Abstract
Antibiotic dosing in septic shock patients poses a challenge for clinicians due to the pharmacokinetic (PK) variability seen in this patient population. Piperacillin-tazobactam is often used for empirical treatment, and initial appropriate dosing is crucial for reducing mortality. Accordingly, we determined the pharmacokinetic profile of piperacillin (4 g) every 8 h, during the third consecutive dosing interval, in 15 patients treated empirically for septic shock. We developed a population pharmacokinetic model to assess empirical dosing and to simulate alternative dosing regimens and modes of administration. Time above the MIC (T>MIC) predicted for each patient was evaluated against clinical breakpoint MIC for Pseudomonas aeruginosa (16 mg/liter). Pharmacokinetic-pharmacodynamic (PK/PD) targets evaluated were 50% fT>4×MIC and 100% fT>MIC. A population PK model was developed using NONMEM, and data were best described by a two-compartment model. Central and intercompartmental clearances were 3.6 liters/h (relative standard error [RSE], 15.7%) and 6.58 liters/h (RSE, 16.4%), respectively, and central and peripheral volumes were 7.3 liters (RSE, 11.8%) and 3.9 liters (RSE, 9.7%), respectively. Piperacillin plasma concentrations varied considerably between patients and were associated with levels of plasma creatinine. Patients with impaired renal function were more likely to achieve predefined PK/PD targets than were patients with preserved or augmented renal function. Simulations of alternative dosing regimens showed that frequent intermittent bolus dosing as well as dosing by extended and continuous infusion increases the probability of attaining therapeutic plasma concentrations. For septic shock patients with preserved or augmented renal function, dose increment or prolonged infusion of the drug needs to be considered. (This study has been registered at ClinicalTrials.gov under registration no. NCT02306928.)
INTRODUCTION
Appropriate early antimicrobial therapy is of utmost importance for reducing mortality in critically ill patients with sepsis and septic shock (1–3). Pathophysiological changes associated with the septic process, such as changes in volume of distribution (V), drug clearance (CL), decrease in plasma protein concentration, and organ dysfunction, lead to pharmacokinetic (PK) changes that may alter the efficacy of the antimicrobial given (4). As a consequence, antibiotic plasma concentrations are variable and hard to predict in these patients, which makes optimal antibiotic exposure a challenge, especially in the early phase of treatment (5, 6). In septic shock patients, appropriate dosing is of greater importance, as effective antimicrobial therapy within the first hour of documented hypotension is associated with increased survival to hospital discharge (7).
Piperacillin-tazobactam is a β-lactam–β-lactamase inhibitor combination with a broad spectrum of antibacterial activity (8), frequently used for empirical treatment in critically ill patients. It is an antibiotic with time-dependent antibacterial activity in that the activity is related to the time for which the free, unbound drug concentration is maintained above the MIC (fT>MIC). Maximizing fT>MIC both increases the therapeutic impact and reduces the risk of drug resistance development (9). The exact threshold for antibiotic exposure and clinical effect is still controversial. Retrospective analysis of clinical data shows that fT>MIC of 100% is associated with microbiological and clinical cures (10, 11), whereas plasma concentrations of 4 to 5 times the MIC throughout the dosing interval are needed for maximal bactericidal activity in critically ill patients (12).
In recent years, therapeutic drug monitoring (TDM) has been introduced as a tool to guide antimicrobial treatment in critically ill patients. TDM and subsequent dose adjustments to meet patient PK alterations provide a more individual approach to antimicrobial therapy and are increasingly used in the intensive care unit (ICU) (13). Furthermore, extended or continuous infusion of the antibiotic given has been suggested to maximize antibiotic exposure and to better achieve the pharmacokinetic-pharmacodynamic (PK/PD) targets required (14). These dosing strategies have a PK advantage over intermittent bolus dosing (15), and a recent meta-analysis concludes that prolonged infusion is associated with reduced mortality and improved clinical outcome, compared to intermittent bolus dosing (16).
Because of the PK changes seen in the critically ill, standard dosing of antimicrobials may result in subtherapeutic plasma concentrations (17). It has been suggested that current empirical dosing recommendations for ICU patients are inadequate and need to be reconsidered (18). Patients with septic shock are especially vulnerable (7), and optimal dosing in these patients is crucial for reducing mortality.
The aim of this study was to determine if empirical dosing with piperacillin-tazobactam (4 g/0.5 g) given every 8 h (q8h) results in therapeutic plasma concentrations in septic shock patients within the initial 24 h of therapy. A population PK model was established with the dual purpose of assessing current standard treatment and simulating alternative dosing regimens and modes of administration.
MATERIALS AND METHODS
Study design.
This was a prospective, observational study conducted at the Intensive Care Unit at Aarhus University Hospital in Skejby, Denmark, between September 2014 and January 2015. Since 2013, β-lactam antibiotic TDM has been available at Aarhus University Hospital to optimize antibiotic treatment. Given that this study was undertaken in parallel with standard-of-care treatment of suspected septic shock, the Regional Ethical Committee approved the study without requiring signed informed consent.
Patient population.
Critically ill patients with known or suspected septic shock who required noradrenaline infusion and who were prescribed piperacillin-tazobactam (4 g/0.5 g) (Tazocin) by the treating physician were eligible for the study. All patients had an arterial catheter. Patients on renal replacement therapy and patients under the age of 18 years were excluded. Included patients had plasma concentrations of piperacillin determined. The following data were registered for each enrolled patient: age, gender, body weight, APACHE (acute physiology and chronic health evaluation) score on admission, SOFA (sequential organ failure assessment) score on day of sampling, amount of noradrenalin infusion given during the third dosing interval, and presence of acute kidney injury (AKI).
Study drug and blood sample collection.
