Abstract
We aimed to describe the in vivo activity of humanized pharmacokinetic exposures of meropenem and comparators against Verona integron-encoded metallo-β-lactamase (MBL) (VIM)-producing Enterobacteriaceae in a murine model. Levofloxacin activity was predicted by its MIC, and cefepime activity displayed variability, whereas meropenem produced a >1 log CFU reduction against all isolates despite high MICs indicative of resistance. Our results suggest that despite in vitro resistance, high-dose meropenem may be a possible option against infections caused by Enterobacteriaceae producing MBL-type carbapenemases.
TEXT
The substantial increase in carbapenem resistance secondary to carbapenemase production continues to exhaust the current repository of antibacterial agents (1, 2). Verona integron-encoded metallo-β-lactamase (MBL) (VIM) is one of the emergent MBL carbapenemases. Our group has conducted several studies to characterize the efficacy of carbapenems and other agents against bacteria expressing various carbapenemases, including KPC, NDM, and OXA (3–5). In this study, we aimed to extend these previous observations and assess the efficacy of humanized doses of meropenem, levofloxacin, and cefepime against VIM-producing Enterobacteriaceae using the neutropenic-thigh infection model.
Commercially available levofloxacin (Akorn Inc., Lake Forest, IL), meropenem (Hospira Inc., Lake Forest, IL), and cefepime (Sagent, Schaumberg, IL) were acquired from the Hartford Hospital Pharmacy Department (Hartford, CT). All were reconstituted according the manufacturer's recommendations and further diluted with normal saline solution.
Nine isolates were tested, including a wild-type strain, Klebsiella pneumoniae 454 (KP454), and its corresponding isogenic VIM-producing variant, K. pneumoniae 460, constructed as described previously (6), as well as seven VIM-producing clinical isolates (Table 1). Isolates were frozen at −80°C in double-strength skim milk (Remel, Lenexa, KS) and cultured twice on Trypticase soy agar with 5% sheep blood (BAP; BD Biosciences, Sparks, MD) prior to experimentation. Determinations of the MIC values of levofloxacin, meropenem, and cefepime (Sigma-Aldrich, St. Louis, MO) were performed in triplicate by broth microdilution, and the median MIC values were recorded (7).
TABLE 1.
Listing of test isolates with their respective genotypes and MICsa
| Isolate | Genotype | MIC (mg/liter) |
||
|---|---|---|---|---|
| LEV | FEP | MER | ||
| KP454 WT | CIP 53153 WT | 0.06 | 0.06 | 0.06 |
| KP460 isogenic | CIP 53153, VIM-1 | 0.125 | >512 | 16 |
| KP451 clinical | VIM-1 | >64 | >512 | >512 |
| KP466 clinical | VIM-1 | 16 | >512 | >512 |
| KP467 clinical | VIM-1, SHV-12 | >64 | >512 | >512 |
| KP470 clinical | VIM-1, SHV-31, DHA, ampC | 16 | 32 | 64 |
| CF36b clinical | VIM-4, CTX-M-39, SHV-12, CMY-48 | 16 | 512 | 4 |
| CF37 clinical | VIM-4, CMY-48, TEM-1B | 32 | 4 | 4 |
| ECL85c clinical | VIM-1, SHV-12, OXA-1, CTX-M-9, OXA-1, CTX-M-9, ACT-16 | 32 | >512 | 16 |
KP, Klebsiella pneumoniae; CF, Citrobacter freundii; ECL, Enterobacter cloacae; LEV, levofloxacin; FEP, cefepime; MER, meropenem; DHA, 2,8-dihydroxyadenine.
Institutional designation for MRSN11938.
Institutional designation for MRSN17626.
