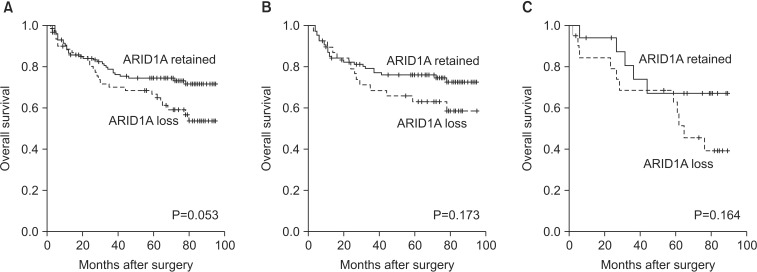
3. Survival analysis
The follow-up period for patients (from surgery to death or the last follow-up) ranged from 4 months to 95 months (median interval: 70 months). During the follow-up period, 58 of the patients (30.4%) died. The median survival of patients with loss of ARID1A expression was 68 months, and the median survival of patients showing retained ARID1A expression was 71 months. There was no statistically significant difference in OS between the two groups; however, patients with retained ARID1A expression showed a tendency toward better OS than those with loss of ARID1A expression (P=0.053). We additionally subdivided cases into MMR-deficient and MMR-proficient groups, in order to exclude the effect of MSI on prognosis and increase the level of homogeneity in each group, before performing survival analysis. In both the MMR-deficient and MMR-proficient groups, the log-rank test for survival analysis failed to show differences related to ARID1A expression status (P=0.229 and P=0.169 for the MMR-deficient and MMR-proficient groups, respectively) (Fig. 3). Additionally, no significant difference was observed in RFS between the two groups (P=0.947). Analysis of RFS, performed after subdividing cases into MMR-proficient and MMR-deficient groups, failed to show differences related to ARID1A expression status (P=0.649 and P=0.622 for the MMRdeficient and MMR-proficient groups, respectively) (Supplementary Fig. 1).
Fig. 3. Kaplan-Meier survival analysis with log-rank test. (A) Overall survival curve of the loss of expression (n=62) vs. retained expression groups (n=129) in all patients. (B) Overall survival curve of the loss of expression (n=39) vs. retained expression groups (n=110) in with mismatch repair (MMR)-deficient patients. (C) Overall survival curve of the loss of expression (n=20) vs. retained expression groups (n=17) in MMR-proficient patients.
Discussion
In the present study, loss of ARID1A expression was observed in 32.6% of patients with GC. This finding is roughly consistent with the results of previous studies, which showed a wide range of values, ranging from 11.0%17 to 30.3%,18 for ARID1A expression in GCs. Recently, it has been reported that the presence of mutations in ARID1A is closely related to immunoreactivity in GCs.11 Moreover, Wang et al.14 revealed that ARID1A mutations were found in 29.4% of GCs. Additionally, TCGA data sets indicate that the frequency of ARID1A gene mutations in GCs is 31.1%.15 However, to date, the expression of ARID1A and its associated clinicopathologic and prognostic implications have rarely been explored in GCs.
In the present study, we performed IHC of MMR proteins as a substitute for MSI testing. Although MSI testing is more widely used for detecting the MMR gene status in patients, IHC is recognized to achieve a similar level of sensitivity and specificity as MSI testing.19 Therefore, IHC may be used as a simple and valuable tool for screening for MMR deficiency in GCs. The immunohistochemical profiles of MMR proteins may be summarized as four phenotypes: MLH1-negative/PMS2-negative, PMS2-negative only, MSH2-negative/MSH6-negative, and MSH6-negative only.20 Changes in PMS2 or MSH6 levels show minor effects on the MSI-H phenotype; the majority of MSI-H GCs are induced by alteration of MLH1 or MSH2. Generally, the loss of MLH1 and MSH2 expression are considered mutually exclusive events; however, concurrent loss of the expression of both proteins has also been reported in rare instances.12 The relatively high incidence of concurrent inactivation of MLH1 and MSH2 observed in the present study may be due to the fact that not all tumor sections were evaluated. Instead, TMAs with a 2-mm diameter core were used to investigate MMR protein expression. Although loss of MMR protein is considered to be mostly homogeneous within a tumor region,12 assessment of the whole section of the tumor area is necessary for more precise evaluation of the MMR status. In addition, the assessment of PMS2 and MSH6 inactivation should aid in the detection of rare MSI-H GCs, and enable more precise evaluation of the MSI phenotype.
