Abstract
A 78-year-old man underwent laparoscopy-assisted total gastrectomy for gastric cancer (pT3N0M0). Multiple port sites were used, including a 10 mm port for a videoscope at the umbilical point and three other working ports. During the six-month follow-up evaluation, a 2 cm enhancing mass confined to the muscle layer was found 12 mm from the right lower quadrant port site, suggesting a metastatic or desmoid tumor. Follow-up computed tomography imaging two months later showed that the mass had increased in size to 3.5 cm. We confirmed that there was no intra-abdominal metastasis by diagnostic laparoscopy and then performed a wide resection of the recurrent mass. The histologic findings revealed poorly differentiated adenocarcinoma, suggesting a metastatic mass from the stomach cancer. The postoperative course was uneventful, and the patient completed adjuvant chemotherapy with TS-1 (tegafur, gimeracil, and oteracil potassium). There was no evidence of tumor recurrence during the 50-month follow-up period.
Keywords: Laparoscopy, Recurrence, Metastasectomy
Introduction
Laparoscopic gastrectomy is widely used for the treatment of gastric cancer and has comparable survival and several early surgical benefits compared with open gastrectomy.1,2 In addition, its application has expanded from early gastric cancer to advanced gastric cancer.3,4 With the increased use of laparoscopic gastrectomy, it appears that port-site recurrence (PSR) may be a new type of recurrence pattern that is not observed after open surgery.
There have been reports of PSR from various types of malignancies. However, most PSRs are accompanied by other factors that make the lesions unresectable, such as combined metastatic lesions or carcinomatosis, and the finding of a single PSR suitable for curative resection is rare. Herein, we report the case of an advanced gastric cancer patient who survived more than 50 months without tumor recurrence following PSR metastasectomy.
Case Report
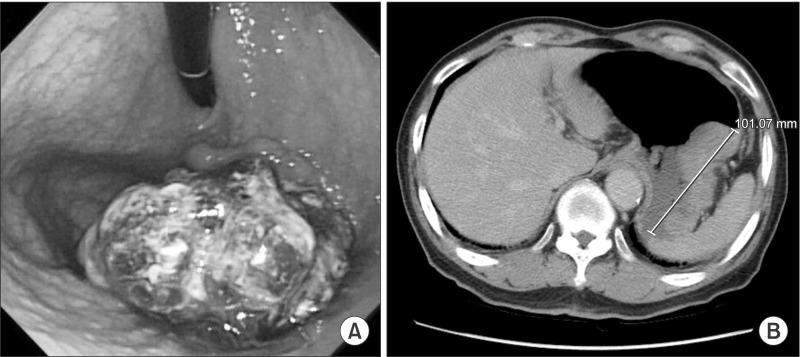
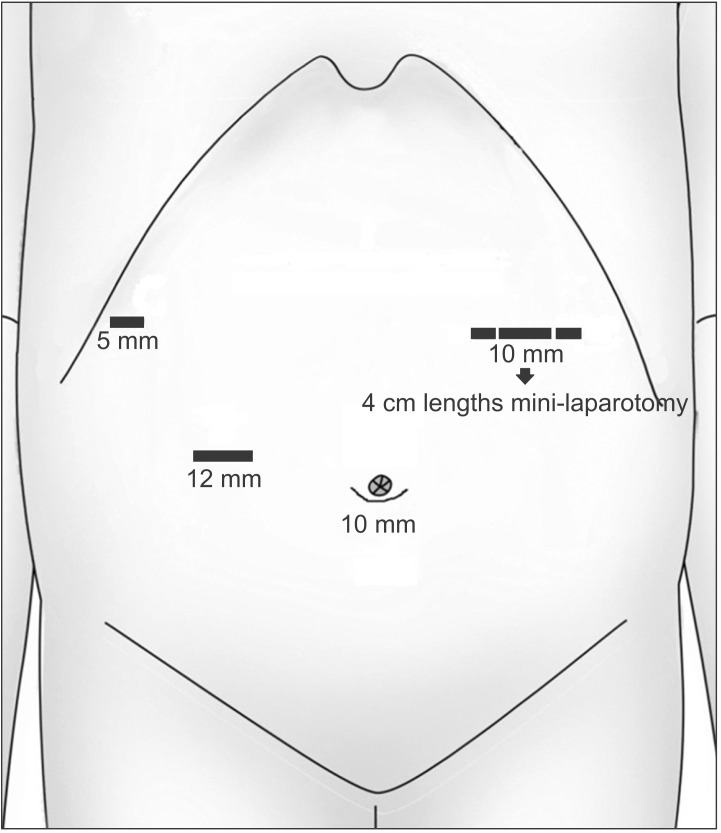
