Abstract
Spillage of cyst contents during surgery is the major cause of recurrences of hydatidosis, also called cystic echinococcosis (CE). Currently, many scolicidal agents are used for inactivation of the cyst contents. However, due to complications in the use of those agents, new and more-effective treatment options are urgently needed. The aim of this study was to investigate the in vitro efficacy of arsenic trioxide (ATO) against Echinococcus granulosus protoscolices. Protoscolices of E. granulosus were incubated in vitro with 2, 4, 6, and 8 μmol/liter ATO; viability of protoscolices was assessed daily by microscopic observation of movements and 0.1% eosin staining. A small sample from each culture was processed for scanning and transmission electron microscopy. ATO demonstrated a potent ability to kill protoscolices, suggesting that ATO may represent a new strategy in treating hydatid cyst echinococcosis. However, the in vivo efficacy and possible side effects of ATO need to be explored.
INTRODUCTION
Hydatidosis, also called cystic echinococcosis (CE), is a severe zoonotic disease caused by the tapeworm Echinococcus granulosus at its larval stage. E. granulosus is distributed worldwide (1). Currently, chemotherapy, puncture with aspiration, and surgery are the three main treatments for hydatid cysts (2), with surgery being one of the best choices for treating hydatidosis (3). The possibility of cyst rupture and protoscolex dissemination can result in secondary CE caused by spillage of a large number of protoscolices; spillage of cyst contents during surgical operation is the major cause of recurrences of the disease (4).
Instillation of a scolicidal agent into a hepatic hydatid cyst is the most commonly employed measure to prevent their reoccurrence and attendant complications. Many scolicidal agents have been used for inactivation of cyst contents. The agents can destroy parasites (5), but their use can result in some complications, including acute hypernatremia and intracranial bleeding, leading to convulsions and myelinolysis (6). Therefore, there is a clinical need for safe and effective scolicidal solutions.
Arsenic trioxide (As2O3 [ATO]) is the main component of arsenolite, an ancient drug used in traditional Chinese medicine for over 5,000 years (7); it was the first effective chemotherapy against cancer, syphilis, parasites, and leukemia and especially in the treatment of patients suffering from acute promyelocytic leukemia (APL) (8). The solution gained popularity as a feed additive for chickens and pigs to prevent parasitic infestations and weight gain (9). In 1905, Harold Thomas of Liverpool used atoxyl, an arsenic derivative, to kill trypanosomes. From 1830 to 1930, it was used to treat African sleeping sickness (10). Moreover, ATO has been shown to be effective, particularly in combination with other drugs, in chronic myeloid leukemia therapy (11, 12).
Currently, ATO is approved by the FDA for the treatment of acute promyelocytic leukemia (13). Arsenical drugs are still used for treatment of parasitic diseases such as African sleeping sickness, amoebic dysentery, and filariasis in dogs (14). Recently, some researchers found that ATO was effective not only in treatment of acute promyelocytic leukemia but also in inhibiting several solid-cancer cell lines such as those associated with primary prostate cancer (13), breast cancer (15), and gastric cancer (16). Arsenic compounds are also used to manufacture agricultural products such as insecticides, sheep dips, and medicines for the eradication of tapeworms in sheep and cattle (17). However, some researchers found that arsenic toxicity caused apoptosis in hepatocytes and that arsenic cytotoxicity transcriptionally induced a number of stress genes expressed in transformed human liver cells (18, 19). Therefore, to better apply ATO as a scolicidal agent preventing hepatic hydatid cyst recurrence, we examine the effect of ATO on Echinococcus granulosus protoscolices in vitro.
The purpose of this study was to examine the effect of ATO as a scolicidal agent to prevent hepatic hydatid cyst recurrence.
MATERIALS AND METHODS
Materials.
ATO was purchased from Sigma-Aldrich (USA) and was dissolved in 1.65 M NaOH at 1 × 10−1 M as a stock solution. Dilutions were prepared freshly on the day of treatment and filter sterilized (0.22 μm pore size) prior to use. RPMI 1640 medium was also purchased from Sigma.
