Abstract
Apart from inadequate antimicrobial treatment, specific virulence factors contribute to the high attributable mortality of infections caused by multidrug-resistant (MDR) Klebsiella pneumoniae. We explored the roles of MDR and clones as virulence determinants of K. pneumoniae and their interaction with innate immunity. Twenty isolates were studied and characterized by resistance phenotype and multilocus sequence type (MLST). Human peripheral blood mononuclear cells (PBMCs) were stimulated for the production of proinflammatory cytokines by live and heat-killed isolates and plasmid DNA; modulation by cellular pathway inhibitors was explored. Survival of 30 mice was recorded after intraperitoneal challenge with susceptible and K. pneumoniae carbapenemase (KPC)-producing isolates. Splenocytes of mice were stimulated for the production of pro- and anti-inflammatory cytokines. Isolates were divided into different patterns of production of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) poststimulation in relation to both the MLST clone and resistance phenotype. The sequence type 383 (ST383) clone producing Verona integron-encoded metallo-β-lactamase (VIM) stimulated high production of both TNF-α and IL-1β. Clone ST17 producing KPC elicited low TNF-α production; this was reversed by Toll-like receptor 9 (TLR9) antagonists, indicating an effect of plasmid DNA. This isolate was linked with early death of mice compared to high-TNF-α-producing isolates. We conclude that KPC-producing isolates seem to be highly virulent in a low-TNF-α-release environment, suggesting an immunoparalysis induction mechanism. KPC plasmids may directly contribute to the immune system stimulation.
INTRODUCTION
Klebsiella pneumoniae has emerged over the past decades as a major pathogen associated with hospital-acquired and opportunistic infections, such as pneumonia, bacteremia, and urinary tract infection. K. pneumoniae is the principal causative pathogen of community-acquired pyogenic liver abscess (1). The acquisition of multidrug resistance (MDR) by K. pneumoniae has been one of the critical features that have enabled it to dominate as a health-care-associated pathogen. MDR in K. pneumoniae is steadily emerging. A recent study conducted in 31 Greek hospitals postulated that the incidence of K. pneumoniae isolates producing carbapenemases (KPC) and extended-spectrum β-lactamases (ESBL), both in the general ward (GW) and in the intensive care unit (ICU), has considerably increased during the period 2010 to 2013: KPC-producing isolates have increased from 5% to almost 25% of all cases of K. pneumoniae infections in the GW, and more than 70% of K. pneumoniae infections in the ICU are caused by KPC-producing isolates (2).
Traditionally, wild-type K. pneumoniae generates an indolent presentation that if left untreated leads to fulminant sepsis and death. However, clinical practice teaches that patients residing in the GW or in the ICU colonized by MDR K. pneumoniae often present with mild clinical symptoms (3, 4). This generates the hypothesis that acquisition of resistance genes may modulate the susceptibility of the host to K. pneumoniae. The aim of the present study was to explore how the acquisition of the MDR phenotype by K. pneumoniae modulates the clinical severity of infection through its interaction with the innate immune system.
MATERIALS AND METHODS
Selection of isolates.
Twenty isolates of K. pneumoniae were studied, derived from the following clinical specimens: blood (n = 7), quantitative cultures of bronchoalveolar lavage fluid (n = 7), urine (n = 3), and pus (n = 3). Each isolate was derived from a different patient; isolates were genetically distinct as defined by pulsed-field gel electrophoresis (PFGE) of their DNA (see Fig. S1 in the supplemental material). The phenotype of resistance to antimicrobials was determined by measurement of the MICs of 16 antimicrobials (see Table S1 in the supplemental material). The MIC was determined by a microdilution technique at a final volume of 0.1 ml using a 5 × 105-CFU/ml log-phase inoculum. Results were interpreted in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (5). All isolates were screened for metallo-β-lactamase (MBL) and class A carbapenemase production with EDTA-meropenem and meropenem-boronic acid disk synergy tests (6), respectively. The presence of KPC and Verona integron-encoded metallo-β-lactamase (VIM) genes was confirmed by PCR with specific primers (7, 8) (provided in Table S2 in the supplemental material). ESBL production was tested with the double disk (DD) approximation test (9). To run the PFGE, after bacterial cell lysis by sonication, genomic DNA was isolated, digested by the restriction endonuclease SpeI, and subjected to electrophoresis on 1.5% agarose gel, the voltage was adjusted to 200 V, the temperature was set to 12°C, and two phases of ramping (3 to 15 s for 15 h and 1 to 27 s for 23 h) were performed in a Gene Navigator apparatus (Pharmacia, Uppsala, Sweden).
MLST.
Genomic DNA was extracted using the NucleoSpin Tissue kit (Macherey-Nagel, Duren, Germany). Multilocus sequence typing (MLST) was performed as described by Diancourt et al. (10), detecting sequences of the seven conserved housekeeping genes rpoB, gapA, mdh, pgi, phoE, infB, and tonB. PCRs were carried out using primers (listed in Table S3 in the supplemental material) and applying conditions detailed on the KP-MLST database (http://bigsdb.web.pasteur.fr/klebsiella/klebsiella.html). PCR products were purified using NucleoSpin gel and PCR cleanup kit (Macherey-Nagel). Sequencing was performed using an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA) and a BigDye Terminator DNA sequencing kit (Applied Biosystems). The obtained sequences were queried against the pubMLST database to determine the allele designations and sequence type (ST) of each isolate.
