Abstract
Determination of the neutrophil gelatinase-associated lipocalin (NGAL) level can be used to detect acute kidney injury (AKI) earlier than determination of the serum creatinine (SCr) level in settings such as cardiac surgery, contrast nephropathy, and intensive care units. We hypothesized that urine NGAL (UrNGAL) would be an early biomarker of drug nephrotoxicity. To test this, we studied hemodynamically stable patients treated with amphotericin B (AmB). We measured the SCr and UrNGAL levels at the baseline and daily after initiation of AmB up to day 14 or development of AKI by the use of the SCr criterion. AKI was defined according to a Kidney Disease: Improving Global Outcomes (KDIGO) criterion (an increase in the SCr level by ≥0.3 mg/dl within 48 h or an SCr level ≥1.5 times the baseline level within 7 days). We studied 24 patients with a mean age of 48.4 ± 16.4 years. Most patients were male, and the patients received AmB (12 received AmB deoxycholate and 12 received liposomal AmB) for the treatment of leishmaniasis (91.7%). Overall, 17/24 patients fulfilled a KDIGO criterion for AKI. Peak UrNGAL levels were higher in patients with AKI than in patients without AKI and in recipients of AmB deoxycholate than in recipients of liposomal AmB. The diagnostic performance of the UrNGAL level on day 5 for the detection of AKI was moderate, with the area under the curve (AUC) being 0.68 (95% confidence interval [CI], 0.41 to 0.95). In the subgroup receiving AmB deoxycholate, however, the AUC rose to 0.89 (95% CI, 0.67 to 1.00). In a patient-level analysis, we found that AKI could be detected 3.2 days earlier by the use of the UrNGAL criterion than by the use of the SCr criterion (times to AKI by the UrNGAL and SCr criteria, 3.7 ± 2.5 versus 6.9 ± 3.3 days, respectively; P = 0.001). Future studies should evaluate if a treatment strategy oriented toward evaluation of UrNGAL levels will improve outcomes. These findings for AmB-induced AKI in leishmaniasis patients could serve as a basis for the investigation of urine biomarkers in the early detection of drug nephrotoxicity in other clinical settings.
INTRODUCTION
Acute kidney injury (AKI) is a frequent clinical syndrome in hospitalized patients (1). There are many recognized etiologies of AKI, but most cases are due to ischemic and/or toxic insults (2). Several interventions that attenuate AKI in experimental models have been proven to be ineffective when carried into clinical practice (3). In part, this inefficiency has been attributed to the late detection of AKI after the occurrence of irreversible acute tubular necrosis.
In the last decade, the nomenclature for what was once called acute renal failure syndrome was changed to AKI, with the term failure being reserved for the more severe cases, and steps have been taken to expedite the diagnosis. Consensus criteria indicating AKI, such as risk, injury failure, loss, and end stage (RIFLE) criteria (4) and the criteria of the Acute Kidney Injury Network (AKIN) (5) and Kidney Disease: Improving Global Outcomes (KDIGO) (6), were developed. Nowadays, the presence of either a urine volume of <0.5 mg/kg of body weight/h for 6 h or an abrupt (within 48 h) increase in the serum creatinine (SCr) level of as little as 0.3 mg/dl is sufficient to establish a diagnosis of AKI (7).
Nevertheless, diagnostic problems remain. Assessment of the urine volume might be confounded by inadequate measurement or the use of loop diuretics and is not useful in cases of nonoliguric AKI. Determination of the SCr level also has significant restrictions because SCr levels can be affected by factors unrelated to the glomerular filtration rate, such as catabolism, rhabdomyolysis, certain antibiotics, hemodilution, and muscle mass. In addition, a rise in the SCr level requires a significant decrease in the glomerular filtration rate and is thus considered a late marker for AKI. Consequently, there has been an intense search for better biomarkers of AKI (8).
In humans, the AKI biomarker that has been most studied is neutrophil gelatinase-associated lipocalin (NGAL) (9). Most NGAL studies have been performed in the context of large surgeries (10–12) and contrast nephropathy (13–17) and with critically ill patients (18–21); collectively, they suggest that NGAL is a promising early biomarker of AKI. However, there are no intervention studies demonstrating that the early detection of renal damage in these situations results in a reduced incidence of clinically manifest AKI or the associated morbidity and mortality (22).
One promising application for biomarkers of AKI is monitoring of drug nephrotoxicity (23–25). In this setting, early knowledge of ongoing AKI may enable the physician to take measures to avoid additional damage before the progression to full-blown acute tubular necrosis. Preliminary studies in rodents have shown that NGAL is a promising biomarker of AKI secondary to cisplatin (26, 27), amphotericin B (AmB) (28), colistin (29), and gentamicin (30) treatment, but few studies have explored this application in humans. In 12 patients with cancer receiving cisplatin infusions, the urinary NGAL (UrNGAL) level rose 4.5 days earlier than the time that the peak SCr level was achieved (31). However, in patients with acute bacterial infections, the ability of plasma NGAL and UrNGAL levels to predict the nephrotoxicity of vancomycin (32) and colistin (33), respectively, was compromised.