Collection of protoscolices.
Protoscolices were aspirated from hepatic cysts of naturally infected sheep slaughtered at Shihezi, Xinjiang Province, western China. The surface of the infected liver was disinfected, and the hydatid fluid was transferred into a test tube under sterile conditions and allowed to sit for 15 min. After the protoscolices settled in the test tube, the supernatant was discarded. The sedimental protoscolices were washed three times with phosphate-buffered saline (PBS) (pH 7.2). Protoscolices were incubated in RPMI 1640 culture medium containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% calf serum in a 5% CO2 atmosphere at 37°C. Each experiment was repeated three times.
In vitro ATO treatment of protoscolices.
In this study, a total of five groups were tested: a control group and four groups administered arsenic trioxide at 2, 4, 6, or 8 μmol/liter. Protoscolices were collected after 3 days of culture and were washed three times in PBS; the protoscolex energy level was close to 100%, using a reaction mixture of RPMI 1640 medium plus protoscolices. Dilutions were prepared in medium, and ATO was added to the reaction mixture containing the protoscolices at a concentration of 2, 4, 6, or 8 μmol/liter. As positive controls, nontreated protoscolices were kept in the RPMI 1640 medium. In short, arsenic trioxide (RPMI medium, 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate) was added to identical volumes of protoscolices and distributed to 6-well plates (Costar, USA) (5 ml per well, 2,000 to 2,500 protoscolices). Each of the six plates represented a concentration of arsenic. In parallel, protoscolices were viewed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to morphologically assess potential drug-induced damage.
Viability test.
To assay the viability of protoscolices, 100 μl of pooled protoscolices was transferred to a slide and mixed with 100 μl 0.1% eosin. After 15 min, the dead protoscolices stained red and the surviving protoscolices remained colorless as observed under an inverted microscope (Olympus, Japan). The viability test was carried out on days 1 to 14, and the medium was changed every 6 days.
Morphological and ultrastructural investigations of ATO-treated protoscolices.
To visualize structural alterations in protoscolices in response to ATO treatment, parasites were processed for scanning electron microscopy and transmission electron microscopy (SEM and TEM, respectively) at different time points after the initiation of treatment with different concentrations of ATO. Fixed specimens were then washed in distilled water, treated with 1% uranyl acetate for 30 min, subsequently washed extensively in distilled water, and dehydrated by incubation in sequentially increasing concentrations (50%, 70%, 80%, and 90%) of ethanol. Samples were then washed in PBS (pH 7.2) and treated with 1% uranyl acetate for 30 min. They were then sputter coated with gold and inspected on a LEO1430VP scanning electron microscope operating at 20 kV. For TEM, specimens were fixed and dehydrated as described above and subsequently embedded in Epon 812 resin (20, 21). Polymerization of the resin was carried out at 65°C overnight. Sections were cut on a Reichert and Jung ultramicrotome and were placed onto 300-mesh copper grids. Ultrathin sections of 80 to 100 nm thickness were prepared for TEM. Staining with uranyl acetate and lead citrate was performed as described previously (22–24).
RESULTS
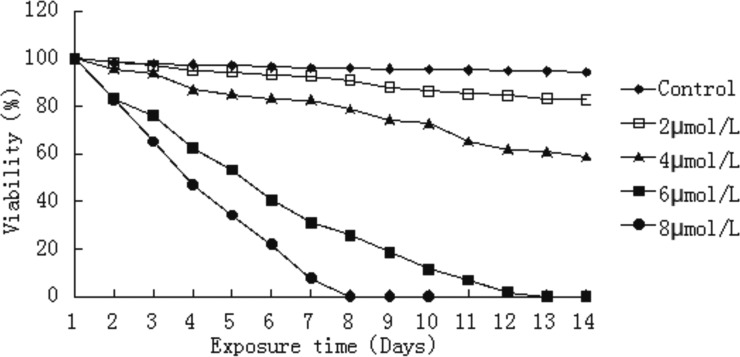
ATO affects the viability of E. granulosus protoscolices.