Piperacillin-tazobactam (4 g/0.5 g) was administered as a 3-min infusion intravenously (i.v.) every 8 h. Due to the fact that the patients occasionally receive the first and sometime second dose of piperacillin-tazobactam before they arrive at the ICU, blood samples (4 ml) were collected by trained staff from an arterial catheter during the administration of the third consecutive infusion. Each patient had a total of eight blood samples drawn: before administration of the drug (time zero) and at 10, 20, and 30 min and 1, 2, 4, and 8 h after administration of the drug. Samples were stored at −20°C until analysis. To assess a possible influence on the piperacillin PK parameters, plasma creatinine (p-creatinine) and plasma albumin (p-albumin) levels were determined on the same day as the plasma concentrations of piperacillin.
Microbiological analysis.
In the Department of Clinical Microbiology, whole-blood samples were cultured according to standard operating procedures (i.e., whole-blood cultures are incubated in BacT/ALERT for up to 6 days). From some of the patients, urine, sputum, and pleural effusion samples were also received for culture. Urine, sputum, pleural effusion samples, and positive blood cultures were streaked onto agar plates, incubated overnight, and investigated for growth of bacteria. Identification of the bacteria was performed by standard methods, including matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Germany). The MIC of piperacillin-tazobactam was obtained by using Etests (AB Biodisk, Solna, Sweden) on Mueller-Hinton agar plates incubated at 35 ± 2°C with inoculum, incubation time, and atmosphere in accordance with the Etest application guide.
UHPLC analysis.
The free concentrations of piperacillin in sera were assessed using ultrahigh-performance liquid chromatography (UHPLC; Agilent 1290; Agilent Technologies, USA). Before UHPLC analysis, 300 μl of serum was placed in a 96-well ultrafilter plate, with a 30-kDa-molecular-mass cutoff (Acroprep 30K Omega; Pall Corporation, USA). After centrifugation for 30 min at 1,000 × g, 15 μl filtrate was mixed with 20 μl of 10 mM phosphate buffer, pH 3 (NaH2PO4, H2O adjusted with HCl; Merck, Germany). For analysis, 5 μl was injected into the UHPLC system equipped with a 1.7-μm, 100-mm by 2.1-mm C18 column (Kinetex; Phenomenex, USA), which was preheated to 40°C. Piperacillin was isolated with a gradient of acetonitrile (Sigma-Aldrich, Denmark) in phosphate buffer, changing from 20% to 30% over 2.5 min. Piperacillin was eluted with a typical retention time of 2.2 min and was measured with UV detection at 210 nm. Calculation of the concentrations was based on the piperacillin peak areas and was done with ChemStation software (Agilent Technologies, USA). Intrarun (total) imprecisions (percent coefficients of variation [% CV]) were 10.2% (15.3%) at 4.5 mg/liter and 4.7% (8.2%) at 15.6 mg/liter. The limit of quantification was defined as the lowest concentration with a CV of <20% and was found to be 0.5 mg/liter.
PK/PD targets.
The following PK/PD targets were evaluated: 50% fT>4×MIC (free piperacillin concentration maintained at a level 4 times the MIC for at least 50% of the dosing interval) and 100% fT>MIC (free piperacillin concentration maintained above the MIC throughout the dosing interval).
These targets were recently evaluated in a large study by Roberts et al., in which β-lactam dosing and plasma concentrations in critically ill patients were evaluated (19). The piperacillin clinical breakpoint MIC for Pseudomonas aeruginosa (16 mg/liter) published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) was used to evaluate PK/PD targets (20). P. aeruginosa is frequently seen in the ICU, and the clinical breakpoint MIC reflects a worst-case scenario regarding bacterial susceptibility, which is important to consider when patients are treated empirically. The upper limit of the piperacillin susceptibility range is 16 mg/liter. If a patient had one or more pathogens detected in sputum, pleural effusion, urine, or blood cultures, piperacillin-tazobactam MICs for these were also evaluated against predefined PK/PD targets.
Pharmacokinetic modeling and covariate screening.
Population PK analysis of concentration-time profiles was performed with nonlinear mixed effects modeling using NONMEM 7.2 (Icon Development Solutions, Hanover, MD, USA). Executions of runs were performed using Pirana and PsN (21). Estimations of typical parameter value, interindividual variability (IIV), covariates, and residual variability were done using the first-order conditional estimation method with interaction (FOCE INTER). Pharmacokinetic parameters were specified as Pi = Ppop · eη · β1 · β2 · … · βn, where Pi is the individual parameter value, Ppop is the typical (population) parameter value, η is the interindividual variability assuming a log-normal distribution, and β denotes the covariates for that parameter. Continuous covariates were parameterized as [1 + βi · (Xi − Xi,median)], and dichotomous covariates were parameterized as (βi · Xi). Additive and/or proportional residual variability models were evaluated.
Model selection and evaluation was guided by goodness-of-fit (GOF) plots and visual predictive checks (VPCs), performed with Pirana and PsN (21) and R (R Foundation for Statistical Computing, Vienna, Austria). Comparison of the statistical fits of nested models was performed using the objective function value (OFV) reported by NONMEM.
A pharmacokinetic model was developed to describe the plasma concentration-time profiles of piperacillin after the third consecutive infusion, and the exact timing of first and second doses was accounted for. The disposition and elimination model was evaluated among one and two-compartment models with first-order elimination and saturable Michaelis-Menten-type elimination.
Identification of significant predictors of variability in pharmacokinetics was investigated in a covariate model. The following variables were investigated as continuous covariates on clearance and central volume of distribution: age, body weight, plasma creatinine level, plasma albumin level, APACHE score, SOFA score, and amount of noradrenalin infusion given during the third dosing interval. The following variables were investigated as dichotomous covariates: gender and presence of acute kidney injury (AKI). Covariates were selected according to a conservative stepwise covariate modeling approach (22) with a criterion of ΔOFV of <−3.84 (P < 0.05) for forward inclusion and ΔOFV of >6.64 (P < 0.01) for backward deletion. For predictive covariate analysis, a sample size of >50 patients is usually recommended (23). However, such a large sample size was not possible due to difficulties recruiting these patients. In each forward selection step, only the strongest covariate (with the greatest reduction in ΔOFV) was selected. The remaining covariates were retested in the next step, unless they had a strong correlation (r > 0.5) with any covariate already in the model. This was done to avoid introducing colinearity into the model (24).