In vitro stimulation of cytokine production.
Human peripheral blood mononuclear cells (PBMCs) were isolated from 10 healthy adult volunteers after gradient centrifugation of heparinized whole blood over Ficoll-Hypaque (Biochrom, Berlin, Germany). The separated mononuclear cells were washed three times with phosphate-buffered saline (PBS [pH 7.2]) (Merck, Darmstadt, Germany) and resuspended in RPMI 1640 (Biochrom) supplemented with 10% fetal bovine serum (Biochrom) in the presence of 100 U/ml penicillin G and 0.1 mg/ml of streptomycin (Sigma, St. Louis, MO). PBMCs were counted in a Neubauer chamber after trypan blue exclusion of dead cells.
Initially, PBMCs were stimulated by live isolates. A log-phase inoculum of each isolate was grown after incubation of 3 to 5 colonies for 12 h in 10 ml of Mueller-Hinton broth (Oxoid, London, United Kingdom) at 37°C in a shaking water bath. The bacterial pellet was washed with phosphate-buffered saline (PBS [pH 7.2]) and reconstituted in RPMI 1640. PBMCs were distributed into a 12-well plate at a final density of 5 × 106 CFU/ml; isolates were added at a final inoculum of 2 × 105 CFU/ml. Incubation was stopped after 1.5, 2.5, and 4 h, when plates were centrifuged. The supernatants were collected and stored at −70°C until assayed for human tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6. Furthermore, quantitative cultures of the wells after 1.5 h of incubation were performed in order to assess growth differences between the isolates.
Separate experiments were done using heat-killed isolates and plasmid DNA. Heat killing was achieved by incubating a log-phase inoculum of each isolate in a shaking water bath at 70°C for 3 h; absence of growth was confirmed by quantitative cultures. PBMCs were stimulated by 5 × 103 CFU/ml of each heat-killed isolate. After 24 h of incubation, the supernatants were collected and assayed for TNF-α, IL-1β, and IL-6. Some experiments were repeated using 5 × 103 CFU/ml of heat-killed isolates in the absence or presence of 10 and 100 μg/ml of crystals of monosodium urate monohydrate (MSU), which is nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) inflammasome stimulus. MSU crystals were prepared as described elsewhere (11).
Plasmid DNA of the studied isolates was extracted using the Plas/mini isolation kit XL (AppliChem, Darmstatd, Germany) and quantitated by spectrophotometer. Ten microliters of plasmid DNA from each isolate was used for the stimulation of 5 × 106 CFU/ml PBMCs per well. After 24 h of incubation, supernatants were collected and assayed for cytokines. Experiments were also repeated in the presence of the following inhibitors of different pathways of cytokine production: SB203580 (InvivoGen, San Diego, CA), which is a p38/RK mitogen-activated protein kinase (MAPK) inhibitor, at a final concentration of 3 μmol/liter; Escherichia coli K-12 lipopolysaccharide (LPS) (InvivoGen), which is a Toll-like receptor 4 (TLR4) antagonist, at a final concentration of 1 μg/ml; polymyxin B (Norma Hellas, Greece), which is an LPS binder, at a final concentration of 90 IU/ml; oligodeoxynucleotide (ODN) 4084-F (InvivoGen), which is a TLR9 antagonist at a final concentration of 1 μΜ; c-Jun N-terminal kinase (JNK) inhibitor (SP600125; Biomol, United Kingdom) at a final concentration of 3 μmol/liter; and spleen tyrosine kinase (SYK) inhibitor (catalog no. 574711; Calbiochem, San Diego, CA) at a final concentration of 3 μmol/liter.
TNF-α, IL-1β, and IL-6 were measured in culture supernatants in duplicate by an enzyme-linked immunosorbent assay (eBioscience, Inc., San Diego, CA) according to the instructions of the manufacturer. The lowest limit of detection was 20 pg/ml.
Animal study.
Thirty male C57BL/6 mice (body weight, 18 to 22 g; 8 to 10 weeks of age) were used for survival experiments. Animals were kept at three mice per cage in individually ventilated cages (IVC) with a HEPA filter and had access to tap water and food ad libitum. The temperature ranged between 18 and 22°C, the relative humidity ranged between 55 and 65%, and the light/dark cycle was 6 a.m./6 p.m. The experimental protocol was conducted in 2012, and it was approved by the Veterinary Directorate of the Prefecture of Athens, according to Greek legislation in conformance with the 160/91 Directive Council of the EU (approval no. 2113/2009). Ten isolates of K. pneumoniae (five susceptible and five KPC producing) were used. Single colonies of each isolate were incubated in Mueller-Hinton broth at 37°C for 12 h. A total inoculum of 5 × 106 CFU/g from each isolate was injected intraperitoneally into three animals. Survival of the animals was recorded every 12 h for a total of 7 days. Sacrifice of the remaining mice was done by inhalation of diethyl ether. Quantitative liver cultures of all animals were performed as described elsewhere (12).