We also quantified the extent of the viability of protoscolices in the presence of ATO using an eosin exclusion assay. Light microscopy was used to distinguish viable from nonviable protoscolices. Control protoscolices incubated in the absence of drug were not altered and remained viable (94.1%) after 14 days of incubation. Protoscoleces cultured with 8 μmol/liter ATO were killed considerably faster than protoscolices cultured with 2, 4, or 6 μmol/liter ATO. After 2 days of exposure to 8 μmol/liter ATO, viability was approximately 65%, and it was reduced to 20% after 6 days of incubation. At 6 μmol/liter, ATO had clearly decreased efficacy, with 50% of protoscolices still viable after 5 days of treatment. The rate of protoscolex death increased with time. Only a small fraction of protoscolices were viable in cultures treated with 6 μmol/liter ATO after 5 days. After 9 days of treatment with 6 μmol/liter ATO, the viability was approximately 13.5%. ATO at 2 and 4 μmol/liter had an effect on protoscolices, but the effect was not as pronounced as that seen with 8 μmol/liter ATO (P < 0.01; Fig. 1).
FIG 1.
Loss of viability of E. granulosus protoscolices during in vitro ATO treatment. Viability was determined through 0.1% eosin staining. Note the dose-dependent effect of ATO.
ATO-induced morphological and ultrastructural alterations.
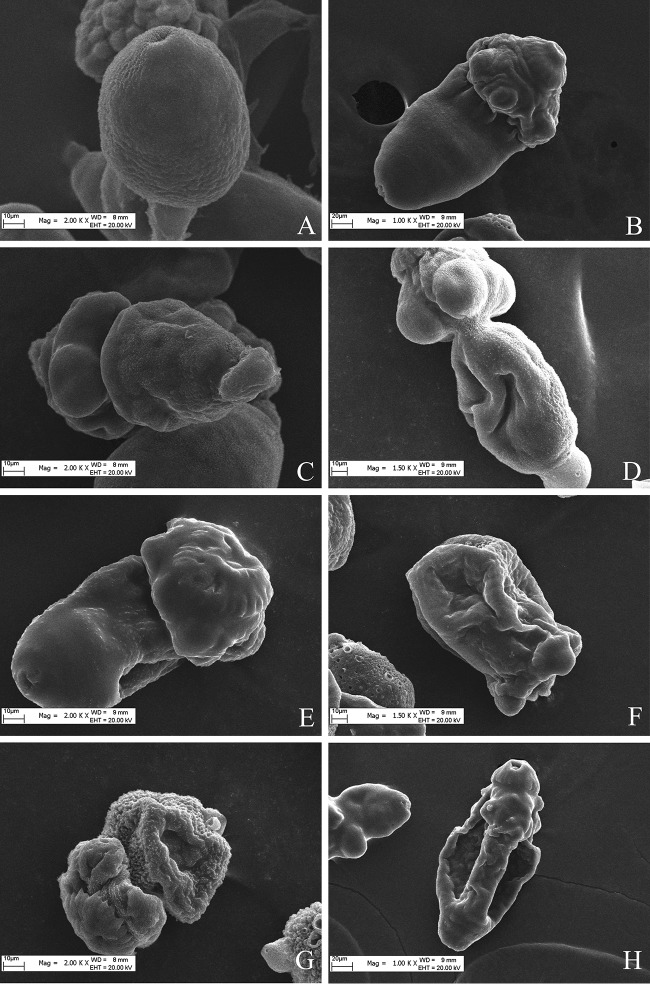
To visualize the structural alterations in protoscolices imposed by ATO treatment, parasites were processed for SEM after 3 days of treatment with 4, 6, and 8 μmol/liter ATO (Fig. 2C, E, and G) and 5 days of treatment with 2, 4, 6, and 8 μmol/liter ATO (Fig. 2B, D, F, and H). Control protoscolices incubated in RPMI 1640 medium demonstrated no ultrastructural alterations throughout the incubation period, exhibiting an intact morphology. SEM analysis showed that nontreated protoscolices exhibited a largely intact germinal layer composed of a multitude of different cell types. Similarly, ultrastructures were also observed with invaginated protoscolices and in the corresponding microscopic view (Fig. 2A). In contrast, morphological damage and ultrastructural damage were detected in ATO-treated protoscolices. On days 3 and 5 of ATO treatment, SEM revealed ultrastructural changes, including multiple pits arising on the outer surfaces of the