Subsequent animal experiments or models were approved by the Institutional Animal Care and Use Committee at Hartford Hospital, Hartford, CT. ICR mice were obtained from Harlan Laboratories (Indianapolis, IN) and prepared by intraperitoneal injection with cyclophosphamide at 150 and 100 mg/kg of body weight at day 4 and day 1, respectively, prior to inoculation (8); uranyl nitrate was administered at 5 mg/kg 3 days prior to inoculation to produce a controlled degree of renal impairment. Animals were inoculated in each thigh with 0.1 ml of bacterial suspension containing 107 CFU/ml to target a thigh bacterial density of 105 to 106 cells, and treatment was started 2 h later. Humanized doses of levofloxacin at 500 mg every 24 h (q24h) designed to achieve area under the concentration-time curve (AUC) values of 42 to 53 mg · h/liter (9), meropenem at 2 g q8h as a 30-min infusion (10), and cefepime at 2 g q8h as a 30-min infusion (11), as previously developed and validated by our group, were administered as 0.2-ml subcutaneous injections to groups of three animals over 24 h to simulate the pharmacodynamic profile observed in humans (Table 2). Three mice were harvested as a group at the beginning of dosing (0 h), and another control group was given 0.2 ml subcutaneous normal saline solution. After the 24-h study period, animals were euthanized by CO2 exposure followed by cervical dislocation; next, thighs were excised and homogenized and serial dilutions were plated on BAP for bacterial enumeration. Antibiotic efficacy was calculated as the change in bacterial density relative to the 0-h controls.
TABLE 2.
Expected free drug time above the MIC at different values of MIC for cefepime and meropenema
| MIC (μg/ml) | % fT>MICa |
|
|---|---|---|
| Cefepime | Meropenem | |
| 4 | 100 | 58 |
| 8 | 83 | 45 |
| 16 | 65 | 30 |
| 32 | 38 | 18 |
| 64 | 20 | 5 |
| 128 | 1 | 0 |
To ensure enzyme production across experiments, thigh homogenates from 0-h and 24-h control animals as well as from meropenem-treated mice were plated on agar containing meropenem concentrations equivalent to the MIC of the test strain.
The genotypic profiles and in vitro susceptibilities of the study isolates are listed in Table 1. The values for mean bacterial density ± standard deviation (SD) for 0-h control animals were 5.79 ± 0.26 log CFU, which increased in the 24-h controls to 7.92 ± 0.94 log CFU (Fig. 1). As expected from its phenotypic profile, humanized levofloxacin exhibited activity against the wild-type and isogenic strains but not against the clinical strains. Cefepime manifested mean reductions in CFU against all strains except KP467 and KP466, both of which showed drug MICs of >512 mg/liter. Meropenem was effective against all strains irrespective of MIC. Each isolate showed growth on meropenem-containing agar, confirming VIM expression during experimentation.
FIG 1.
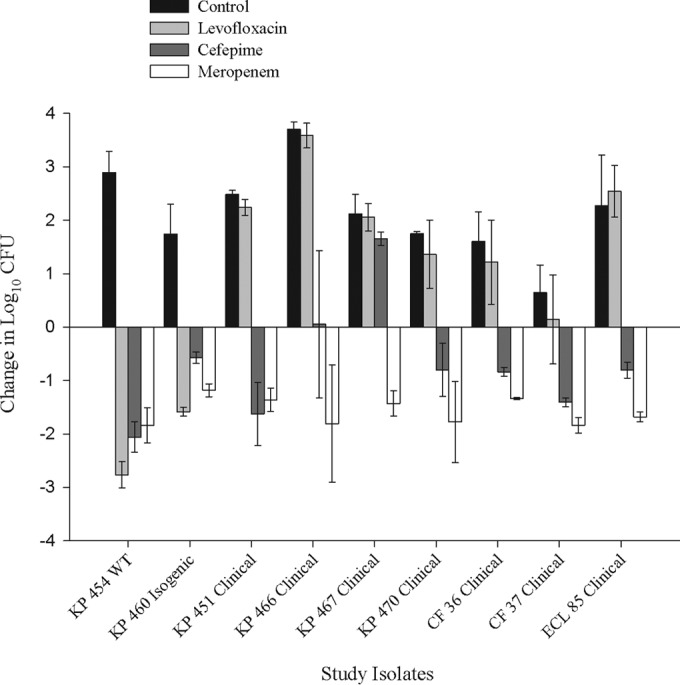
Efficacy of humanized levofloxacin administered at 500 mg q24h and of cefepime administered at 2 g q8h (30-min infusion) and meropenem administered at 2 g q8h (30-min infusion) against Enterobacteriaceae isolates producing VIM in a neutropenic murine thigh model. WT, wild type.