Aiming to evaluate the role of UrNGAL as an early biomarker of drug-induced AKI, we used the treatment of nonseptic, hemodynamically stable patients with AmB as a unique model. AmB is a highly nephrotoxic drug with antiparasitic and antifungal properties. At the Hospital Universitário Professor Edgard Santos, the main indication for the use of AmB on the hospital wards is leishmaniasis (34). Patients with leishmaniasis usually do not present with an inflammatory response syndrome or renal dysfunction, are hemodynamically stable (and, hence, are not subject to ischemic insults), and require in-house treatment with AmB for prolonged periods.
MATERIALS AND METHODS
Study site.
The study was performed at the Hospital Universitário Professor Edgard Santos, a tertiary care facility affiliated with the Medical School of the Federal University of Bahia, located in Salvador, Bahia, Brazil.
Population.
All adult inpatients initiating treatment with AmB in the medical wards were considered potentially eligible. We did not include intensive care unit patients, as they are usually exposed to ischemic renal insults due to septic or cardiogenic shock. Exclusion criteria were signs and symptoms of urinary tract infection, renal transplantation, ongoing acute kidney injury, advanced (stage IV or stage V) chronic kidney disease, and AmB use for less than 3 days.
Study design.
This study was a prospective cohort study. All patients were followed from the time of initiation of AmB until death or hospital discharge. From the viewpoint of treatment, the study was observational, without any deviation from standard practice. The indication for treatment as well as the dose and type of AmB was the responsibility of the medical staff assisting the patient, without any interference from the research team. Similarly, decisions to discontinue or reduce the dose of AmB were the prerogatives of the primary care team. Our intervention was purely diagnostic. Blood and urine samples were collected prior to the initiation of AmB therapy and daily thereafter until day 14 or the development of AKI by the SCr criterion using the KDIGO definition. The primary care team had no knowledge of UrNGAL levels because testing was done in batches at a later point in time.
Protection of human subjects.
The Hospital Universitário Professor Edgard Santos Institutional Review Board approved the study (protocol number 08087412.1.0000.0049), and all participants provided written informed consent.
Measurements.
We collected data on patient demographics, date of admission, indication for AmB use, type and dose of AmB, comorbid conditions, and the use of other potentially nephrotoxic drugs and obtained blood and urine samples daily for the collection of laboratory data.
Laboratory methods.
Blood samples were collected in the morning by the hospital's laboratory personnel through peripheral venipuncture and immediately sent to the clinical laboratory of the Hospital Universitário Professor Edgard Santos for analysis of SCr levels according to the usual routine for inpatients. Twenty milliliters of freshly voided urine was collected in the morning by the research team; 10 ml was sent to the hospital clinical laboratory for urine creatinine analysis, and the remaining 10 ml was immediately stored in a −80°C freezer (Sanyo VIP series). Frozen urine samples were shipped on dry ice to a commercial laboratory (Science Pro Laboratories, São Caetano do Sul, Brazil). Urine samples were centrifuged, and supernatants were diluted 1/50 for UrNGAL measurement using an NGAL rapid enzyme-linked immunosorbent assay kit (catalog number KIT037RUO) according to the manufacturer's instructions (BioPorto Diagnostics, Gentofte, Denmark). Each subject's longitudinal samples were assayed in the same batch. Quantitative UrNGAL results were obtained in nanograms per milliliter (assay range, 0.2 to 20.0 ng/ml; limit of detection, <0.1 ng/ml).
AmB treatment regimen.
At the Hospital Universitário Professor Edgard Santos, the usual protocol for initiating AmB deoxycholate is to start with 0.25 mg/kg of body weight/day and increase the dose by 5 to 10 mg/day to a maximum of 1.5 mg/kg/day. For the liposomal AmB preparation, we start with 3 to 5 mg/kg/day. Both types of AmB preparations are diluted in 500 ml of 5% dextrose in water and infused intravenously over 1 to 2 h. In addition, patients receive 500 to 2,000 ml of 0.9% NaCl over 24 h, at the discretion of the attending physician. Pretreatment with acetaminophen, antihistamines, or hydrocortisone is reserved for patients who develop reactions during the infusion.
Definitions.