germinal membrane, disruption of the external plasma membrane, collapse of the sucker region, and protoscolex contraction (Fig. 2C, B, D, E, and F). Five days of 4 μmol/liter ATO resulted in invaginated protoscolices with extensive damage to the tegument (Fig. 2D); 3 days of 8 μmol/liter ATO treatment caused extensive damage to the tegument, including loss of microtriches on the rostellum, a contracted soma region in the sucker region (Fig. 2G); and 5 days of 8 μmol/liter ATO resulted in complete destruction of the rostellum and the germinal layer (Fig. 2H).
FIG 2.
Representative images from scanning electron microscopy (SEM) of protoscolices. (A) Protoscolices cultured in vitro for 5 days in the presence of medium containing RPMI 1640. (B) Collapse of sucker region (after 5 days with 2 μmol/liter ATO treatment). (C) Invaginated protoscolices, clearly altered after culture in the presence of ATO (3 days with 4 μmol/liter ATO). (D) Altered and contracted soma (5 days with 4 μmol/liter ATO). (E) Altered protoscolices after 3 days with 6 μmol/liter ATO. (F) The ultrastructural changes included collapse of the soma region and shedding of microtriches of the scolex region (8 μmol/liter ATO, 3 days). (G) Loss of hooks and shedding of microtriches (8 μmol/liter ATO, 3 days). (H) The internal tissue was severely affected, resulting in the loss of its integrity (3 days with 8 μmol/liter ATO).
Ultrastructural assessment of ATO-induced damage in E. granulosus protoscolices.
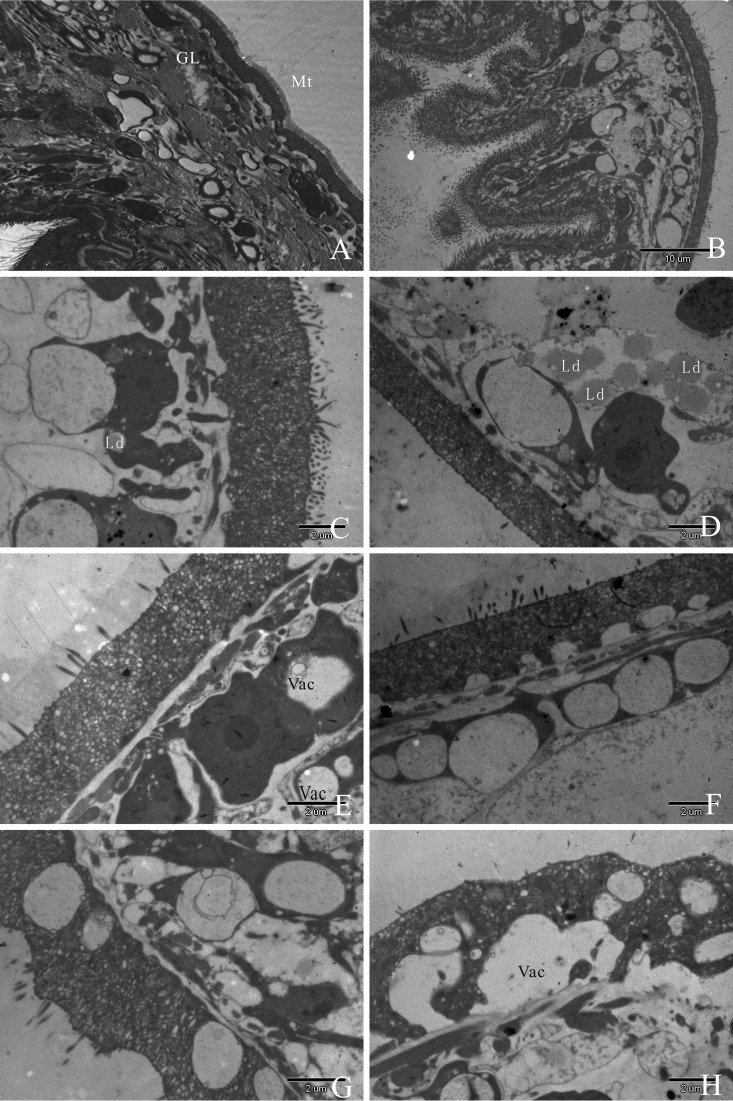
Untreated E. granulosus protoscolices obtained from in vitro cultures exhibited features typical of protoscolices, with a distinct acellular outer laminated layer and an intact germinal layer comprised of a multitude of different, morphologically intact cell types, and the tegument is replaced by the germinal layer, which contains a number of different cell types, including a number of undifferentiated cells with a large nucleus and nucleolus cell types (Fig. 3A). After five days of treatment with 2 μmol/liter ATO, partial loss of microtriches was seen within the germinal layer (Fig. 3B). After 3 days of treatment with 4 μmol/liter ATO, the tegument was present, but truncated microtriches and lipid droplets of the germinal layer could be observed (Fig. 3C); after 5 days of treatment with 4 μmol/liter ATO, the