Area under the free plasma concentration-time curve (fAUC0–8) was calculated using the differential equation solver in NONMEM as
| (1) |
where Cp is the plasma concentration and t is time. Maximum plasma concentration (Cmax) was determined as the maximum value of Cp reported by the differential equation solver in NONMEM, and Cmin was determined as the value immediately before the next dose. Terminal half-life (t1/2) for the two-compartment model was calculated as in reference 25:
| (2) |
where k12, k21, and k10 are rate constants defined as k12 = Q/V1, k21 = Q/V2, and k10 = CL/V1. The targets 50% fT>4×MIC and 100% fT>MIC were calculated as the time above MIC threshold for each individual using the differential equation solver of NONMEM.
Simulation of alternative dosing regimens and probability of target attainment (PTA).
To assess alternative dosing regimens, 24-h piperacillin pharmacokinetic profiles following intermittent bolus administration (IA), extended infusion (EI), and continuous infusion (CI) were predicted in NONMEM. The IA dosing regimens were 4 g every 6 h (q6h), 4 g every 8 h (q8h), and 4 g every 12 h (q12h). The EI dosing regimens were 4 g q6h (infusion over 3 h), 4 g q8h (infusion over 4 h), and 4 g q12h (infusion over 6 h). The CI dosing regimens were 16 g/day, 12 g/day, and 8 g/day, including a loading bolus dose of 4 g. Two independent predictions were performed for all the dosing regimens listed above: (i) predictions for an average patient in our population, having a population mean PK profile; (ii) predictions for a patient exhibiting a low PK profile, equivalent to the patient in the population with the largest piperacillin clearance. This patient also had the lowest model-predicted T>MIC and a plasma creatinine level within normal range (53 μmol/liter). The PK/PD target evaluated in the predictions was 100% fT>MIC for P. aeruginosa (EUCAST clinical breakpoint MIC, 16 mg/liter).
Calculations of probability of target attainment (PTA) for pathogen MICs ranging from 0.125 to 256 mg/liter and VPCs were done based on 1,000 simulations of PK in the population using the final model. PTA calculations for the first day of treatment were done for the following simulated dosing regimens: 16 g/day (CI of 16 g, EI of 4 g q6h, and IA of 4 g q6h), 12 g/day (CI of 12 g, EI of 4 g q8h, and IAs of 4 g q8h and 2 g q4h), and 8 g/day (CI of 8 g, EI of 4 g q12h, and IAs of 4 g q12h and 2 g q6h). To visualize the impact of p-creatinine level, we also calculated PTA from simulations with p-creatinine levels fixed at 80 μmol/liter, 150 μmol/liter, and 250 μmol/liter for all subjects in the population. In these calculations, the third consecutive dose of the intermittent bolus dosing, 4 g q8h, was simulated. For all PTA calculations performed, the PK/PD targets evaluated were the same as previously mentioned: 50% fT>4×MIC and 100% fT>MIC.
Statistical analysis.
Demographic data were analyzed using Stata version 13 (StataCorp, College Station, TX). Continuous variables were defined as median and interquartile range (IQR).
RESULTS
Patient characteristics and microbiology.
Patient characteristics are shown in Table 1. A total of 15 patients, 11 men and four women, were included in the study. Only two patients had a p-creatinine level within the normal range (45 to 90 μmol/liter for women and 60 to 105 μmol/liter for men).
TABLE 1.
Patient characteristics (n = 15)
| Parameter | Value |
|---|---|
| Age (yr) [median (IQR)] | 66 (59; 79) |
| Gender [n (%)] | |
| Male | 11 (73) |
| Female | 4 (27) |
| Body wt (kg) [median (IQR)] | 80 (70.2; 95) |
| AKI [n (%)] | 10 (67) |
| Plasma creatinine level (μmol/liter) [median (IQR)] | 170 (119; 282) |
| Plasma albumin level (g/liter) [median (IQR)] | 30 (27; 32) |
| APACHE score [median (IQR)] | 19 (14; 23) |
| SOFA score [median (IQR)] | 9 (7; 10) |
| Noradrenalinea (μg/kg/min) [median (IQR)] | 0.1 (0.04; 0.24) |
Median amount of noradrenaline infused during the third dosing interval.
Four patients had one or more of the following pathogens detected in blood cultures or sputum or pleural effusion samples: three Escherichia coli (MICs, 1.5, 2, and 64 mg/liter), one Enterococcus faecalis (MIC, 3 mg/liter), one Staphylococcus aureus (MIC, 0.5 mg/liter), one Streptococcus pneumoniae (MIC, 0.016 mg/liter), and one Klebsiella oxytoca (MIC, 256 mg/liter).
PK/PD analysis.
The piperacillin plasma concentration-time profiles were best described by a two-compartment model with linear elimination and interindividual variability (IIV) adequately described as variance in clearance and central volume of distribution. A two-compartment model led to a ΔOFV of −48.5 versus a one-compartment model. The linear elimination model was equivalent to a saturable Michaelis-Menten-type elimination model (ΔOFV of −2.5) and was chosen due to the parsimony principle. Residual variability was best described by a proportional residual error model. In two patients, a clear absorption phase extending beyond the infusion period was present. In order to avoid bias in estimates of systemic parameters, the absorption was described individually for these two patients by a first-order absorption rate with lag time. The VPC, demonstrating adequate prediction of the final model relative to the observed data, and corresponding pharmacokinetic parameters are shown in Fig. 1 and Table 2.
FIG 1.