AKI was defined according to a KDIGO criterion, which requires an absolute increase in the SCr level by ≥0.3 mg/dl within 48 h or an increase in the SCr level to ≥1.5 times the baseline level which is known or presumed to have occurred within the prior 7 days (this binary definition is referred to here as the KDIGObin criterion and is equivalent to KDIGO stage 1 or greater). We did not have rigorous measurements of urine volume. AKI was staged according to KDIGO: AKI was considered to be stage 1 when there was an absolute increase in the SCr level to ≥0.3 mg/dl or an increase in the SCr level to 1.5 to 1.9 times the baseline level, stage 2 required an increase in the SCr level 2.0 to 2.9 times the baseline level, and stage 3 required an increase in the SCr level to ≥3.0 times the baseline level or the initiation of dialysis. For comparison, we examined two definitions of AmB nephrotoxicity commonly used in the literature: an absolute increase in the SCr level by ≥0.5 mg/dl (NT0.5) and a doubling of the baseline SCr level (NT2×). The latter is equivalent to KDIGO stage 2 or greater. There is currently no consensus definition of AKI on the basis of UrNGAL levels.
Outcomes.
The primary endpoint was the difference between the average time to detection of AKI by the use of the SCr (KDIGObin) criterion and the average time to detection of AKI by the use of the empirically derived UrNGAL criterion. We also evaluated the sensitivity, specificity, positive and negative predictive values, and accuracy of the UrNGAL level for the detection of AmB-induced AKI.
Statistical analyses.
Data were summarized by counts, relative frequencies, and measures of central tendency and dispersion (mean ± standard deviation or median and interquartile range [IQR], according to the shape of the distribution). NGAL data were analyzed in nanograms per milliliter and micrograms per gram of urine creatinine. Given the asymmetry of the UrNGAL data, we also analyzed log-transformed data. To account for differences in baseline values, we also expressed UrNGAL as a relative value by dividing the values obtained on days 1 through 14 by the baseline (day 0) value. The correlation between data points was assessed by the use of Spearman's correlation coefficient. Comparisons of continuous variables between two groups were made with the Wilcoxon rank sum test or, for log-transformed UrNGAL data, the Student t test. The trend across ordered groups was tested using a nonparametric test developed by Cuzick, which is an extension of the Wilcoxon rank sum test. Receiver operating characteristic (ROC) curves were generated at all time points to determine the best sensitivity/specificity cutoffs for the detection of AKI according to the UrNGAL level. Individual patient-level analyses were also carefully conducted to identify the best cutoff UrNGAL level for the early diagnosis of AKI. Two-by-two tables were constructed, and sensitivity, specificity, positive and negative predictive values, and accuracy were determined. The time to the peak UrNGAL concentration versus the time to the peak SCr concentration in patients with AKI and the time to AKI determined by use of the empirically derived UrNGAL criterion versus the time to AKI determined by use of the SCr (KDIGObin) criterion in matched pairs of concordant cases (true positives) were compared by the use of the one-sample paired t test. A P value of <0.05 in the final analyses was considered statistically significant. All analyses were performed using Stata (version 12.1) and IBM SPSS Statistics (version 20.0) software.
Sample size.
The minimum sample size required to detect a difference between the mean time to AKI by use of the UrNGAL criterion and the mean time to AKI by use of the SCr (KDIGObin) criterion was calculated using free online software. A priori sample size calculation was based on the following assumptions: the mean time to AKI by use of the UrNGAL criterion of 3 ± 1 days and the mean time to AKI by use of the SCr criterion of 5 ± 2 days. A sample size of 20 AKI cases would provide us with an 80% power to detect an average difference of 2 days with an alpha level of 0.05.
RESULTS
Patient population.
We studied 24 patients with a mean age of 48.4 ± 16.4 years. Most were male and from rural areas, and the patients received AmB (12 received AmB deoxycholate and 12 received liposomal AmB) predominantly for the treatment of leishmaniasis (91.7%). Three patients had HIV infection. The mean length of stay in the hospital was slightly over 1 month. Mean baseline renal function, acid-base status, and electrolyte levels were within normal limits (Table 1).
TABLE 1.