Another 24 mice were sacrificed 12 h after intraperitoneal challenge by three susceptible and three KPC-producing isolates, with 12 mice per group. From each mouse, the spleen was removed, and splenocytes were isolated after gentle squeezing of the spleen through a sterile filter (250 mm, 12 by 13 cm; Alter Chem Co., Athens, Greece). After being counted in a Neubauer chamber, isolated splenocytes were stimulated for the production of TNF-α after 24 h of incubation in the absence or presence of 10 ng/ml of LPS from E. coli O55:B5, as previously described, or for 5 days in the absence or presence of 5 × 105 CFU/ml heat-killed Candida albicans, and supernatants were assayed for IL-10, IL-17, and interferon gamma (IFN-γ). Quantitative liver and kidney cultures were performed.
Statistical analysis.
The study endpoint was to explore the combination of MLST cloning and MDR phenotyping that is linked with high virulence of K. pneumoniae. To this end, four isolates that failed to be typed were excluded from analysis. Isolates were categorized into groups according to their potency for TNF-α production by PBMCs. Percentiles of concentrations were calculated: isolates eliciting production distributed at the upper percentile were considered high TNF-α inducers, those at the 2nd and 3rd percentiles were considered moderate TNF-α-inducers, and those at the lower percentile were considered low TNF-α-inducers. The same was done for IL-1β production to group isolates into high and low inducers. In order to evaluate inflammasome activation, the percentage of change in IL-1β after addition of MSU was estimated. For the determination of the mechanism of TNF-α modulation, the percentage of change in TNF-α after addition of the various inhibitors of receptors or of intracellular pathways was estimated. Comparisons between groups were performed by the Mann-Whitney U test. Survival of the animals was assessed by Kaplan-Meier analysis; comparisons between groups were done by the log-rank test. Any P value below 0.05 after Bonferroni's adjustment for multiple comparisons was considered significant.
RESULTS
Sixteen of the studied isolates belonged to nine discrete MLST clones: ST147 (n = 4), ST258 (n = 3), ST383 (n = 2), ST17 (n = 2), ST1237 (n = 1), ST340 (n = 1), ST528 (n = 1), ST347 (n = 1), and ST643 (n = 1). Clones of four isolates could not be defined (see Table S1 in the supplemental material), and they were excluded from further analysis.
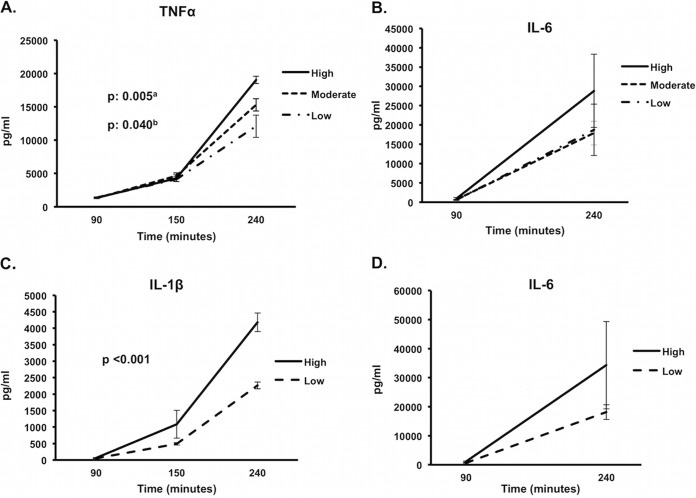
In vitro stimulation of PBMCs by live isolates revealed three different patterns of TNF-α production over time in relation to both MLST clone and resistance phenotype: (i) those that stimulated high TNF-α production, comprising ST383 VIM, ST258 ESBL, and ST528 susceptible; (ii) those that stimulated moderate TNF-α production, comprising ST258 KPC, ST147 susceptible, ST147 ESBL, ST17 VIM, ST643 susceptible, ST1237 VIM, and ST347 KPC; and (iii) those that stimulated low TNF-α production, comprising ST147 VIM, ST340 KPC, ST383 ESBL, and ST17 KPC (Fig. 1A). IL-6 production followed the pattern of TNF-α production (Fig. 1B).
FIG 1.