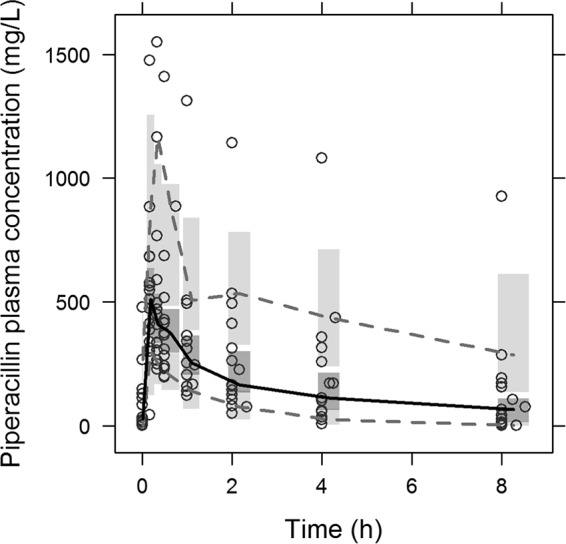
Visual predictive check (VPC) of final PK model based on 1,000 simulations. Open circles, observations; solid black line, median interpolated observations; gray dashed lines, 10th and 90th percentiles of observations. Shaded areas are the 90% model prediction interval of the median (dark) and the 10th and 90th percentiles (light).
TABLE 2.
Pharmacokinetic parameters corresponding to the two-compartment population model depicted in Fig. 1
| Population parametera | Value |
|
|---|---|---|
| Typical | IIV (%) | |
| CL (liters/h) (RSE%) | 3.6 (15.7) | 71.2 |
| V1 (liters) (RSE %) | 7.3 (11.8) | 57.8 |
| Q (liters/h) (RSE %) | 6.58 (16.4) | |
| V2 (liters) (RSE %) | 3.9 (9.7) | |
| βPcrea (liters/h)/(μmol/liter) (RSE %) | −0.011 (11.9) | |
| Proportional error (%) (RSE %) | 14.7 (14.4) | |
| Cmax (mg/liter) [median (IQR)] | 546 (363; 668) | |
| Cmin (mg/liter) [median (IQR)] | 51.7 (10.7; 159.4) | |
| AUC0–8 (mg/liter · h) [median (IQR)] | 1,148 (739; 2,492) | |
| t1/2 (h) [median (IQR)] | 3.49 (1.62; 4.47) | |
RSE, relative standard error reported on the approximate standard deviation scale; IIV, interindividual variability expressed as coefficient of variation; CL, clearance; V1, central volume; Q, intercompartmental clearance; V2, peripheral volume; βPcrea, covariate between clearance and plasma creatinine level (Pcrea), with clearance given by CLi = CL + βPcrea · (Pcrea − 170 μmol/liter); Cmax, maximum predicted plasma concentration; Cmin, trough predicted plasma concentration; AUC0–8, area under the plasma concentration-time curve from 0 to 8 h after the studied dose; t1/2, terminal half-life.
Plasma creatinine level was found to be the strongest covariate with significant linear relationship to clearance of piperacillin (P = 0.005). The inclusion of this covariate led to a reduction in IIV on clearance from a CV of 114.3% to a CV of 70.6%. No other covariate passed the backward inclusion criterion of P < 0.01. Age, APACHE score, and SOFA score were highly correlated (r > 0.5) with p-creatinine level and were therefore excluded after the first forward inclusion step of the covariate analysis, since p-creatinine level was then included in the model.
Each individual model-predicted T>MIC was compared to the clinical breakpoint MIC for P. aeruginosa (16 mg/liter). Twelve patients (80%) achieved 50% fT>4×MIC, and 10 patients (67%) achieved 100% fT>MIC. Four patients (27%) did not achieve any of the predefined targets. These patients had a p-creatinine value below 150 μmol/liter. Except for Klebsiella oxytoca (MIC, 256 mg/liter), one or both predefined PK/PD targets were achieved for the pathogens actually detected (Table 3).
TABLE 3.
Percentage of time above MIC (% T>MIC) and PK/PD target achievement for pathogens actually detected (n = 7)a
| Patient and pathogen (MIC, mg/liter) | PK/PD target |
|||
|---|---|---|---|---|
| % T>4×MIC | Target achieved | % T>MIC | Target achieved | |
| Patient 1, E. coli (1.5) | 100 | Yes | 100 | Yes |
| Patient 8, E. coli (64) | 18 | No | 100 | Yes |
| Patient 10, E. coli (2) | 52 | Yes | 74 | No |
| Patient 15 | ||||
| S. pneumoniae (0.016) | 100 | Yes | 100 | Yes |
| S. aureus (0.5) | 100 | Yes | 100 | Yes |
| E. faecalis (3) | 64 | Yes | 93 | No |
| K. oxytoca (256) | 0 | No | 7 | No |
For each pathogen, the model-predicted % T>MIC for that particular individual was calculated.
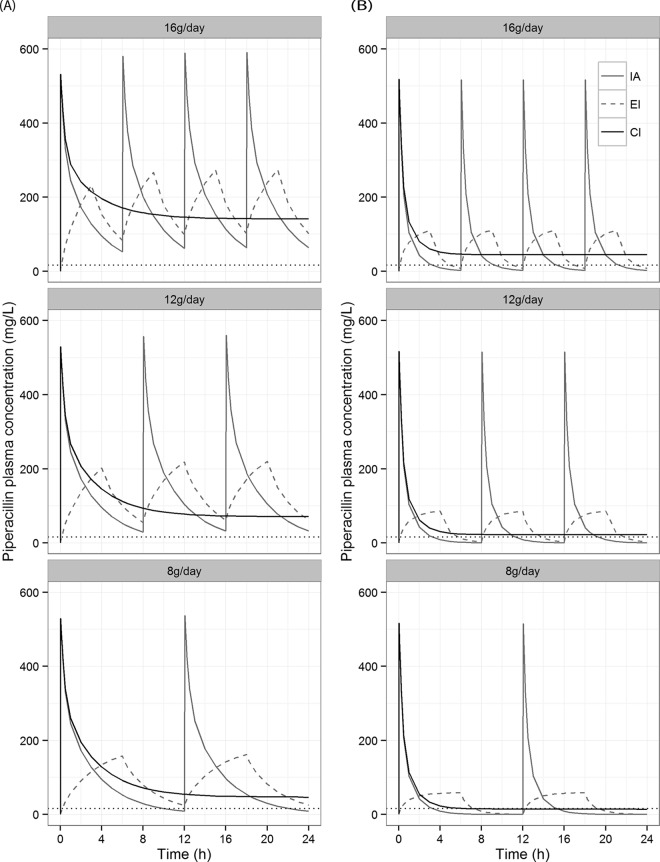
Predictions of piperacillin 24-h pharmacokinetic profiles following IA, EI, and CI are depicted in Fig. 2. Results for % fT>MIC varied greatly between the patient with an average PK profile for the current population and the patient with a low PK profile. In both types of patients, prolonged infusion resulted in a higher % fT>MIC than did intermittent bolus infusion, with the biggest differences seen in the patient with a low PK profile (Table 4).