Demographic, clinical, and baseline laboratory variables for 24 patients treated with AmBa
| Variable | Value (n = 24) |
|---|---|
| Age (yr) | 48.4 ± 16.4 |
| No. of patients with the following characteristic/total no. of patients tested (%): | |
| Gender | |
| Male | 19/24 (79.0) |
| Female | 5/24 (21.0) |
| Place of residence | |
| Salvador, Brazil (capital of Bahia) | 3/24 (12.5) |
| Rural areas | 21/24 (87.5) |
| Reason for AmB treatment | |
| Leishmaniasisb | 22/24 (91.7) |
| Histoplasmosis | 1/24 (4.17) |
| Paracoccidioidomycosis | 1/24 (4.17) |
| Significant comorbidities | |
| HIV infection | 3/24 (12.5) |
| Systemic lupus erythematosus | 1/24 (4.17) |
| Hemophagocytic syndrome | 1/24 (4.17) |
| Cirrhosis | 1/24 (4.17) |
| AmB formulation | |
| Deoxycholate | 12/24 (50.0) |
| Liposomal | 12/24 (50.0) |
| Length of hospital stay (days) | 36.2 ± 15.4 |
| Baseline serum laboratory values | |
| Serum creatinine concn (mg/dl) | 0.8 ± 0.2 |
| Serum potassium concn (meq/liter) | 4.3 ± 0.6 |
| Serum magnesium concn (mg/dl) | 2.0 ± 0.3 |
| Serum bicarbonate concn (meq/liter) | 28.0 ± 4.8 |
| Baseline urine creatinine concn (mg/dl) | 102.2 ± 69.2 |
| No. of patients with the following baseline UrNGAL concn stratum/total no. tested (%): | |
| 0.01–1.00 ng/ml | 18/24 (75.0) |
| 1.00–2.00 ng/ml | 3/24 (12.5) |
| ≥2.00 ng/ml | 3/24 (12.5) |
| Baseline UrNGAL concn | |
| UrNGAL concn (ng/ml) | 1.2 ± 2.9 |
| Log UrNGAL concn (ng/ml) | −1.2 ± 1.7 |
| UrNGAL concn (μg/g creatinine) | 3.1 ± 11.0 |
| Log UrNGAL concn (μg/g creatinine) | −1.1 ± 1.9 |
Continuous data are presented as means ± standard deviations. AmB, amphotericin B; UrNGAL, urine neutrophil gelatinase-associated lipocalin.
Of the 22 cases of leishmaniasis, 5 were visceral (kala-azar) and 17 were tegumentary (localized cutaneous leishmaniasis, n = 2; disseminated cutaneous leishmaniasis, n = 6; mucosal leishmaniasis, n = 3; disseminated cutaneous leishmaniasis with mucosal involvement, n = 6).
At the baseline, UrNGAL levels were low in the majority of patients, with the median level being 0.23 ng/ml [IQR, 0.11 to 0.93 ng/ml]; in six patients, the baseline UrNGAL level was above 1.0 ng/ml; one of them had a baseline level of 13.9 ng/ml in the absence of clinically manifest AKI. Due to the skewness of the UrNGAL data, we also performed a log transformation (Table 1).
Summary of AmB treatment.
For AmB deoxycholate, the mean starting, maintenance, and total doses were 26 ± 10 mg/day (minimum, 15 mg/day; maximum, 50 mg/day), 47 ± 18 mg/day (minimum, 15 mg/day; maximum, 75 mg/day), and 730 ± 731 mg (minimum, 75 mg; maximum, 2,485 mg), respectively. For liposomal AmB, the mean starting, maintenance, and total doses were 116 ± 38 mg/day (minimum, 50 mg/day; maximum, 150 mg/day), 211 ± 85 mg/day (minimum, 50 mg/day; maximum, 300 mg/day), and 2,560 ± 1314 mg (minimum, 450 mg; maximum, 5,250 mg), respectively.
Kinetics of SCr during AmB treatment.
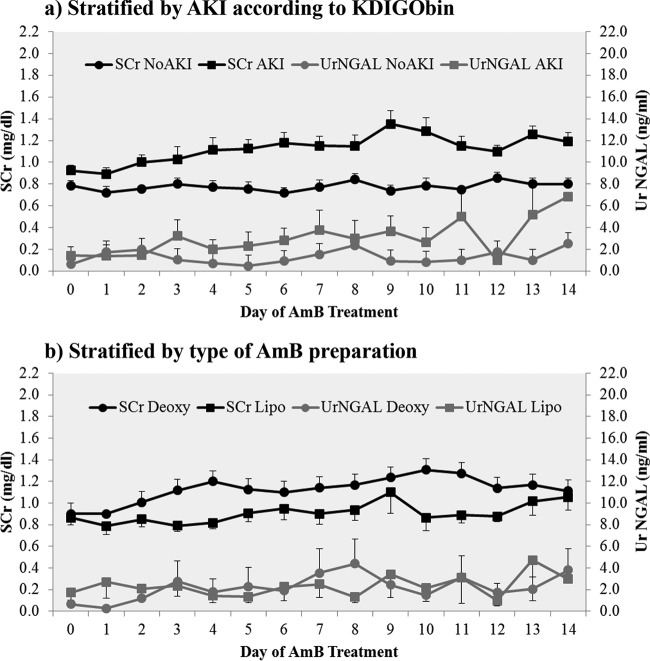
Figure 1a shows the mean SCr and UrNGAL levels over time for the entire group of 24 patients stratified by the presence or absence of AKI according to the KDIGObin criterion. The mean SCr levels among the groups were similar at days 0, 1, and 3; at day 2 and from day 4 to day 14, SCr levels were significantly higher in the AKI group (P ≤ 0.04 for days 2 and 8; P ≤ 0.01 for all other time points). The highest SCr level in our data set was 2.2 mg/dl, which was reached on day 9 in a patient receiving AmB deoxycholate.