A 78-year-old man presented to the hospital with a history of epigastric pain for six months. Esophagogastroduodenoscopy and computed tomography (CT) showed a 9 cm Borrmann-I gastric carcinoma at the fundus without distant metastasis, which indicated clinical stage T3N0M0 (Fig. 1). The expression of tumor markers (carcinoembryonic antigen and carbohydrate antigen 19-9) was within normal limits. The patient was treated with laparoscopy-assisted total gastrectomy (LATG). A 10 mm umbilical port was inserted to explore the intra-abdominal status. There was no evidence of peritoneal seeding or serosal invasion of the primary tumor. Additional ports (12 mm×1, 10 mm×1, 5 mm×1) were inserted as shown in Fig. 2. The LATG procedures were similar to previously reported methods.5 D2 lymph node dissection with splenectomy was carried out. For the esophagojejunostomy (EJ) procedure, an OrVil™ (Covidien, Mansfield, MA, USA) anvil was used. The EJ was performed with a 25 mm circular stapler through the left upper quadrant port site extension. The pancreas was preserved and a combined splenectomy was performed for the complete resection of the No. 10 lymph nodes. The final pathologic report revealed Stage IIA (T3N0M0) gastric cancer. The tumor histologic type was poorly differentiated (PD) adenocarcinoma.
Fig. 1. (A) Gastrofiberscopy showing a large fungating mass at the fundus. (B) Computed tomography shows a 10 cm fungating mass without definite lymph node enlargement.
Fig. 2. Port placements for the laparoscopy-assisted total gastrectomy.
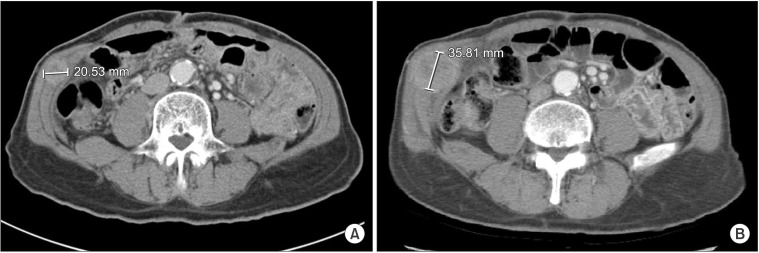
Six months after the surgery, the patient complained of a palpable mass located in the right lower quadrant port insertion site. The CT scan showed a 2 cm enhancing mass confined to the abdominal muscle layer and located in the right lateral abdominal wall (Fig. 3A). The mass was located at the level of the external and internal oblique muscles above the transversus abdominis. Because it was difficult to differentiate between an abdominal wall metastasis and a desmoid tumor, we decided to observe the patient for two more months and perform follow-up CT scanning with the patient's informed consent.
Fig. 3. (A) The follow-up computed tomography 6 months after surgery showed a 2 cm abdominal wall mass at the right lower quadrant port site. (B) The mass increased in size to nearly 3.5 cm during the 2-month observation period.