Pattern of overtime cytokine production by Klebsiella pneumoniae in relation to multilocus sequence typing (MLST) cloning and resistance phenotype. (A) High, high-tumor necrosis factor alpha (TNF-α)-producing isolates (ST383 VIM, ST258 ESBL, ST528 susceptible); Moderate, moderate-TNF-α-producing isolates (ST258 KPC, ST147 susceptible, ST147 ESBL, ST17 VIM, ST643 susceptible, ST1237 VIM, ST347 KPC); Low, low-TNF-α-producing isolates (ST147 VIM, ST340 KPC, ST383 ESBL, ST17 KPC); (C) High, high-interleukin-1β (IL-1β)-producing isolates (ST383 VIM, ST347 KPC); Low, low-IL-1β-producing isolates (all other except nontypeable). a, high versus low; b, high versus moderate. (B and D) IL-6 kinetics in relation to TNF-α and IL-1β production. Comparisons were performed by the Mann-Whitney U test with Bonferroni's correction. Six experiments were run using cells from two healthy volunteers. Abbreviations: ST, sequence type; VIM, Verona integron-encoded metallo-β-lactamase; ESBL, extended-spectrum β-lactamases; KPC, Klebsiella pneumoniae carbapenemase.
Two different patterns of IL-1β production could be detected: (i) high IL-1β inducers, comprising ST383 VIM and ST347 KPC, and (ii) low IL-1β inducers, comprising all other isolates (Fig. 1C). IL-6 production followed the pattern of IL-1β production (Fig. 1D) as well. These results showed that production of IL-6 was driven through TNF-α and IL-1β. From the total of studied isolates, ST383 VIM was the only isolate that stimulated high production of both TNF-α and IL-1β (Fig. 2).
FIG 2.
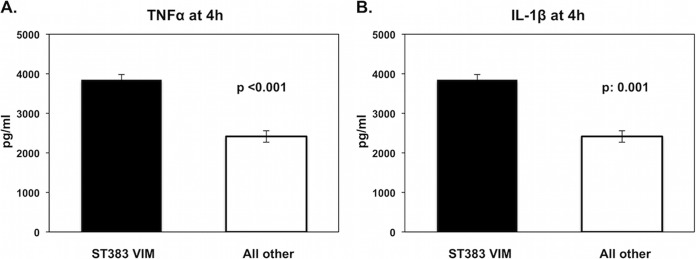
Tumor necrosis factor alpha (TNF-α) (A) and interleukin-1β (IL-1β) (B) stimulation at 4 h by sequence type 383 (ST383) Verona integron-encoded metallo-β-lactamase (VIM) versus stimulation by all other isolates. Comparisons were performed by the Mann-Whitney U test. Six experiments were run using cells from two healthy volunteers.
Bacterial growth at 1.5 h did not differ significantly between groups (Fig. 3). This allowed us to exclude difference in bacterial replication as an explanation of the diversity in cytokine production.
FIG 3.
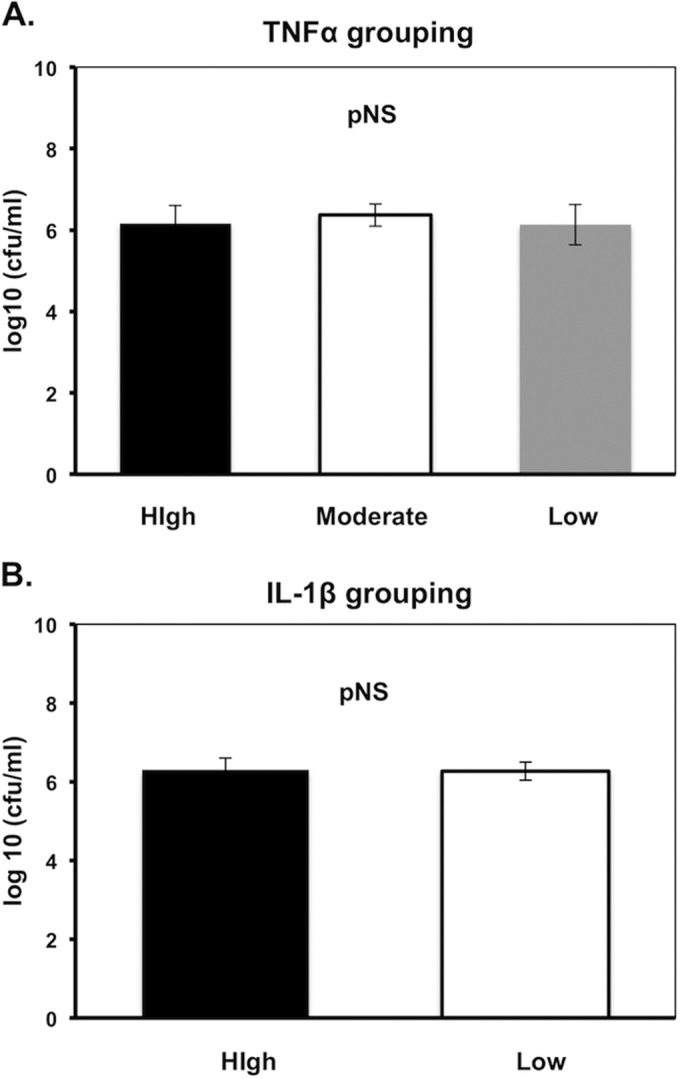
Growth at 1.5 h of incubation of Klebsiella pneumoniae isolates in relation to the pattern of tumor necrosis factor alpha (TNF-α) (A) and interleukin-1β (IL-1β) (B) production. (A) High, high-TNF-α-producing isolates; Moderate, moderate TNF-α-producing isolates; Low, low-TNF-α-producing isolates; (B) High, high-IL-1β-producing isolates; Low, low-IL-1β-producing isolates. pNS, difference nonsignificant. Comparisons were performed by the Mann-Whitney U test. Five experiments were run using cells from two healthy volunteers.