tegument was still present, but effects included increased numbers of lipid droplets of the germinal layer and complete shedding of microtriches (Fig. 3D). After 3 days of treatment with 6 μmol/liter ATO, there was a loss of microtriches, partial separation from the tegument layer, formation of vacuolation of the distal cytoplasm, and increased occurrence of small lipid vesicles in the laminated layer (Fig. 3E); after 5 days of treatment with 6 μmol/liter ATO, a large part of the germinal layer collapsed and aggregated (Fig. 3F). TEM micrograph images at 3 days of treatment with 8 μmol/liter ATO revealed that most of the parasite tissue, including the tegument, had been destroyed (Fig. 3G). Five days of 8 μmol/liter ATO treatment had a devastating impact on protoscolices, with complete shedding of microtriches over a major portion of the tegument layer and only tissue residues present (Fig. 3H).
FIG 3.
TEM of Echinococcus granulosus protoscolices after 3 days of in vitro culture in the presence of RPMI 1640 (A) and in the presence of ATO for 3 days or 5 days (B to F). (A) Note the distinct features of the protoscolices such as the germinal layer (GL) and intact microtriches (Mt) (arrows), which together comprise the whole-cell type. (B) Five days of 2 μmol/liter ATO exposure resulted in partial loss of microtriches. (C and D) Protoscolices cultured in vitro in the presence of 4 μmol/liter ATO for 3 days (C) or 5 days (D). Note the morphological changes of E. granulosus protoscolices upon treatment, including shedding of microtriches (indicated by arrows in panels B and D) and occurrence of lipid droplets (indicated by “Ld” in panels C and D). (E) After 3 days of 6 μmol/liter ATO exposure, shortening of microtriches and vacuolation (indicated by “Vac” in panels E and H) of the distal cytoplasm were found in the germinal layer. (F) Five days of 6 μmol/liter ATO exposure resulted in small vesicular bodies within the laminated layer. (G) Five days of exposure to 6 μmol/liter ATO. Note the separation of the laminated layer and the tegument. (H) Substantial portions of the germinal layer already showed massive signs of cellular destruction after 5 days of drug treatment with 8 μmol/liter ATO and were detached from the laminated layer.
DISCUSSION
Current state of treatment of E. granulosis protoscolices.