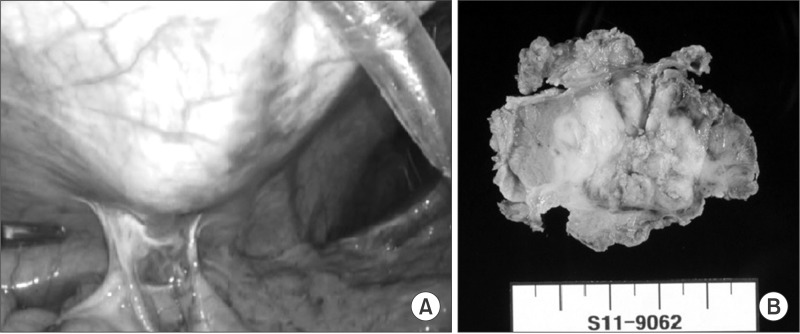
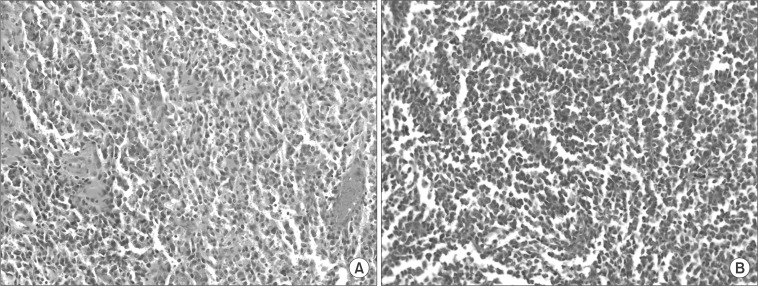
The follow-up CT showed that the mass had increased in size to 3.5 cm (Fig. 3B). A metastasectomy was planned based on the hypothesis that the tumor was an abdominal wall metastasis. A diagnostic laparoscopy confirmed there were no peritoneal seeding lesions and no tumor invasion to the peritoneal surface (Fig. 4A). The metastatic lesion was resected with a proper safety margin. The abdominal wall was reconstructed using synthetic mesh. The gross metastasectomy specimen was a 4 cm firm and whitish mass surrounded by muscle, and there was no continuity found with the peritoneum (Fig. 4B). The histologic type of the tumor was PD adenocarcinoma, which was the same type as the primary gastric tumor (Fig. 5). The postoperative course was uneventful. The patient received adjuvant chemotherapy with TS-1 (tegafur, gimeracil, and oteracil potassium; Taiho Pharmaceutical Co. Ltd., Tokyo, Japan) after the second operation and experienced no cancer recurrence during the 50-month follow-up period.
Fig. 4. (A) Diagnostic laparoscopy revealed no peritoneal metastasis or peritoneal invasion of the recurrent mass. (B) The metastasectomy specimen shows a 3.5×3.0 cm poorly defined white soft mass without invasion of the peritoneum.
Fig. 5. The histologic findings of the primary tumor (A) and abdominal wall tumor (B) were similar and suggested poorly differentiated adenocarcinoma in both samples (A, B: H&E, ×200).
Discussion
The development of PSR is caused by several mechanisms. The main mechanism is direct seeding of the tumor cells rather than systemic circulation.6 In addition, carbon dioxide (CO2) has a stimulating effect on tumor growth. Furthermore, gas leakage through and around the port site is called the 'chimney effect' and is suggested to cause PSR.7
There have been several cases of PSR reported since the first PSR case, involving rapid abdominal wall metastasis due to ovarian cancer in 1978.6,7,8,9,10,11 However, most PSRs develop following exploratory laparoscopy for carcinomatosis patients.8,9,10,11,12 The incidence of PSR after laparoscopic gastrectomy ranges from 0.4% to 11.0%.6,13,14,15 However, the development of PSR following curative resection is rare. Two case reports described subcutaneous metastasis following curative resection for gastric cancer. One case developed in a patient with early gastric cancer (T1bN0M0) eight months after laparoscopic distal gastrectomy.13 Although the abdominal wall mass was resected, the patient developed peritoneal metastasis and died one year after the second operation. The other case developed in an advanced gastric cancer patient with serosal invasion and extensive lymph node metastasis (pT4aN3M0) 12 months after LATG.16 The prognosis was not reported in this case.
Most PSRs are accompanied by other metastatic lesions, especially in cases with carcinomatosis. The patient in the present case had a single PSR and experienced long-term survival following metastasectomy, which is extremely rare.
There are two important issues in this case report. First, it is a rare case of gastric cancer without serosal invasion or peritoneal dissemination. Although there were no metastatic lymph nodes, lymphatic invasion existed in this case. Therefore, cancer cells from the lymphatic vessel could be a possible cause of the PSR. Second, this patient survived for more than 50 months following metastasectomy. Thus, the present case was unique because most PSRs are accompanied by carcinomatosis and have poor prognoses.
In the present case, we did not perform positron emission tomography (PET)-CT or ultrasound-guided core needle biopsy when the recurrent mass was initially found, for two reasons. First, the newly developed lesion could be a benign condition. Second, it would be important to evaluate for the presence of peritoneal metastasis if the abdominal wall lesion was a metastatic one. However, because no abnormality was seen on the CT scan, PET-CT might not effectively detect peritoneal metastasis in such an early period. For these reasons, we decided to perform a follow-up CT scan in two months, after thorough discussion regarding the situation with the patient and his family.