At the next step, we tried to explore the underlying mechanism of high or low TNF-α/IL-1β production by the studied isolates. We hypothesized that specific pathogen-associated molecular patterns (PAMPs) of susceptible K. pneumoniae and of K. pneumoniae isolates carrying the MDR phenotype may be responsible for the observed differences. These PAMPs may differ in kinetics of release from one isolate to the other. These PAMPs may be LPS, exotoxins, or plasmid DNA itself. To demonstrate this, we used inhibitors of intracellular monocyte pathways.
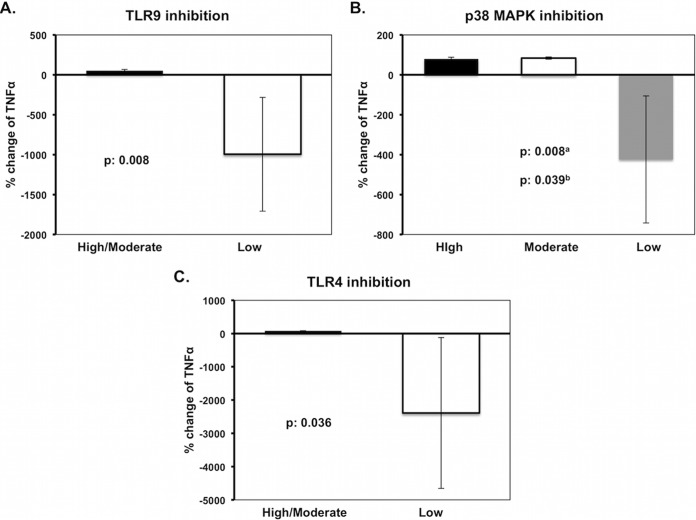
Stimulation of TNF-α production by the low-TNF-α-producing isolates was mainly driven through TLR9, as shown after TLR9 blockade (P = 0.008 versus high- and moderate-TNF-α-producing isolates), indicating plasmid DNA as one responsible PAMP. Similar differences were produced after p38 MAPK inhibition (P = 0.008 for low versus moderate, and P = 0.039 for low versus high) and by TLR4 inhibition (P = 0.036) (Fig. 4), thus indicating the rate of LPS release as another mechanism.
FIG 4.
Mechanism of tumor necrosis factor alpha (TNF-α) modulation by Klebsiella pneumoniae in relation to multilocus sequence typing (MLST) cloning and resistance phenotype. (A) TNF-α production after stimulation of mononuclear cells with plasmid DNA in the presence of Toll-like receptor 9 (TLR9) inhibitor; (B and C) TNF-α production after stimulation of mononuclear cells by heat-killed isolates in the presence of the respective inhibitors. High, high-TNF-α-producing isolates; Moderate, moderate-TNF-α-producing isolates; Low, low-TNF-α-producing isolates. a, high versus low; b, high versus moderate. Comparisons were performed by the Mann-Whitney U test with Bonferroni's corrections. Five experiments were run using cells from two healthy volunteers.
Since production of IL-1β is driven through modulation of pro-IL-1β cleavage after activation of the NLRP3 inflammasome, experiments were repeated in which production of IL-1β was assayed after stimulation in the presence of the NLRP3 agonist MSU. A substantially different pattern of IL-1β production was observed between high- and low-IL-1β-producing heat-killed isolates. More precisely, stimulation with high-IL-1β-producing isolates and MSU induced a 10.4% reduction in IL-1β levels, whereas mean release of IL-1β stimulated by low-IL-1β-producing isolates and MSU was increased by 23.8%, denoting significant synergy at the NLRP3 stimulation level (P = 0.025).