E. granulosus protoscolex infections in humans cause cystic echinococcosis, in which protoscolex development in visceral organs often results in particular organ failure. Furthermore, in farm animals, cystic hydatidosis causes severe economic losses. Although benzimidazole derivatives such as albendazole are used in treatments, there is often an absence of complete recovery after treatment (25). Surgery remains the most effective treatment. Protection of the operation field is necessary before removing or emptying the cyst.
Scolicidal agents are an essential part of the treatment for hydatid cyst disease. To date, several parasiticidal substances have been used to inactivate hydatid cyst contents; this procedure helps to prevent the illness from reoccurring. Besim et al. (26) reported that cetrimide-chlorhexidine was the most effective scolicidal agent in vitro. However, metabolic acidosis, leading to methemoglobinemia, has been reported for this agent (27). The use of 20% hypertonic saline solution has been found to be 100% effective against scolices of a hydatid cyst after 6 min of treatment, but disadvantages, namely, acute hypernatremia, intracranial bleeding, necrosis, convulsions, and myelinolysis, have been reported (6, 26). Although 50 μg/ml albendazole sulfoxide was reported to be a powerful scolicidal agent in vitro, fatal hepatic lesions have been reported. Finally, it has been demonstrated that 10% H2O2 killed 100% of scolices in 15 min (27). Injection of scolicidal solution in the cysts leads to air embolism and anaphylactic shock.
An ideal parasiticidal substance would be able to effectively kill E. granulosus protoscolices with a low morbidity rate, would be inexpensive, and would lack local and systemic side effects (28).
Arsenic is one of the oldest drugs in both Western and traditional Chinese medicine. Traditional Chinese medicine has used arsenic as a therapeutic agent to treat several diseases under the principle of “combat poison with poison” (29). ATO has been shown to be active against a broad spectrum of other diseases such as parasite infections, hematologic cancer, and solid tumors (30). Melarsoprol, an organic arsenical, has primarily been used for the treatment of African trypanosomiasis (31).
ATO negatively affects E. granulosus in vitro.
In line with these studies, the present study demonstrated that ATO is effective against cultured E. granulosus protoscolices in vitro. Moreover, as the ATO concentration was increased, there were increasing numbers of dead protoscolices, and 8 μmol/liter ATO killed protoscolices considerably faster than 2, 4, and 6 μmol/liter ATO. After 3 days of exposure to 8 μmol/liter ATO, protoscolex viability was approximately 47.1%, falling to 22% after 5 days of incubation. In contrast, the viability of control parasites cultured in RPMI 1640 was not significantly altered.
Protoscolices cultured with 8 μmol/liter ATO had more obvious damage than those cultured with 2, 4, and 6 μmol/liter ATO. At 3 days of 8 μmol/liter ATO treatment, SEM showed loss of hooks, shedding of microtriches, and reduced volume. In addition, TEM clearly revealed ultrastructural changes such as shedding of surface microtriches, destruction of the rostellum, membrane blebbing, and cell volume reduction. Under control conditions, protoscolices incubated in RPMI 1640 had a normal morphological and ultrastructural appearance.
The present results demonstrate that in vitro treatment with ATO induces a number of significant alterations in E. granulosus protoscolices that could eventually impair parasite viability and lead to parasite death. Future studies will investigate the efficacy of ATO and its derivatives against E. granulosus protoscolex development in the human liver as well as the mechanism of action.
In conclusion, we have demonstrated here the in vitro efficacy and parasiticidal activity of ATO against E. granulosus protoscolices. The present results demonstrate the therapeutic potential of ATO therapy and indicate that ATO use may represent a new idea for the treatment of E. granulosus.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (grant no. 81360410, 81560334, and U1303121).
We thank Feng Sun for her guidance in the experiments. We also thank Ying Lei and Hongjuan Shi for their help in culture of protoscolices.