We found a significant correlation between loss of ARID1A expression and the MMR-deficient phenotype. This phenomenon has also been observed in various other tumor sites. Bosse et al.11 showed a strong association between ARID1A loss and sporadic MSI-H phenotype endometrial cancers. Additionally, Han et al.21 reported that loss of ARID1A was positively correlated with MSI-H status and loss of MMR protein in GCs. As previously mentioned, Wang et al.14 reported a higher somatic mutation rate in MSI-H phenotype tumors compared with other molecular subtypes of GCs, demonstrating that ARID1A is targeted by MSI. Among the two MMR genes, loss of expression of MLH1, but not MLH2, was significantly associated with ARID1A expression loss, which is in line with results in CRCs.10 However, the biological relevance of this correlation is not clear. Sporadic MSI-H GCs with loss of MLH1 expression, which exhibit MLH1 promoter hypermethylation in association with the CpG island methylator phenotype (CIMP), represent the majority of MSI-H GCs. It has been suggested that ARID1A promoter hypermethylation induced by CIMP may account for the correlation between ARID1A expression loss and MMR deficiency in sporadic MSI-H cancers in other organs such as the endometrium.11 Moreover, the ARID1A gene has been known to harbor CpG islands in its promoter, and ARID1A promoter hypermethylation has also been described in breast carcinoma.22 However, in GCs, promoter hypermethylation of ARID1A has not been reported, and even TCGA data of GCs did not reveal evidence of ARID1A promoter hypermethylation in the 322 GC patients studied.15 In addition, an inactivation mechanism involving promoter hypermethylation does not explain the high occurrence rate of somatic mutation of the ARID1A gene, and its strong correlation with ARID1A loss of expression, reported in previous studied. Several researchers have suggested an alternative mechanism whereby MLH1 promoter hypermethylation occurs due to aberrant epigenetic alterations mediated by a deficient SWI/SNF complex.11 However, to the best of our knowledge, no studies on the correlation between the SWI/SNF complex and MLH1 promoter methylation have been carried out. Further research is required for elucidation of the association between ARID1A expression and the MSI phenotype, especially in sporadic MSI-H GCs.
The current study shows that loss of ARID1A expression is significantly associated with vascular invasion. Moreover, tumors with loss of ARID1A expression tended to exhibit more poorly differentiated histology, suggesting more aggressive behavior. Furthermore, GCs with ARID1A loss were more frequently observed in the upper third of the stomach, which is consistent with other recent findings.12
Studies have yielded controversial results regarding the prognostic role of ARID1A expression in GCs. Some authors suggest that loss of ARID1A expression is associated with poor survival.23 Abe et al.17 reported that loss of ARID1A expression was significantly associated with lower rates of disease-free survival in patients with Epstein-Barr virus-negative (EBV[-])/MLH1-preserved GCs. However, Wang et al.14 described a trend of prolonged, RFS in patients with GCs with altered ARID1A expression. Lee et al.24 failed to show a significant difference in surviv al between the two groups. These differences in prognostic significance may be attributed to various factors such as characteristics of the study groups, sample size, type of antibody, and the assessment system applied. For example, the cohort studied by Wang et al.14 included an exaggerated number of MSI-H GCs. Therefore, the observation of better prognosis in patients with ARID1A expression loss may be attributed to a link between ARID1A alteration and MSI status in the study by Wang et al.14 In our study, loss of ARID1A was associated with a tendency towards shorter OS; however, this correlation was not strong enough to be utilized as a prognostic indicator. On subdivision into MMR-deficient and MMR-proficient groups, no difference was noted in OS between the groups in which ARID1A expression had been lost or retained. It is postulated that the number of cases examined in the present study was not sufficiently high and that the study population consisted of an excessively high proportion of early gastric carcinomas (EGCs) and early stage tumors, which hindered determination of the exact prognostic role of ARID1A in GCs. EGCs and stage I cases accounted for 34.0% (n=65) and 40.3% (n=77) cases, respectively, in the prenset cohort. Therefore, the recruitment of a larger number of patients is needed to clarify the function of the ARID1A gene in gastric carcinogenesis.
In conclusion, our results demonstrate that loss of expression of ARID1A, which occurs in approximately 32% of GCs, is closely associated with the MMR-deficient phenotype, as established by IHC. In addition, the loss of ARID1A expression appears to be closely linked to sporadic MSI-H GCs. Although the difference in survival outcomes between patients with GCs exhibiting loss or retention of ARID1A expression was not statistically significant, the latter group showed a trend toward better OS outcomes
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
Electronic Supplementary Material
This online version of this article (doi: 10.5230/jgc.2015.15.3.201) contains supplementary materials.