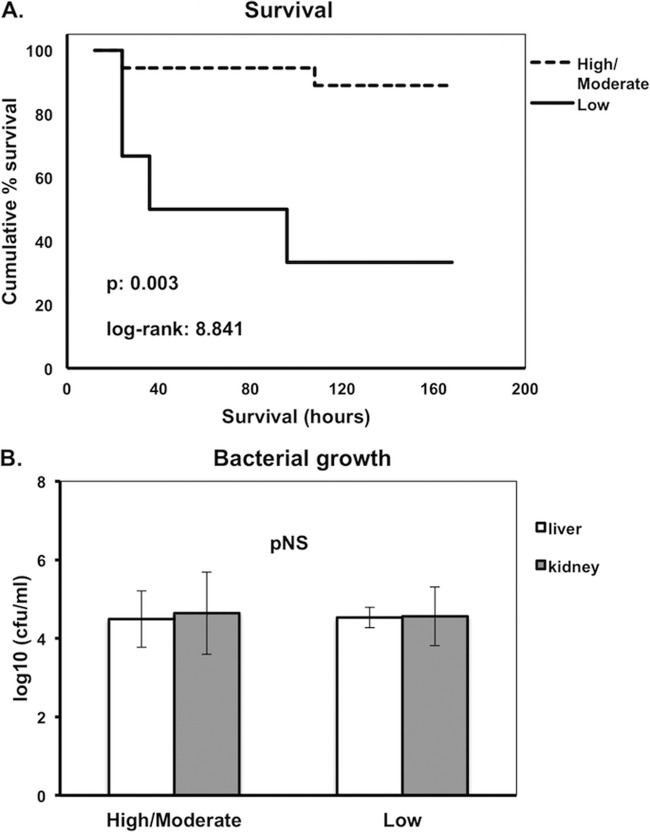
In the next step, we explored whether the observed differences in cytokine stimulation between different isolates of K. pneumoniae may reflect differences in survival after challenge of mice. Indeed, survival of mice challenged by high-TNF-α-producing isolates was significantly lower than that of mice challenged by moderate- and/or low-TNF-α-producing isolates (P = 0.003, log rank, 8.841) (Fig. 5A). In contrast, no significant differences in survival were observed between high- and low-IL-1β-inducing isolates (data not shown).
FIG 5.
(A) Kaplan-Meier survival curves of mice after intraperitoneal challenge by Klebsiella pneumoniae in relation to patterns of tumor necrosis factor alpha (TNF-α) production. High, high-TNF-α-producing isolates; Moderate, moderate-TNF-α-producing isolates; Low, low-TNF-α-producing isolates. Comparisons were performed by the log rank test. Six experiments were run. (B) Bacterial growth of K. pneumoniae in liver and kidney of mice sacrificed 24 h after intraperitoneal challenge by high/moderate- and low-TNF-α-producing isolates. pNS, difference nonsignificant. Comparisons were performed by the Mann-Whitney U test. Six experiments were run.
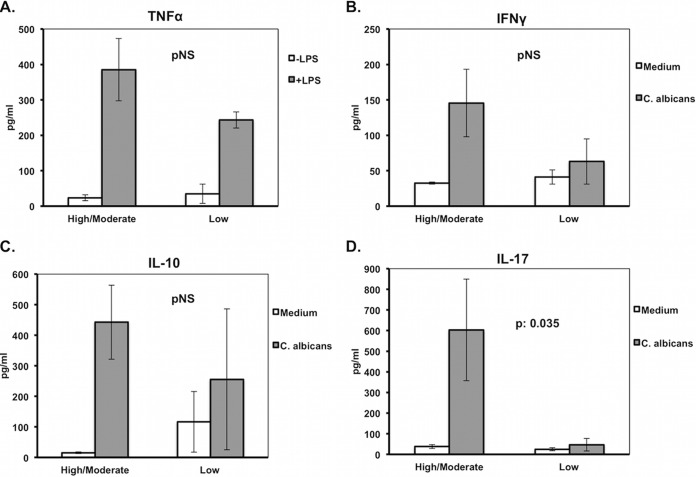
Quantitative liver and kidney cultures of animals sacrificed 24 h after intraperitoneal challenge did not show any statistical difference in bacterial growth between high/moderate- and low-TNF-α-producing isolates that could possibly attribute the difference in TNF-α production to the rate of bacterial replication (Fig. 5B). However, stimulation of murine splenocytes revealed decreased production of IL-17 by the low-TNF-α-inducing isolates, whereas similar changes were not found for TNF-α, IFN-γ, and IL-10 (Fig. 6). At the end of the 7-day follow-up for mouse survivors or at the time of animal death, tissue bacterial growth was measured. Survival time was negatively correlated with the tissue outgrowth (Fig. 7). This led us to consider defective production of IL-17 after stimulation by the low-TNF-α-inducing isolates as a cytokine effect modulating tissue growth and subsequent survival.
FIG 6.
Release of interleukin-10 (IL-10), IL-17, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ) after stimulation of murine splenocytes. P values represent comparisons of the percentage of change of TNF-α production after lipopolysaccharide (LPS) stimulation for high/moderate- versus low-TNF-α-producing isolates (A), IFN-γ production after Candida albicans stimulation for high/moderate- versus low-TNF-α-producing isolates (B), IL-10 production after C. albicans stimulation for high/moderate- versus low-TNF-α-producing isolates (C), and IL-17 production after C. albicans stimulation for high/moderate- versus low-TNF-α-producing isolates (D). pNS, difference nonsignificant. Comparisons were performed by the Mann-Whitney U test. Six experiments were run.
FIG 7.