REFERENCES
- 1.Qu H, Tong D, Zhang Y, Kang K, Zhang Y, Chen L, Ren L. 2013. The synergistic antitumor activity of arsenic trioxide and vitamin K2 in HL-60 cells involves increased ROS generation and regulation of the ROS-dependent MAPK signaling pathway. Pharmazie 68:839–845. [PubMed] [Google Scholar]
- 2.Lv H, Jiang Y, Liao M, Sun H, Zhang S, Peng X. 2013. In vitro and in vivo treatments of Echinococcus granulosus with Huaier aqueous extract and albendazole liposome. Parasitol Res 112:193–198. doi: 10.1007/s00436-012-3125-1. [DOI] [PubMed] [Google Scholar]
- 3.Topcu O, Sumer Z, Tuncer E, Aydin C, Koyuncu A. 2009. Efficacy of chlorhexidine gluconate during surgery for hydatid cyst. World J Surg 33:1274–1280. doi: 10.1007/s00268-009-9971-z. [DOI] [PubMed] [Google Scholar]
- 4.Moro P, Schantz PM. 2009. Echinococcosis: a review. Int J Infect Dis 13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Moazeni M, Larki S. 2010. In vitro effectiveness of acidic and alkline solutions on scolices of hydatid cyst. Parasitol Res 106:853–856. doi: 10.1007/s00436-010-1723-3. [DOI] [PubMed] [Google Scholar]
- 6.Albi A, Baudin F, Matmar M, Archambeau D, Ozier Y. 2002. Severe hypernatremia after hypertonic saline irrigation of hydatid cysts. Anesth Analg 95:1806–1808, table of contents. doi: 10.1097/00000539-200212000-00062. [DOI] [PubMed] [Google Scholar]
- 7.Chen SJ, Zhou GB, Zhang XW, Mao JH, de The H, Chen Z. 2011. From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood 117:6425–6437. doi: 10.1182/blood-2010-11-283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes A, Falcao AS, Silva RF, Brito MA, Brites D. 2007. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur J Neurosci 25:1058–1068. doi: 10.1111/j.1460-9568.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 9.Scheindlin S. 2005. The duplicitous nature of inorganic arsenic. Mol Interv 5:60–64. doi: 10.1124/mi.5.2.1. [DOI] [PubMed] [Google Scholar]
- 10.Au WY. 2011. A biography of arsenic and medicine in Hong Kong and China. Hong Kong Med J 17:507–513. [PubMed] [Google Scholar]
- 11.Baj G, Arnulfo A, Deaglio S, Mallone R, Vigone A, De Cesaris MG, Surico N, Malavasi F, Ferrero E. 2002. Arsenic trioxide and breast cancer: analysis of the apoptotic, differentiative and immunomodulatory effects. Breast Cancer Res Treat 73:61–73. doi: 10.1023/A:1015272401822. [DOI] [PubMed] [Google Scholar]
- 12.Yu D, Wang ZH, Cheng SB, Li HK, Chan HB, Chew EC. 2001. The effect of arsenic trioxide on the expression of Hsc and HNF4 in nuclear matrix proteins in HepG2 cells. Anticancer Res 21:2553–2559. [PubMed] [Google Scholar]
- 13.Uslu R, Sanli UA, Sezgin C, Karabulut B, Terzioglu E, Omay SB, Goker E. 2000. Arsenic trioxide-mediated cytotoxicity and apoptosis in prostate and ovarian carcinoma cell lines. Clin Cancer Res 6:4957–4964. [PubMed] [Google Scholar]
- 14.Beauchamp EM, Uren A. 2012. A new era for an ancient drug: arsenic trioxide and Hedgehog signaling. Vitam Horm 88:333–354. doi: 10.1016/B978-0-12-394622-5.00015-8. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sanchez L, Cebrian ME. 22 July 2014. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol doi: 10.1016/j.taap.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Zhang TC, Cao EH, Li JF, Ma W, Qin JF. 1999. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer 35:1258–1263. doi: 10.1016/S0959-8049(99)00106-9. [DOI] [PubMed] [Google Scholar]