Kaplan-Meier survival analysis with log-rank test. (A) Recurrence free survival curve of the loss of expression (n=60) vs. retained expression groups (n=128) in all patients. (B) Recurrence free survival curve of the loss of expression (n=38) vs. retained expression groups (n=109) in mismatch repair (MMR)-deficient patients. (C) Recurrence free survival curve of the loss of expression (n=19) vs. retained expression groups (n=17) in MMR-proficient patients.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Choi YY, Bae JM, An JY, Kwon IG, Cho I, Shin HB, et al. Is microsatellite instability a prognostic marker in gastric cancer? A systematic review with meta-analysis. J Surg Oncol. 2014;110:129–135. doi: 10.1002/jso.23618. [DOI] [PubMed] [Google Scholar]
- 3.Velho S, Fernandes MS, Leite M, Figueiredo C, Seruca R. Causes and consequences of microsatellite instability in gastric carcinogenesis. World J Gastroenterol. 2014;20:16433–16442. doi: 10.3748/wjg.v20.i44.16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu RC, Wang TL, Shih IeM. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther. 2014;15:655–664. doi: 10.4161/cbt.28411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Lee C, Suh JH, Chae JY, Kim HW, Moon KC. Decreased ARID1A expression correlates with poor prognosis of clear cell renal cell carcinoma. Hum Pathol. 2015;46:454–460. doi: 10.1016/j.humpath.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Faraj SF, Chaux A, Gonzalez-Roibon N, Munari E, Ellis C, Driscoll T, et al. ARID1A immunohistochemistry improves outcome prediction in invasive urothelial carcinoma of urinary bladder. Hum Pathol. 2014;45:2233–2239. doi: 10.1016/j.humpath.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Liu C, Zhao Z. ARID1A: a potential prognostic factor for breast cancer. Tumour Biol. 2014;35:4813–4819. doi: 10.1007/s13277-014-1632-7. [DOI] [PubMed] [Google Scholar]
- 10.Ye J, Zhou Y, Weiser MR, Gönen M, Zhang L, Samdani T, et al. Immunohistochemical detection of ARID1A in colorectal carcinoma: loss of staining is associated with sporadic microsatellite unstable tumors with medullary histology and high TNM stage. Hum Pathol. 2014;45:2430–2436. doi: 10.1016/j.humpath.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Bosse T, ter Haar NT, Seeber LM, v Diest PJ, Hes FJ, Vasen HF, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod Pathol. 2013;26:1525–1535. doi: 10.1038/modpathol.2013.96. [DOI] [PubMed] [Google Scholar]
- 12.Inada R, Sekine S, Taniguchi H, Tsuda H, Katai H, Fujiwara T, et al. ARID1A expression in gastric adenocarcinoma: clinicopathological significance and correlation with DNA mismatch repair status. World J Gastroenterol. 2015;21:2159–2168. doi: 10.3748/wjg.v21.i7.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allo G, Bernardini MQ, Wu RC, Shih IM, Kalloger S, Pollett A, et al. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high-grade endometrial carcinomas. Mod Pathol. 2014;27:255–261. doi: 10.1038/modpathol.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KJ, Lee KS, Cho HJ, Kim YH, Yang HK, Kim WH, et al. Prognostic implications of tumor-infiltrating FoxP3+ regulatory T cells and CD8+ cytotoxic T cells in microsatellite-unstable gastric cancers. Hum Pathol. 2014;45:285–293. doi: 10.1016/j.humpath.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Abe H, Maeda D, Hino R, Otake Y, Isogai M, Ushiku AS, et al. ARID1A expression loss in gastric cancer: pathway-dependent roles with and without Epstein-Barr virus infection and microsatellite instability. Virchows Arch. 2012;461:367–377. doi: 10.1007/s00428-012-1303-2. [DOI] [PubMed] [Google Scholar]
- 18.Yan HB, Wang XF, Zhang Q, Tang ZQ, Jiang YH, Fan HZ, et al. Reduced expression of the chromatin remodeling gene ARID1A enhances gastric cancer cell migration and invasion via downregulation of E-cadherin transcription. Carcinogenesis. 2014;35:867–876. doi: 10.1093/carcin/bgt398. [DOI] [PubMed] [Google Scholar]
- 19.Shia J, Ellis NA, Klimstra DS. The utility of immunohistochemical detection of DNA mismatch repair gene proteins. Virchows Arch. 2004;445:431–441. doi: 10.1007/s00428-004-1090-5. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol. 2014;20:4230–4243. doi: 10.3748/wjg.v20.i15.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han N, Kim MA, Lee HS, Kim WH. Loss of ARID1A expression is related to gastric cancer progression, epstein-barr virus infection, and mismatch repair deficiency. Appl Immunohistochem Mol Morphol. 2015 doi: 10.1097/PAI.0000000000000199. [In print] [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Sun Q, Shan M, Niu M, Liu T, Xia B, et al. Promoter hypermethylation of ARID1A gene is responsible for its low mRNA expression in many invasive breast cancers. PLoS One. 2013;8:e53931. doi: 10.1371/journal.pone.0053931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang DD, Chen YB, Pan K, Wang W, Chen SP, Chen JG, et al. Decreased expression of the ARID1A gene is associated with poor prognosis in primary gastric cancer. PLoS One. 2012;7:e40364. doi: 10.1371/journal.pone.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SY, Kim DW, Lee HS, Ihn MH, Oh HK, Park do J, et al. Loss of AT-rich interactive domain 1A expression in gastrointestinal malignancies. Oncology. 2015;88:234–240. doi: 10.1159/000369140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier survival analysis with log-rank test. (A) Recurrence free survival curve of the loss of expression (n=60) vs. retained expression groups (n=128) in all patients. (B) Recurrence free survival curve of the loss of expression (n=38) vs. retained expression groups (n=109) in mismatch repair (MMR)-deficient patients. (C) Recurrence free survival curve of the loss of expression (n=19) vs. retained expression groups (n=17) in MMR-proficient patients.