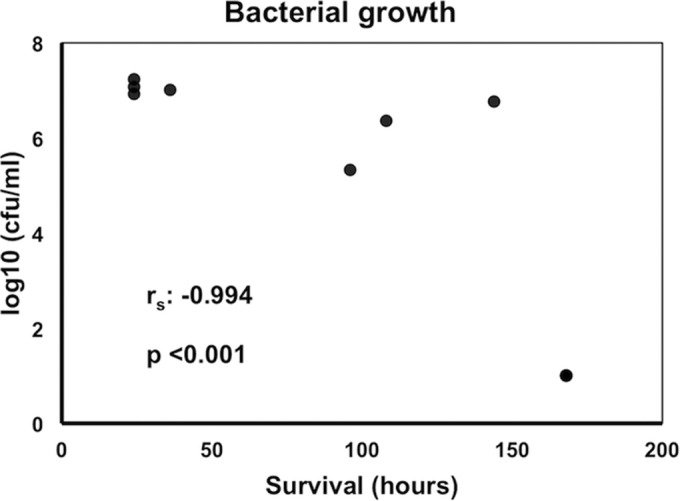
Correlation between bacterial growth of Klebsiella pneumoniae in liver of mice after intraperitoneal challenge and survival time. Comparisons were performed by Spearman's rank correlation. Six experiments were run.
DISCUSSION
The present study suggests that both the resistance profile and clone of K. pneumoniae isolates are involved in the interaction with the host immune system and determine the outcome of infection. Virulence of K. pneumoniae is determined by the stimulation of TNF-α production. More precisely, KPC-producing isolates of the ST17 clone are highly virulent by a mechanism associated with low release of both TNF-α and IL-17 mediated through stimulation with plasmid DNA on TLR9. Existing evidence for TNF-α as a driver of IL-17 production explains this (13). The remaining isolates differ in cytokine production capacity: more precisely, their LPS-releasing capacity and NLRP3 inflammasome interaction highly vary from one clone to the other; the resistance mechanism may also play some role. Since the studied isolates came from different clinical specimens (blood, tracheobronchial secretions, urine, and pus), it can be speculated the results are highly generalizable.
Two main limitations of the present study should be acknowledged. The first limitation is the use of PBMCs isolated from healthy volunteers and of immunocompetent mice, whereas infections by MDR K. pneumoniae strains are more frequent in immunosuppressed or elderly patients. Therefore, it is possible that cytokine production may differ in that setting. Traditionally, the early hyperinflammatory response of severe infections is followed by a period of immunosuppression when monocytes and macrophages have defective cytokine production. Immunosuppression is associated with an unfavorable outcome (14). Highly virulent ST17 KPC emerges through the elicitation of a state of low cytokine production and mimics the state of sepsis-induced immunosuppression. The second limitation of the study is the lack of data reporting on the expression of HLA-DR on monocytes, which is an index of infection-induced immunosuppression.
Several attempts to correlate resistance and individual isolate characteristics to virulence and immune system stimulation have been made in the past. Giamarellos-Bourboulis et al. (15) demonstrated that significant differences are encountered in host-pathogen interactions between MDR and susceptible Pseudomonas aeruginosa isolates; susceptible isolates induced a greater release of proinflammatory cytokines accompanied by increased mortality in rodents. The opposite was found when the study design was repeated with E. coli species. More precisely (16), MDR E. coli induced a potent early production of proinflammatory cytokines, followed by low release of IL-10; this was accompanied by prolonged survival in an experimental sepsis model in rabbits much resembling the findings of K. pneumoniae. Peres et al. (17) showed that Staphylococcus aureus isolates have differential capacities to induce pro- and anti-inflammatory cytokine production by human monocytes, implicating that these responses may determine colonization and disease tolerance versus pathogenicity and disease.
To our knowledge, the current study is the first to evaluate the combined role of MDR and clone in virulence of K. pneumoniae. Siu et al. (18) investigated virulence and murine mortality of KPC-producing K. pneumoniae strains; they failed to detect any known virulence factors and suggested that increased mortality in KPC-producing infections may not be attributed to increased virulence of these isolates. Tzouvelekis et al. (19) evaluated the virulence of KPC-producing ST258 in an animal model and concluded that high mortality is largely due to inadequate antimicrobial treatment.
The present results suggest that the clinical course of K. pneumoniae infections seems to be an unpredictable phenomenon that involves more than one virulence mechanism. Taking into consideration the risk for empirical inappropriate antimicrobial therapy associated with unfavorable outcome of the patients (20), it is suggested that aggressive broad-spectrum antimicrobial coverage be mandated for hospital-acquired K. pneumoniae infections. The high mortality associated with KPC-producing K. pneumoniae has been demonstrated in several studies in the past (21, 22). In a recent study, Daikos et al. (4) showed that combination therapy with carbapenem-containing regimens provides a significant survival benefit in KPC-producing K. pneumoniae infections.
Using an integrative approach, it is concluded that the high mortality associated with MDR K. pneumoniae may be attributed, among other things, to the intense immunoparalysis caused by such isolates. The increasing resistance to antibiotics among K. pneumoniae isolates urges the need of extending our knowledge on the mechanisms of pathogenesis of infections by these bacteria in order to develop new therapies. Part of future studies should rely on the effect of MDR K. pneumoniae on the adaptive immune responses.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Hellenic Institute for the Study of Sepsis.
The authors of this article have no commercial or other associations that might pose a conflict of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01405-15.