- 17.Tchounwou PB, Centeno JA, Patlolla AK. 2004. Arsenic toxicity, mutagenesis, and carcinogenesis—a health risk assessment and management approach. Mol Cell Biochem 255:47–55. doi: 10.1023/B:MCBI.0000007260.32981.b9. [DOI] [PubMed] [Google Scholar]
- 18.Ray A, Roy S, Agarwal S, Bhattacharya S. 2008. As2O3 toxicity in rat hepatocytes: manifestation of caspase-mediated apoptosis. Toxicol Ind Health 24:643–653. doi: 10.1177/0748233708100370. [DOI] [PubMed] [Google Scholar]
- 19.Tchounwou PB, Wilson BA, Ishaque AB, Schneider J. 2001. Atrazine potentiation of arsenic trioxide-induced cytotoxicity and gene expression in human liver carcinoma cells (HepG2). Mol Cell Biochem 222:49–59. doi: 10.1023/A:1017903005541. [DOI] [PubMed] [Google Scholar]
- 20.Stettler M, Fink R, Walker M, Gottstein B, Geary TG, Rossignol JF, Hemphill A. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob Agents Chemother 47:467–474. doi: 10.1128/AAC.47.2.467-474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duke BO. 1977. The effects of some drugs—pentamidine, stibocaptate, Hoechst 33258, F 151, compound ‘E’ and Nifurtimox—on Onchocerca volvulus in chimpanzees. Tropenmed Parasitol 28:447–455. [PubMed] [Google Scholar]
- 22.Milord F, Pepin J, Loko L, Ethier L, Mpia B. 1992. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 340:652–655. doi: 10.1016/0140-6736(92)92180-N. [DOI] [PubMed] [Google Scholar]
- 23.Sonişik M, Korkmaz A, Besim H, Karayalçin K, Hamamci O. 1998. Efficacy of cetrimide-chlorhexidine combination in surgery for hydatid cyst. Br J Surg 85:1277. doi: 10.1046/j.1365-2168.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 24.Naguleswaran A, Spicher M, Vonlaufen N, Ortega-Mora LM, Torgerson P, Gottstein B, Hemphill A. 2006. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob Agents Chemother 50:3770–3778. doi: 10.1128/AAC.00578-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erzurumlu K, Hokelek M, Baris S, Sahin M, Birinci A, Amanvermez R, Tac K. 1998. Effect of albendazole sulfoxide solution on the scolices and the hepatobiliary system. Eur Surg Res 30:433–438. doi: 10.1159/000008610. [DOI] [PubMed] [Google Scholar]
- 26.Besim H, Karayalcin K, Hamamci O, Gungor C, Korkmaz A. 1998. Scolicidal agents in hydatid cyst surgery. HPB Surg 10:347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urrea-París MA, Moreno MJ, Casado N, Rodriguez-Caabeiro F. 2000. In vitro effect of praziquantel and albendazole combination therapy on the larval stage of Echinococcus granulosus. Parasitol Res 86:957–964. doi: 10.1007/PL00008526. [DOI] [PubMed] [Google Scholar]
- 28.Yetim I, Erzurumlu K, Hokelek M, Baris S, Dervisoglu A, Polat C, Belet U, Buyukkarabacak Y, Guvenli A. 2005. Results of alcohol and albendazole injections in hepatic hydatidosis: experimental study. J Gastroenterol Hepatol 20:1442–1447. doi: 10.1111/j.1440-1746.2005.03843.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoudvand H, Fasihi HM, Shakibaie M, Aflatoonian MR, ZiaAli N, Makki MS, Jahanbakhsh S. 2014. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg 12:399–403. doi: 10.1016/j.ijsu.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Wang ZY, Chen Z. 2008. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 31.Tchounwou PB, Patlolla AK, Centeno JA. 2003. Carcinogenic and systemic health effects associated with arsenic exposure–a critical review. Toxicol Pathol 31:575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]