FIG 2.
Illustrations of 24-h piperacillin pharmacokinetic profiles following intermittent bolus administration (IA), extended infusion (EI), and continuous infusion (CI) at 16 g/day, 12 g/day, and 8 g/day. Each profile is predicted from the PK model presented in Fig. 1. (A) Predictions for an average patient in the population; (B) prediction for a patient with PK parameters equivalent to the patient with the lowest PK profile in the population. The dotted line represents a MIC of 16 mg/liter.
TABLE 4.
Estimates of percentage of time above MIC, derived from predictions of alternative dosing regimens
| Mode of administration |
T>MICa (%) at first dose for patient with PK profile, by dose per 24 h |
|||||
|---|---|---|---|---|---|---|
| Population avg |
Low |
|||||
| 16 g | 12 g | 8 g | 16 g | 12 g | 8 g | |
| IA | 100 | 100 | 84 | 54 | 40 | 27 |
| EI | 98 | 100 | 98 | 82 | 70 | 58 |
| CI | 100 | 100 | 100 | 100 | 100 | 54 |
EUCAST clinical breakpoint MIC for P. aeruginosa (16 mg/liter).
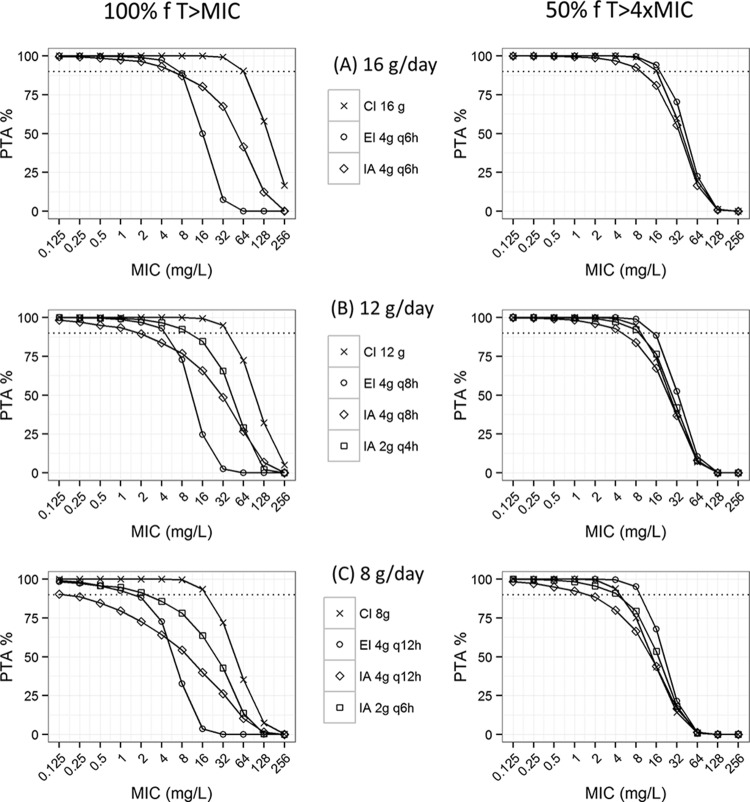
The PTA as a function of MIC for the first day of treatment is illustrated in Fig. 3. Eleven different treatment regimens were simulated using IA, EI, or CI in total daily doses of 8 g/day, 12 g/day, and 16 g/day. Continuous infusion resulted in a higher PTA than did extended and intermittent bolus dosing, for all doses simulated. The benefit of a more frequent bolus dosing is seen in Fig. 3B and C; IA of 2 g q4h and IA of 2 g q6h resulted in a higher PTA for both PK/PD targets evaluated, compared to IA of 4 g q8h and IA of 4 g q12h.
FIG 3.
Probability of target attainment (PTA) versus MIC for different dosing regimens and modes of administration, for the first day of piperacillin treatment. Calculations were done based on 1,000 simulations of PK in the population using the final model. IA, intermittent bolus administration; EI, extended infusion; CI, continuous infusion. The dotted black lines represent a PTA of 90%.
The PTA of the third consecutive dose (IA of 4 g q8h) as a function of MIC, for different estimates of p-creatinine level, is shown in Fig. 4. For a pathogen with a MIC of 16 mg/liter, the standard dose of piperacillin of 4 g q8h resulted in target attainment below the accepted 90% target for all p-creatinine level estimates simulated. However, the probability of targeting 100% fT>MIC was notably higher when p-creatinine level was fixed to 250 μmol/liter (PTA, 77.6%) than when it was fixed to 80 μmol/liter (PTA, 49.9%). The maximal MICs resulting in PTAs of ≥90% were higher in the 50% fT>4×MIC simulations than in the 100% fT>MIC simulations.
FIG 4.
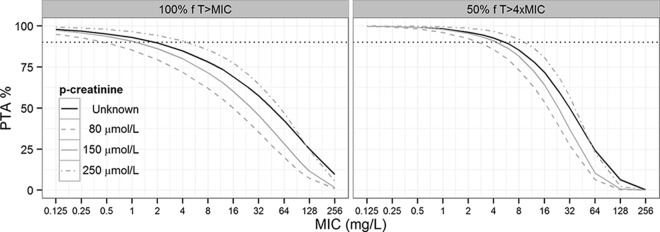
Probability of target attainment (PTA) versus MIC for different estimates of p-creatinine level, each derived from 1,000 simulations of data (the third consecutive dose of intermittent bolus dosing of 4 g q8h). The black lines demonstrate PTA when patient p-creatinine level is unknown but within the range observed in this population (53 to 446 μmol/liter). The gray lines demonstrate PTA in three scenarios where patient p-creatinine level is known (80, 150, and 250 μmol/liter). The dotted black lines represent a PTA of 90%.