FIG 1.
Mean SCr and UrNGAL levels during 2 weeks of treatment with intravenous AmB. (a) SCr and UrNGAL levels stratified by AKI according to the KDIGObin criterion; (b) SCr and UrNGAL levels stratified by type of AmB preparation. SCr, serum creatinine; UrNGAL, urine neutrophil gelatinase-associated lipocalin; AKI, acute kidney injury; AmB, amphotericin B; Deoxy, AmB deoxycholate; Lipo, liposomal AmB; KDIGObin criterion, the Kidney Disease: Improving Global Outcomes criterion that requires an increase in the SCr level by ≥0.3 mg/dl within 48 h or an increase in the SCr to ≥1.5 times the baseline level which is known or presumed to have occurred within the prior 7 days. Squares and circles, means; bars, standard errors of the means. SCr levels (black) are plotted on the primary y axis (left), and UrNGAL levels (gray) are plotted on the secondary y axis (right).
Figure 1b shows the mean SCr and UrNGAL levels over time for the entire group of 24 patients stratified by the type of AmB preparation. Mean SCr levels were significantly higher in the AmB deoxycholate group than in the liposomal AmB group on days 4, 10, 11, and 12 (P = 0.02, 0.01, 0.03, and 0.01, respectively).
Kinetics of UrNGAL during AmB treatment.
Two-hundred fifty-six measurements for determination of UrNGAL levels were performed over 14 days in 24 patients.
Mean UrNGAL levels (in nanograms per milliliter) were not significantly different at any point in time when stratified by the presence or absence of AKI according to the KDIGObin criterion (Fig. 1a) or by the type of AmB preparation (Fig. 1b) (P > 0.05 for all comparisons using nonparametric tests).
Incidence and time to AKI according to SCr (KDIGObin) criterion.
As shown in Table 2, the SCr (KDIGObin) criterion was more sensitive than the traditional nephrotoxicity criterion, with more cases of AmB-induced AKI being detected at earlier time points according to the SCr (KDIGObin) criterion. In addition, use of the KDIGO system allowed AKI staging. Most cases of AmB-induced AKI were mild; there were no cases of stage 3 AKI. AKI was more common and occurred earlier in recipients of AmB deoxycholate than in recipients of liposomal AmB.
TABLE 2.
Incidence and time to AmB-induced AKI according to different definitions, stratified by type of AmB preparationa
| Patient group | No. of patients with AKI/total no. tested (%) by: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Binary criteria |
KDIGO stage |
Time to AKI (days) by the following criterion: |
|||||||
| NT2× | NT0.5 | KDIGObin | Stage 1 | Stage 2 | Stage 3 | NT2× | NT0.5 | KDIGObin | |
| All (n = 24) | 4/24 (16.7) | 15/24 (62.5) | 17/24 (70.8) | 13/24 (54.2) | 4/24 (16.7) | 0/24 (0.0) | 10.3 ± 3.4 (n = 4) | 8.0 ± 3.4 (n = 15) | 7.2 ± 3.1 (n = 17) |
| Deoxycholate AmB (n = 12) | 3/12 (25.0) | 9/12 (75.0) | 10/12 (83.3) | 7/12 (58.3) | 3/12 (25.0) | 0/12 (0.0) | 9.3 ± 3.5 (n = 3) | 7.3 ± 3.5 (n = 9) | 7.1 ± 3.6 (n = 10) |
| Liposomal AmB (n = 12) | 1/12 (8.3) | 6/12 (50.0) | 7/12 (58.3) | 6/12 (50.0) | 1/12 (8.3) | 0/12 (0.0) | 13.0 (n = 1) | 9.0 ± 3.4 (n = 6) | 7.3 ± 2.6 (n = 7) |
Continuous data are expressed as means ± standard deviations. AmB, amphotericin B; NT2×, traditional nephrotoxicity criterion that requires at least doubling of the SCr level; NT0.5, traditional nephrotoxicity criterion that requires an absolute increase in the SCr level of at least 0.5 mg/dl; KDIGObin, Kidney Disease: Improving Global Outcomes criterion that requires an increase in the SCr level by ≥0.3 mg/dl within 48 h or an increase in the SCr level to ≥1.5 times the baseline level which is known or presumed to have occurred within the prior 7 days. KDIGO stage 1 required an increase in the SCr level by ≥0.3 mg/dl or to a level 1.5 to 1.9 times the baseline level; stage 2 required an increase in the SCr levels to 2.0 to 2.9 times the baseline level, and stage 3 required an increase in the SCr level to a level ≥3.0 times the baseline level or initiation of dialysis.