While levofloxacin efficacy was predictable based on MIC, this was not true of the β-lactams. Cefepime manifested efficacy against KP454, KP470, and CF37 which was pharmacodynamically predictable, namely, MIC values of ≤32 μg/ml and free drug time above the MIC (fT>MIC) values of ≥35% (12). However, efficacy also was apparent for 4 isolates with MIC values of >512 μg/ml (0% fT>MIC). Evaluating these isolates genetically, it is apparent that the β-lactam MIC of each is driven almost entirely by VIM; such is the case of isogenic strain KP460 and clinical strain KP451, which produce only VIM, while Citrobacter freundii 36 (CF36) and Enterobacter cloacae 85 (ECL85) produce VIM and a wide array of β-lactamases that poorly hydrolyze cefepime (13, 14). There were, however, two strains against which cefepime had little activity (KP466 and KP467). While it is not clear why these strains differ from the other VIM producers, it is possible that nonenzymatic resistance mechanisms are contributing to the cefepime MIC (15). As a collective, these findings suggest that although VIM is highly active in vitro, it does not confer in vivo resistance to cefepime. In a previous neutropenic-thigh study, similar activity was observed for ceftazidime against an isogenically created NDM-producing strain, despite an MIC of >128 mg/liter; however, unlike cefepime, ceftazidime was minimally active against clinical NDM-producing strains, likely due to coexpression of an extended-spectrum β-lactamase, against which ceftazidime is readily hydrolyzed. Interestingly, the addition of avibactam to ceftazidime, through inhibition of non-MBL enzymes, restored the activity of ceftazidime against the clinical NDM producers (16).
In the current study, meropenem, secondary to its stability against ESBL enzymes, was active against all isolates irrespective of their MIC, further substantiating the diminished role of VIM in vivo, as noted above. Similar observations were also made for ertapenem and doripenem against NDM-producing K. pneumoniae whereby efficacy was observed despite established pharmacodynamic (40% fT>MIC) exposures not being achieved (17). While this phenomenon seems to hold across the MBL enzyme class, experiments with other classes of carbapenemases yielded differing results. Previous work by our group using ertapenem against OXA-48-producing K. pneumoniae in the neutropenic-thigh model found MIC to be predictive of in vivo activity (4). Similarly, when KPC-producing K. pneumoniae strains were studied, results showed that carbapenems were active only when the MIC was ≤4 μg/ml (i.e., when the pharmacodynamic targets were met) (5).
While the majority of the literature on the treatment of VIM-producing organisms is based on murine infection models, some clinical data are available. One such study was a prospective observational study of bloodstream infections caused by K. pneumoniae strains, 41% of which produced VIM-1. Although it is unclear what treatment the patients with VIM-related infections received, that study found that VIM production had no effect on mortality compared to the results seen with non-VIM-producing strains (18). A case series of patients infected with VIM-producing Enterobacteriaceae found that 12 of 17 were successfully treated with carbapenem-containing combinations, regardless of the carbapenem MIC (19). Clearly, additional data are required to validate our in vivo model-derived findings with respect to applicability to clinical practice.