DISCUSSION
Efficacious antibiotic dosing in early-phase septic shock treatment remains a pivotal challenge for clinicians. To address this problem, we performed a PK population model analysis for piperacillin in septic shock patients prior to consideration of renal replacement therapy. Our study demonstrates extensive interindividual variations in piperacillin pharmacokinetics in this patient population. Furthermore, our simulations indicate that this variation can be overcome through the incorporation of prolonged antibiotic infusion into the initial treatment of septic shock patients.
The variations in piperacillin pharmacokinetics ranged from near-complete drug clearance to persistently high concentrations throughout the dosing interval. This variability is in agreement with other studies on plasma concentrations of β-lactam antibiotics in critically ill patients (5, 19, 26). However, piperacillin concentrations estimated in this study were generally higher. This may be explained by the high levels of p-creatinine in many of the patients, as our PK model indicates that the individual PK profile is related to the level of p-creatinine. A critical distinction between this and previous work is the inclusion or exclusion of patients who were exhibiting high levels of p-creatinine. In the current study, these patients were included as long as they were not undergoing renal replacement therapy.
Renal function significantly influences plasma concentrations of β-lactam antibiotics, which are predominantly renally cleared. A decrease in β-lactam antibiotic clearance is associated with an increase in p-creatinine level (6). Plasma creatinine level may therefore be of value for clinicians when choosing a suitable dosing regimen for the patient, especially in situations where TDM and estimation of creatinine clearance through urine collection are not available. In patients with AKI, p-creatinine level is increased. Consequently, these patients are more likely to achieve β-lactam antibiotic target concentrations following standard dosing than are patients with preserved or augmented renal clearance (27). This is in accordance with our results; the patients in our population who failed to achieve the predefined PK/PD targets had p-creatinine values below 150 μmol/liter. For these patients, dose increment or continuous infusion would be necessary to maintain a piperacillin plasma concentration of 16 mg/liter or higher throughout the dosing interval.
The influence of p-creatinine level on the piperacillin plasma concentrations is also illustrated in the results from the Monte Carlo simulations of PTA versus MIC (Fig. 4). The likelihood of achieving the PK/PD targets improves with an increase in p-creatinine level. However, even with a p-creatinine level of 250 μmol/liter, a PTA of ≥90% for a bacterium with a MIC of 16 mg/liter is not obtained, which suggests that the standard dose of piperacillin-tazobactam (4 g/0.5 g q8h) is inadequate when given as intermittent bolus infusion.
The benefits of prolonged infusion are demonstrated in the 24-h pharmacokinetic profile predictions (Fig. 2; Table 3). For patients with a low PK profile, continuous infusion of at least 12 g daily is necessary to avoid subtherapeutic concentrations. Moreover, results from the dosing regimens simulated in Fig. 3 (16 g/day, 12 g/day, and 8 g/day) demonstrate that a ≥90% probability of targeting 100% fT>MIC for a bacterium with a MIC of 16 mg/liter was achieved only when the total daily piperacillin dose was simulated as continuous infusion. For the PK/PD target 50% fT >4×MIC, this was achieved for continuous infusion of 16 g/day as well as extended infusion of 4 g q6h.
This is in agreement with other studies reporting pharmacodynamic target advantages of dosing by prolonged infusion. Results from a study by Roberts et al. demonstrated that continuous infusion resulted in superior pharmacodynamic targets compared with intermittent bolus infusion in critically ill patients with sepsis (14), and De Waele et al. found that the use of β-lactam intermittent bolus infusions in critically ill patients was associated with increased risk of PK/PD target nonattainment compared to extended and continuous infusion (18). Furthermore, Felton et al. (28) illustrated that piperacillin-tazobactam (4 g/0.5 g) administered as extended infusion (3 h every 6 h) results in a prominently higher PTA than does intermittent bolus infusion. However, the clinical advantages of prolonged infusion remain controversial. An extensive randomized multicenter study, recently published by Dulhunty et al. (29), found that in critically ill patients with severe sepsis, there was no difference in clinical outcomes between β-lactam administrations by continuous infusion and by intermittent infusion. The advantage of continuous infusion is most pronounced when the causative microorganism has an elevated MIC. In these situations, continuous infusion is more likely to result in efficient plasma concentrations than is intermittent infusion (30, 31). In addition, it is important to consider the practicalities necessary for prolonged infusion to be efficient, such as the use of a loading dose and drug stability (32). Piperacillin-tazobactam has proven stable at 37°C for at least 24 h and is therefore suitable for continuous infusion (33).
The advantage of a more frequent intermittent bolus dosing is illustrated in Fig. 3. For the dosing regimen of 12 g/day, the maximal MIC resulting in a PTA of ≥90% (PK/PD target of 100% fT>MIC) was 10 mg/liter for intermittent bolus dosing of 2 g q4h, compared to 1.86 mg/liter for intermittent bolus dosing of 4 g q8h. This is also higher than the maximal MIC resulting in a PTA of ≥90% (PK/PD target of 100% fT>MIC) for piperacillin of 16 g/day, administered as intermittent bolus dosing of 4 g q6h (MIC, 5.72 mg/liter). For the dosing regimen of 8 g/day, intermittent bolus dosing of 2 g q6h also resulted in a markedly higher MIC (2.37 mg/liter) than did 4 g q12h (0.15 mg/liter) for the PK/PD target of 100% fT>MIC.