Peak UrNGAL levels.
As shown in Fig. 2, peak UrNGAL levels were numerically higher in the group with AKI than in the group without AKI, but the differences between means were not statistically significant (6.70 ± 7.22 versus 2.90 ± 1.90 ng/ml; P = 0.33). When stratified by AKI status across the AmB treatment subgroups, the peak UrNGAL levels were the highest in AKI patients in the AmB deoxycholate subgroup (7.76 ± 7.71 ng/ml) and liposomal AmB subgroup (5.18 ± 6.73 ng/ml), followed by patients without AKI in the AmB deoxycholate subgroup (3.12 ± 2.40 ng/ml) and liposomal AmB subgroup (2.81 ± 1.92 ng/ml) (P for trend across ordered groups = 0.15). The highest UrNGAL level in our data set was 21.88 ng/ml, achieved on day 3 in a patient who developed AKI while receiving deoxycholate AmB. The highest increase over the baseline UrNGAL level in our data set was 9,150.00%, achieved on day 8, also in a patient receiving AmB deoxycholate.
FIG 2.
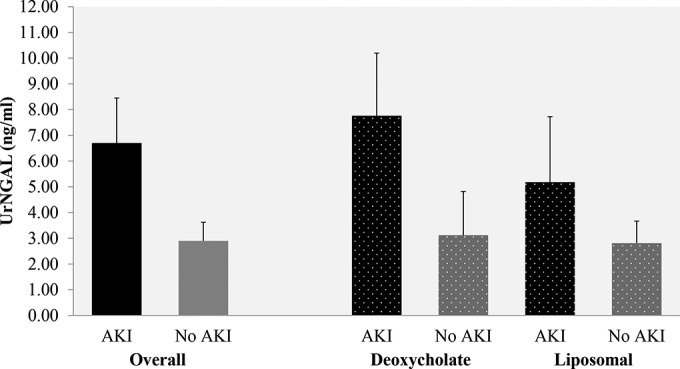
Peak UrNGAL levels stratified by AKI status according to the KDIGObin criterion. Data are presented as the mean and standard error of the mean. The number of cases per group was as follows: n = 17 in the group with AKI and n = 7 in the group without AKI; for the subgroup receiving AmB deoxycholate, n = 10 patients with AKI and n = 2 patients without AKI; for the subgroup receiving liposomal AmB, n = 7 patients with AKI and n = 5 patients without AKI. The P value for trend was 0.15, and the order of the groups for the trend test was patients with AKI receiving AmB deoxycholate → patients with AKI receiving liposomal AmB → patients without AKI receiving AmB deoxycholate → patients without AKI receiving liposomal AmB.
Diagnosis of AKI by UrNGAL level at a specific time point: ROC curves.
To test the diagnostic performance of the UrNGAL level for the detection of AKI using the SCr (KDIGObin) criterion as the “gold standard,” we performed ROC curves at all time points. UrNGAL levels on day 5 were associated with the highest area under the curve (AUC), which was 0.68 (95% confidence interval [CI], 0.41 to 0.95). As shown in Fig. 3, UrNGAL levels on day 5 were better predictors of AKI when analyzed as absolute values (in nanograms per milliliter) rather than relative values (percent change from the baseline). However, this performance of the UrNGAL levels on day 5 was more likely due to the consistently low UrNGAL levels in the group without AKI instead of the very elevated levels in the group with AKI (0.50 ± 0.49 versus 2.32 ± 5.01 ng/ml in the group without AKI versus the group with AKI, respectively; P = 0.21). A level of UrNGAL of ≥0.37 ng/ml on day 5 was associated with a sensitivity of 81%, a specificity of 50%, and an accuracy of 73% for the detection of AKI. Changing the SCr-based AKI criterion (from the KDIGObin criterion to the NT0.5 or NT2× criterion) or the way in which the UrNGAL data were expressed (using log-transformed data or switching from nanograms per milliliter to micrograms per gram of urine creatinine or the percent increase over the baseline) did not result in better diagnostic performance.
FIG 3.