REFERENCES
- 1.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. 2012. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 2.Koupetori M, Retsas T, Antonakos N, Vlachogiannis G, Perdios I, Nathanail C, Makaritsis K, Papadopoulos A, Sinapidis D, Giamarellos-Bourboulis EJ, Pneumatikos I, Gogos C, Armaganidis A, Paramythiotou E, Hellenic Sepsis Study Group. 2014. Bloodstream infections and sepsis in Greece: over-time change of epidemiology and impact of de-escalation on final outcome. BMC Infect Dis 14:272. doi: 10.1186/1471-2334-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giamarellos-Bourboulis EJ, Papadimitriou E, Galanakis N, Antonopoulou A, Tsaganos T, Kanellakopoulou K, Giamarellou H. 2006. Multidrug resistance to antimicrobials as a predominant factor influencing patient survival. Int J Antimicrob Agents 27:476–481. doi: 10.1016/j.ijantimicag.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis A, Goukos D, Skoutelis A. 2014. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; twenty fourth informational supplement. CLSI document M100-S124. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Tsakris A, Poulou A, Pournaras S, Voulgari E, Vrioni G, Themeli-Digalaki K, Petropoulou D, Sofianou D. 2010. A simple phenotypic method for the differentiation of metallo-beta-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J Antimicrob Chemother 65:1664–1671. doi: 10.1093/jac/dkq210. [DOI] [PubMed] [Google Scholar]
- 7.Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin Infect Dis 39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 8.Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo JD, Nordmann P. 2000. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother 44:891–897. doi: 10.1128/AAC.44.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stürenburg E, Mack D. 2003. Extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory, therapy, and infection control. J Infect 47:273–295. doi: 10.1016/S0163-4453(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 10.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis EJ, Mouktaroudi M, Bodar E, van der Ven J, Kullberg BJ, Netea MG, van der Meer JW. 2009. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann Rheum Dis 68:273–278. doi: 10.1136/ard.2007.082222. [DOI] [PubMed] [Google Scholar]
- 12.Louis K, Netea MG, Carrer DP, Kotsaki A, Mylona V, Pistiki A, Savva A, Roditis K, Alexis A, Van der Meer JW, Giamarellos-Bourboulis EJ. 2013. Bacterial translocation in an experimental model of multiple organ dysfunctions. J Surg Res 183:686–694. doi: 10.1016/j.jss.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 13.Onishi RM, Gaffen SL. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelekanou A, Tsangaris I, Kotsaki A, Karagianni V, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ. 2009. Decrease of CD4-lymphocytes and apoptosis of CD14-monocytes are characteristic alterations in sepsis caused by ventilator-associated pneumonia: results from an observational study. Crit Care 13:R172. doi: 10.1186/cc8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giamarellos-Bourboulis EJ, Plachouras D, Tzivras A, Kousoulas V, Bolanos N, Raftogiannis M, Galani I, Dontas I, Dionyssiou-Asteriou A, Giamarellou H. 2004. Stimulation of innate immunity by susceptible and multidrug-resistant Pseudomonas aeruginosa: an in vitro and in vivo study. Clin Exp Immunol 135:240–246. doi: 10.1111/j.1365-2249.2003.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bristianou M, Panagou C, Adamis T, Raftogiannis M, Antonopoulou A, Chrisofos M, Galani I, Kanellakopoulou K, Tsaganos T, Giamarellos-Bourboulis EJ. 2008. The impact of multidrug resistance on the pathogenicity of Escherichia coli: an experimental study. Int J Antimicrob Agents 31:216–223. doi: 10.1016/j.ijantimicag.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Peres AG, Stegen C, Li J, Xu AQ, Levast B, Surette MG, Cousineau B, Desrosiers M, Madrenas J. 2015. Uncoupling of pro- and anti-inflammatory properties of Staphylococcus aureus. Infect Immun 83:1587–1597. doi: 10.1128/IAI.02832-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siu LK, Lin JC, Gomez E, Eng R, Chiang T. 2012. Virulence and plasmid transferability of KPC Klebsiella pneumoniae at the Veterans Affairs Healthcare System of New Jersey. Microb Drug Resist 18:380–384. doi: 10.1089/mdr.2011.0241. [DOI] [PubMed] [Google Scholar]
- 19.Tzouvelekis LS, Miriagou V, Kotsakis SD, Spyridopoulou K, Athanasiou E, Karagouni E, Tzelepi E, Daikos GL. 2013. KPC-producing, multidrug-resistant Klebsiella pneumoniae sequence type 258 as a typical opportunistic pathogen. Antimicrob Agents Chemother 57:5144–5146. doi: 10.1128/AAC.01052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang CE, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Bloodstream infections caused by antibiotic-resistant Gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, Tsioka A, Roilides E, Sofianou D, Gritsi-Gerogianni N. 2010. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 31:1250–1256. doi: 10.1086/657135. [DOI] [PubMed] [Google Scholar]
- 22.Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP, Tarasi A, Parisi G, Lappa A, Carattoli A, Petrosillo N, SEERBIO-GRAB Network . 2013. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect 19:E23–E30. doi: 10.1111/1469-0691.12070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.