Even though dosing predictions for an average patient in our population imply that continuous infusion of piperacillin of 8 g/day would result in therapeutic plasma concentrations, a dose reduction in the early phase of sepsis treatment may be hazardous, as PK profiles vary widely among patients. An elevated p-creatinine level does not guarantee a PK profile in the therapeutic range as there is still a relatively large unexplained variability between individuals. For example, Taccone et al. found that in only 71% of critically ill patients with AKI did the renally adjusted first dose of piperacillin-tazobactam result in target concentrations (5). For safe dose adjustments and optimal antibiotic exposure, TDM must be applied. TDM is increasingly used in the context of critical illness and is a valuable tool to guide dose optimization. De Waele et al. found that meropenem and piperacillin dose adaptation based on TDM in critically ill patients resulted in an increase of PK/PD target attainment, compared to conventional dosing (34). Furthermore, Roberts et al. demonstrated that in order to optimize treatment, dose adjustment was required in 74.2% of patients, following TDM performed in 236 critically ill patients (35).
Lack of succinct PK/PD targets for critically ill patients makes consistency in dosing strategy difficult. Results from an international multicenter survey regarding β-lactam TDM practice in the ICU setting revealed significant differences in PK/PD targets used, emphasizing the need for future clinical studies that robustly define PK/PD targets for β-lactam TDM in the critically ill (36). To account for this uncertainty and the likely variability of antibiotic distribution into tissue, the PK/PD targets chosen in this study were more aggressive than targets evaluated elsewhere.
Tazobactam concentrations were not measured in our study, based on the assumption that β-lactamases remain inhibited throughout the dosing interval without regard to the exponential decay of the tazobactam concentration (37). Exposure to tazobactam is believed to prolong the susceptibility to piperacillin-induced bactericidal effects even when the concentration of the β-lactamase inhibitor is no longer detectable (37, 38). Our recommendations regarding piperacillin-tazobactam dosing regimens are therefore based on piperacillin concentrations only, which is in line with several other studies (14, 39, 40).
Using a population approach, we developed a robust two-compartment model which adequately described the PK of intermittent bolus administration of piperacillin at 4 g. This was accomplished despite the limitations associated with the relatively small sample size in this study. Roberts et al. also developed a population PK model to describe early-phase piperacillin pharmacokinetics in critically ill patients with sepsis (14). Their data were also described with a two-compartment model.
However, they excluded patients with a p-creatinine level of >120 μmol/liter, and the calculated total V and CL estimated in their study (25 liters and 17.2 liters/h, respectively) were higher than ours (3.6 liters/h and 11.2 liters, respectively). The piperacillin CL and total V in the present patient population were also substantially lower than in other patient populations who received piperacillin as intermittent bolus dosing, such as patients with intra-abdominal infections (13.7 liters/h and 22.4 liters, respectively [41]) and patients with nosocomial infections (13.8 liters/h and 21.7 liters, respectively [42]). Jeon et al. (39) measured piperacillin plasma concentrations in burn patients. Their data were also described with a two-compartment population PK model with creatinine clearance and sepsis as significant covariates. CL and total V in the presence of sepsis were 16.6 liters/h and 56.2 liters, respectively. Data on plasma creatinine level were not reported; however, median creatinine clearance in their patient cohort was 132.1 ml/min. The more severe renal impairment and the large variation in PK parameters in our study population are likely to influence these differences. The typical parameter estimates could also be sensitive to outlying individuals in the sampled population. Due to the relatively small study population, an outlier sensitivity analysis was not feasible, which is a limitation of the study.
In conclusion, our results suggest that piperacillin plasma concentrations vary considerably in the early phase of septic shock. Therefore, ensuring optimal drug exposure is a challenge for clinicians. Variations in piperacillin concentrations are largely influenced by variations in renal function, with high levels of p-creatinine increasing the likelihood of PK/PD target achievement. In contrast, patients with preserved or augmented renal function risk being underdosed when current empirical dosing regimens of piperacillin are applied. For these patients, dose increment or prolonged infusion may be required to increase the probability of attaining therapeutic plasma concentrations.
ACKNOWLEDGMENTS
This work was supported by the Aase and Ejnar Danielsen Foundation. Kristina Öbrink-Hansen is supported by a Ph.D. grant from the Faculty of Health Science, University of Aarhus, Denmark. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Janelle Denton for her language editing of the manuscript.
REFERENCES
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, Barchuk W. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 38:284–288. doi: 10.1086/379825. [DOI] [PubMed] [Google Scholar]
- 3.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med 31:2742–2751. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 4.Pinder M, Bellomo R, Lipman J. 2002. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care 30:134–144. [DOI] [PubMed] [Google Scholar]
- 5.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, De Backer D, Layeux B, Wallemacq P, Vincent JL, Jacobs F. 2010. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 14:R126. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blot S, Lipman J, Roberts DM, Roberts JA. 2014. The influence of acute kidney injury on antimicrobial dosing in critically ill patients: are dose reductions always necessary? Diagn Microbiol Infect Dis 79:77–84. doi: 10.1016/j.diagmicrobio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 8.Gin A, Dilay L, Karlowsky JA, Walkty A, Rubinstein E, Zhanel GG. 2007. Piperacillin-tazobactam: a beta-lactam/beta-lactamase inhibitor combination. Expert Rev Anti Infect Ther 5:365–383. doi: 10.1586/14787210.5.3.365. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Kruger P, Paterson DL, Lipman J. 2008. Antibiotic resistance—what's dosing got to do with it? Crit Care Med 36:2433–2440. doi: 10.1097/CCM.0b013e318180fe62. [DOI] [PubMed] [Google Scholar]