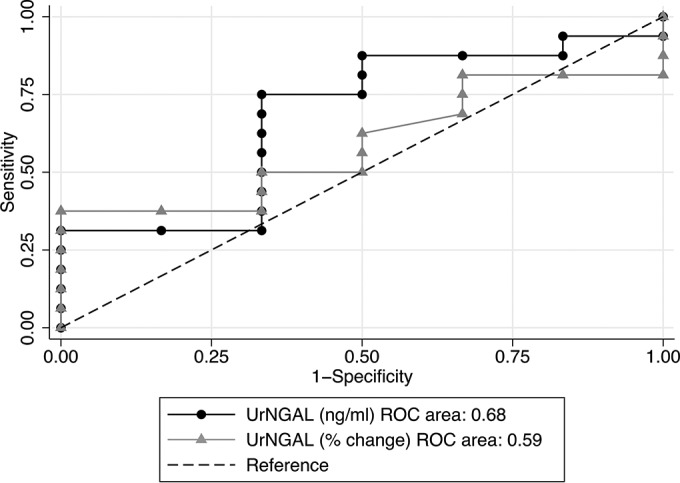
ROC curves analyzing the performance of the UrNGAL criterion on day 5 in detecting AKI using the SCr (KDIGObin) criterion as the gold standard. The UrNGAL level on day 5 was a better predictor of AKI when reported as nanograms per milliliter than when reported as the percent change over the baseline level. Data for all 24 patients (12 receiving AmB deoxycholate and 12 receiving liposomal AmB) were included in this analysis.
Lastly, we repeated the ROC analyses to test the diagnostic performance of the UrNGAL level (expressed in nanograms per milliliter) in the AmB deoxycholate and liposomal AmB treatment subgroups. In the AmB deoxycholate subgroup, the diagnostic performance of the UrNGAL level on day 5 was better than that when the entire group was considered. Using the KDIGObin definition as the gold standard, the AUC for UrNGAL on day 5 was 0.89 (95% CI, 0.67 to 1.00); an UrNGAL level of ≥0.23 ng/ml was associated with a sensitivity of 88.9%, a sensitivity of 100%, and an accuracy of 90.9% for the detection of AKI. Using the NT0.5 criterion, an UrNGAL level of ≥0.23 ng/ml on day 5 resulted in a perfect fit (AUC = 1.00; 100% sensitivity, specificity, and accuracy). This perfect discriminative ability of the UrNGAL level on day 5 when using the NT0.5 criterion as the gold standard was due to consistently low levels in the group without AKI (UrNGAL levels of 0.07 ± 0.08 versus 3.14 ± 6.88 ng/ml in the groups without AKI and with AKI, respectively; P = 0.01).
In the liposomal AmB group, the diagnostic performance of the UrNGAL level was worse. Here, the day associated with the best AUC was day 6. The AUC for UrNGAL levels on day 6 was 0.60 (95% CI, 0.21 to 0.99); the results were the same when the KDIGObin or NT0.5 criterion was used as the gold standard.
Diagnosis of AKI by UrNGAL level: patient-level analyses.
The UrNGAL level cutoffs on day 5 identified by ROC curves were very low; in fact, they were similar to the baseline values. Moreover, by day 5, one-quarter of the patients already had the diagnosis of AKI established by use of the SCr criterion (time to AKI by the KDIGObin criterion, 7.2 ± 3.1 days; median, 7 days; IQR, 5 to 9 days). In some patients, the UrNGAL level had already peaked and declined by day 5; in others, UrNGAL levels had not yet risen by day 5. Since we were interested in finding if the UrNGAL level could be used to detect AKI earlier than the SCr level, we reviewed individual patient-level data. For these analyses, after carefully observing the UrNGAL data, we chose an UrNGAL level cutoff of ≥2.54 ng/ml as the criterion for AKI. As illustrated in Table 3, using this cutoff and the SCr (KDIGObin) criterion as the gold standard, we found true-positive results for 11 patients, false-positive results for 3 patients, false-negative results for 6 patients, and true-negative results for 4 patients (sensitivity, 64.7%; specificity, 57.1%; positive predictive value, 78.6%; negative predictive value, 40.0%; accuracy, 62.5%). A change of the gold standard to the NT0.5 criterion resulted in a worse diagnostic performance due to an increased frequency of false-positive cases (from 3 to 5).
TABLE 3.
Diagnostic performance of UrNGAL: patient-level analysisa
| Criterion for AKI by UrNGAL level | No. of patients with AKI by SCr (KDIGObin) criterion |
||
|---|---|---|---|
| Yes | No | Total | |
| UrNGAL level of ≥2.54 ng/ml | |||
| Yes | 11 | 3 | 14 |
| No | 6 | 4 | 10 |
| Total | 17 | 7 | 24 |
| UrNGAL level ≥3 times baseline level | |||
| Yes | 13 | 5 | 18 |
| No | 4 | 2 | 6 |
| Total | 17 | 7 | 24 |
AKI, acute kidney injury; UrNGAL, urine neutrophil gelatinase-associated lipocalin; SCr, serum creatinine; KDIGObin criterion, Kidney Disease: Improving Global Outcomes criterion that requires an increase in the SCr level to ≥0.3 mg/dl within 48 h or an increase in the SCr level to ≥1.5 times the baseline level which is known or presumed to have occurred within the prior 7 days.