The use of laparoscopic gastrectomy for advanced gastric cancer is increasing and there could be more cases with this type of metastasis. Although the exact mechanism of PSR is not fully understood, efforts to prevent tumor cell dissemination are essential. It is important to handle the stomach carefully, and lymphatic sealing is also important to prevent PSR. In this case, we demonstrated that a solitary PSR without other factors rendering it inoperable can be cured with metastasectomy and adjuvant chemotherapy.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627–633. doi: 10.1200/JCO.2013.48.8551. [DOI] [PubMed] [Google Scholar]
- 2.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 3.Fang C, Hua J, Li J, Zhen J, Wang F, Zhao Q, et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am J Surg. 2014;208:391–396. doi: 10.1016/j.amjsurg.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AC, Kojima K, Inokuchi M, Kato K, Sugihara K. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg Endosc. 2013;27:462–470. doi: 10.1007/s00464-012-2459-1. [DOI] [PubMed] [Google Scholar]
- 5.Jung YJ, Kim DJ, Lee JH, Kim W. Safety of intracorporeal circular stapling esophagojejunostomy using trans-orally inserted anvil (OrVil) following laparoscopic total or proximal gastrectomy: comparison with extracorporeal anastomosis. World J Surg Oncol. 2013;11:209. doi: 10.1186/1477-7819-11-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaeff B, Paolucci V, Thomopoulos J. Port site recurrences after laparoscopic surgery. A review. Dig Surg. 1998;15:124–134. doi: 10.1159/000018605. [DOI] [PubMed] [Google Scholar]
- 7.Kazemier G, Bonjer HJ, Berends FJ, Lange JF. Port site metastases after laparoscopic colorectal surgery for cure of malignancy. Br J Surg. 1995;82:1141–1142. doi: 10.1002/bjs.1800820850. [DOI] [PubMed] [Google Scholar]
- 8.Cava A, Román J, González Quintela A, Martín F, Aramburo P. Subcutaneous metastasis following laparoscopy in gastric adenocarcinoma. Eur J Surg Oncol. 1990;16:63–67. [PubMed] [Google Scholar]
- 9.Nduka CC, Monson JR, Menzies-Gow N, Darzi A. Abdominal wall metastases following laparoscopy. Br J Surg. 1994;81:648–652. doi: 10.1002/bjs.1800810506. [DOI] [PubMed] [Google Scholar]
- 10.Rangarajan M. Multiple port-site and subcutaneous metastases following palliative laparoscopic surgery for advanced gastric cancer. Ann Acad Med Singapore. 2007;36:875–876. [PubMed] [Google Scholar]
- 11.Döbrönte Z, Wittmann T, Karácsony G. Rapid development of malignant metastases in the abdominal wall after laparoscopy. Endoscopy. 1978;10:127–130. doi: 10.1055/s-0028-1098280. [DOI] [PubMed] [Google Scholar]
- 12.Stockdale AD, Pocock TJ. Abdominal wall metastasis following laparoscopy: a case report. Eur J Surg Oncol. 1985;11:373–375. [PubMed] [Google Scholar]
- 13.Sakurai K, Tanaka H, Lee T, Muguruma K, Kubo N, Yashiro M, et al. Port site metastasis after laparoscopic-assisted distal gastrectomy (LADG) Int Surg. 2013;98:363–366. doi: 10.9738/INTSURG-D-13-00049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc. 2009;23:1252–1258. doi: 10.1007/s00464-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 15.Jeong SH, Lee YJ, Park ST, Choi SK, Hong SC, Jung EJ, et al. Risk of recurrence after laparoscopy-assisted radical gastrectomy for gastric cancer performed by a single surgeon. Surg Endosc. 2011;25:872–878. doi: 10.1007/s00464-010-1286-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Ha WS, Park ST, Choi SK, Hong SC. Port-site recurrence after laparoscopy-assisted gastrectomy: report of the first case. J Laparoendosc Adv Surg Tech A. 2007;17:455–457. doi: 10.1089/lap.2006.0216. [DOI] [PubMed] [Google Scholar]