- 10.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. 2002. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother 50:425–428. doi: 10.1093/jac/dkf130. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi Y, Lipman J, Udy AA, Ng M, McWhinney B, Ungerer J, Lust K, Roberts JA. 2013. Beta-lactam therapeutic drug monitoring in the critically ill: optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int J Antimicrob Agents 41:162–166. doi: 10.1016/j.ijantimicag.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. 2010. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents 35:156–163. doi: 10.1016/j.ijantimicag.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Piperacillin penetration into tissue of critically ill patients with sepsis—bolus versus continuous administration? Crit Care Med 37:926–933. doi: 10.1097/CCM.0b013e3181968e44. [DOI] [PubMed] [Google Scholar]
- 16.Teo J, Liew Y, Lee W, Kwa AL. 2014. Prolonged infusion versus intermittent boluses of beta-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents 43:403–411. doi: 10.1016/j.ijantimicag.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Udy AA, Roberts JA, Lipman J. 2013. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 39:2070–2082. doi: 10.1007/s00134-013-3088-4. [DOI] [PubMed] [Google Scholar]
- 18.De Waele JJ, Lipman J, Akova M, Bassetti M, Dimopoulos G, Kaukonen M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Udy AA, Starr T, Wallis SC, Roberts JA. 2014. Risk factors for target non-attainment during empirical treatment with beta-lactam antibiotics in critically ill patients. Intensive Care Med 40:1340–1351. doi: 10.1007/s00134-014-3403-8. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2013. Clinical breakpoints. http://www.eucast.org/clinical_breakpoints/. Accessed 16 April 2015. [Google Scholar]
- 21.Keizer RJ, Karlsson MO, Hooker A. 2013. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahlby U, Jonsson EN, Karlsson MO. 2002. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS Pharm Sci 4:E27. doi: 10.1208/ps040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribbing J, Jonsson EN. 2004. Power, selection bias and predictive performance of the population pharmacokinetic covariate model. J Pharmacokinet Pharmacodyn 31:109–134. doi: 10.1023/B:JOPA.0000034404.86036.72. [DOI] [PubMed] [Google Scholar]
- 24.Bonate PL. 1999. The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res 16:709–717. doi: 10.1023/A:1018828709196. [DOI] [PubMed] [Google Scholar]
- 25.Toutain PL, Bousquet-Melou A. 2004. Plasma terminal half-life. J Vet Pharmacol Ther 27:427–439. doi: 10.1111/j.1365-2885.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 26.Carlier M, Carrette S, Stove V, Verstraete AG, De Waele JJ. 2014. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int J Antimicrob Agents 43:470–473. doi: 10.1016/j.ijantimicag.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, Daali Y, Pugin J, Karmime A, Fathi M, Lew D, Harbarth S. 2015. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents 45:385–392. doi: 10.1016/j.ijantimicag.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Felton TW, Hope WW, Lomaestro BM, Butterfield JM, Kwa AL, Drusano GL, Lodise TP. 2012. Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob Agents Chemother 56:4087–4094. doi: 10.1128/AAC.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, Starr T, Paul SK, Lipman J. 22 July 2015. A multicenter randomized trial of continuous versus intermittent beta-lactam infusion in severe sepsis. Am J Respir Crit Care Med doi: 10.1164/rccm.201505-0857OC. [DOI] [PubMed] [Google Scholar]
- 30.Lodise TP Jr, Lomaestro B, Drusano GL. 2007. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 44:357–363. doi: 10.1086/510590. [DOI] [PubMed] [Google Scholar]
- 31.Lorente L, Jimenez A, Martin MM, Iribarren JL, Jimenez JJ, Mora ML. 2009. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 33:464–468. doi: 10.1016/j.ijantimicag.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 32.De Waele JJ, Lipman J, Carlier M, Roberts JA. 2015. Subtleties in practical application of prolonged infusion of beta-lactam antibiotics. Int J Antimicrob Agents 45:461–463. doi: 10.1016/j.ijantimicag.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Viaene E, Chanteux H, Servais H, Mingeot-Leclercq MP, Tulkens PM. 2002. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob Agents Chemother 46:2327–2332. doi: 10.1128/AAC.46.8.2327-2332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Waele JJ, Carrette S, Carlier M, Stove V, Boelens J, Claeys G, Leroux-Roels I, Hoste E, Depuydt P, Decruyenaere J, Verstraete AG. 2014. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 40:380–387. doi: 10.1007/s00134-013-3187-2. [DOI] [PubMed] [Google Scholar]
- 35.Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL, Lipman J. 2010. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents 36:332–339. doi: 10.1016/j.ijantimicag.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Wong G, Brinkman A, Benefield RJ, Carlier M, De Waele JJ, El Helali N, Frey O, Harbarth S, Huttner A, McWhinney B, Misset B, Pea F, Preisenberger J, Roberts MS, Robertson TA, Roehr A, Sime FB, Taccone FS, Ungerer JP, Lipman J, Roberts JA. 2014. An international, multicentre survey of beta-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 69:1416–1423. doi: 10.1093/jac/dkt523. [DOI] [PubMed] [Google Scholar]
- 37.Strayer AH, Gilbert DH, Pivarnik P, Medeiros AA, Zinner SH, Dudley MN. 1994. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin-resistant and -susceptible organisms in an in vitro model of infection. Antimicrob Agents Chemother 38:2351–2356. doi: 10.1128/AAC.38.10.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okereke C, Dudley MN. 1998. Beta-lactam/beta-lactamase inhibitor combinations: pharmacodynamic considerations and possible role in the management of bacterial infections in the neutropenic host. J Antimicrob Chemother 41(Suppl D):43–49. [DOI] [PubMed] [Google Scholar]
- 39.Jeon S, Han S, Lee J, Hong T, Paek J, Woo H, Yim DS. 2014. Population pharmacokinetic analysis of piperacillin in burn patients. Antimicrob Agents Chemother 58:3744–3751. doi: 10.1128/AAC.02089-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodise TP Jr, Lomaestro B, Rodvold KA, Danziger LH, Drusano GL. 2004. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrob Agents Chemother 48:4718–4724. doi: 10.1128/AAC.48.12.4718-4724.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Kuti JL, Nightingale CH, Mansfield DL, Dana A, Nicolau DP. 2005. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intra-abdominal infection. J Antimicrob Chemother 56:388–395. doi: 10.1093/jac/dki243. [DOI] [PubMed] [Google Scholar]
- 42.Chen R, Qian Q, Sun MR, Qian CY, Zou SL, Wang ML, Wang LY. 18 April 2015. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with nosocomial infections. Eur J Drug Metab Pharmacokinet. doi: 10.1007/s13318-015-0276-3. [DOI] [PubMed] [Google Scholar]