In our study, we characterized the efficacy of meropenem, levofloxacin, and cefepime against VIM-producing K. pneumoniae. Levofloxacin efficacy paralleled the MIC phenotype, although this was not true of the beta-lactams. Regardless of the MIC, meropenem was effective against all 8 VIM-producing stains and cefepime was active against 5 of these isolates. Our data corroborate the findings of previous studies showing that genotype should be considered in the choice of effective antibiotics and suggest that carbapenems may still be a therapeutic consideration for Enterobacteriaceae producing MBL-type carbapenemases.
ACKNOWLEDGMENTS
This study was conducted using internal funding from the Center for Anti-Infective Research and Development at Hartford Hospital.
We acknowledge Kimelyn Greenwood, Jennifer Hull, Lucinda Lamb, Sara Robinson, Debora Santini, Pamela Tessier, and Abrar Thabit (Center for Anti-Infective Research and Development, Hartford Hospital) for their assistance with animal experiments and MIC determination. VIM-producing strains were kindly provided by Patrice Nordmann and the International Health Management Associates.
REFERENCES
- 1.Centers for Disease Control and Prevention (CDC). 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev 18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiskirchen DE, Crandon JL, Nicolau DP. 2013. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae. Int J Antimicrob Agents 41:582–585. doi: 10.1016/j.ijantimicag.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. Efficacy of humanized carbapenem and ceftazidime regimens against Enterobacteriaceae producing OXA-48 carbapenemase in a murine infection model. Antimicrob Agents Chemother 58:1678–1683. doi: 10.1128/AAC.01947-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagihara M, Crandon JL, Urban C, Nicolau DP. 2013. Efficacy of doripenem and ertapenem against KPC-2-producing and non-KPC-producing Klebsiella pneumoniae with similar MICs. J Antimicrob Chemother 68:1616–1618. doi: 10.1093/jac/dkt056. [DOI] [PubMed] [Google Scholar]
- 6.Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI publication M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother 42:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onyeji CO, Bui KQ, Owens RC, Nicolau SP, Quintiliani R, Nightingale CH. 1999. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicaemia. Int J Antimicrob Agents 12:107–114. doi: 10.1016/S0924-8579(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 10.DeRyke CA, Banevicius MA, Fan HW, Nicolau DP. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob Agents Chemother 4:1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crandon JL, Nicolau DP. 2015. In vivo activity of humanized cefepime and cefepime/AAI101 against multi-drug resistant Gram-negative Enterobacteriaceae. Antimicrob Agents Chemother 59:2688–2694. doi: 10.1128/AAC.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin Infect Dis 44:79–86. [DOI] [PubMed] [Google Scholar]
- 13.Rupp ME, Fey PD. 2003. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs 63:353–365. doi: 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 14.Queenan AM, Shang W, Flamm R, Bush K. 2010. Hydrolysis and inhibition profiles of β-lactamases from molecular classes A to D with doripenem, imipenem, and meropenem. Antimicrob Agents Chemother 54:565–569. doi: 10.1128/AAC.01004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Jiang X, Wang Y, Li G, Tian Y, Liu H, Ai F, Ma Y, Wang B, Ruan F, Rajakumar K. 2014. Contribution of β-lactamases and porin proteins OmpK35 and OmpK36 to carbapenem resistance in clinical isolates of KPC-2-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 58:1214–1217. doi: 10.1128/AAC.02045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. In vivo efficacy of human simulated regimens of carbapenems and comparator agents against NDM-1-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:1671–1677. doi: 10.1128/AAC.01946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, Georgousi K, Tzouvelekis LS, Tassios PT, Bamia C, Petrikkos G. 2009. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 53:1868–1873. doi: 10.1128/AAC.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souli M, Kontopidou FV, Papadomichelakis E, Galani I, Armaganidis A, Giamarellou H. 2008. Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-beta-lactamase in a Greek university hospital. Clin Infect Dis 46:847–854. doi: 10.1086/528719. [DOI] [PubMed] [Google Scholar]