We had two cases in which the baseline UrNGAL level was ≥2.54 ng/ml. In the first of these cases, the baseline UrNGAL level was 4.20 ng/ml, but the levels from days 1 through 5 were all <1.0 ng/ml. On day 6, the UrNGAL level increased to 6.5 ng/ml and peaked at 21.3 ng/ml on day 7. This patient reached AKI according to the KDIGObin criterion on day 10 and was thus considered to have a true-positive case of AKI. In the second case, the baseline UrNGAL level was 13.90 ng/ml. He reached an AKI diagnosis according to the KDIGObin criterion on day 8. UrNGAL levels varied from 16.58 ng/ml on day 1 to 3.58 ng/ml on day 8; the peak UrNGAL level for this patient was 19.63 ng/ml on day 13. Since this was only 1.4 times higher than the baseline value, we considered this patient to have a false-negative case of AKI.
We tested whether analyzing UrNGAL levels as a percent change from the baseline values would influence the diagnostic performance of this biomarker. For these analyses, we chose an UrNGAL level cutoff of ≥3 times the level at the baseline as the criterion for AKI. As illustrated in Table 3, using this cutoff and the SCr (KDIGObin) criterion as the gold standard, we found true-positive results for 13 patients, false-positive results for 5 patients, false-negative results for 4 patients, and true-negatives results for 2 patients (sensitivity, 76.5%; specificity, 28.6%; positive predictive value, 72.2%; negative predictive value, 33.3%; accuracy, 62.5%). A change of the gold standard to the NT0.5 criterion resulted in a worse diagnostic performance due to an increased frequency of false-positive cases (from 5 to 7).
Comparison of time to AKI by use of UrNGAL criterion versus SCr criterion.
Using a cutoff of an UrNGAL level of ≥2.54 ng/ml, 14 cases of AKI were detected. However, 3 of these cases were considered false positive according to the KDIGObin criterion. In 11 cases where the diagnosis of AKI was concordant between the UrNGAL criterion (UrNGAL level, ≥2.54 ng/ml) and the SCr (KDIGObin) criterion, AKI was detected, on average, 1.7 days earlier by use of the UrNGAL level (Table 4). Similar analyses were performed using a cutoff of an UrNGAL level of ≥3 times the baseline level. Even though 18 patients reached this UrNGAL cutoff, the results for 5 were considered false positive according to the KDIGObin criterion. In the 13 concordant cases, AKI was detected, on average, 3.2 days earlier by use of the UrNGAL level (Table 4). Finally, we compared the time to the peak UrNGAL level with the time to the peak SCr level; the analysis was restricted to the 17 patients that developed AKI according to the KDIGObin criterion. As shown in Table 4, the UrNGAL level peaked, on average, 1.5 days earlier than the SCr level; the results were identical when looking at the UrNGAL level as the percent increase over the baseline level.
TABLE 4.
Comparison of time to AKI by UrNGAL criterion versus SCr criteriona
| UrNGAL criterion | No. of patients included in analysis | Time to AKI (days) |
Time to peak concn (days) |
Difference in time to AKI or peak concn (days) | P value | ||
|---|---|---|---|---|---|---|---|
| UrNGAL criterion | KDIGObin criterion | UrNGAL | SCr | ||||
| UrNGAL concn | |||||||
| ≥2.54 ng/ml | 11 | 5.2 ± 3.8 | 6.9 ± 3.5 | −1.7 | 0.060 | ||
| ≥3 times baseline level | 13 | 3.7 ± 2.5 | 6.9 ± 3.3 | −3.2 | 0.001 | ||
| Peak UrNGAL concn | 17 | 6.8 ± 3.3 | 8.2 ± 3.4 | −1.5 | 0.035 | ||
AKI, acute kidney injury; UrNGAL, urine neutrophil gelatinase-associated lipocalin; SCr, serum creatinine; KDIGObin criterion, Kidney Disease: Improving Global Outcomes criterion that requires an increase in the SCr level by ≥0.3 mg/dl within 48 h or an increase in the SCr level to ≥1.5 times the baseline level which is known or presumed to have occurred within the prior 7 days.
DISCUSSION
Our group has recently shown that the use of newer AKI diagnostic criteria (RIFLE, AKIN, and KDIGO) is able to shorten the time to detection of AmB-induced AKI compared to the use of traditional criteria (34). Herein, we showed that determination of the UrNGAL level has the potential to detect AmB-induced AKI even earlier than use of the most sensitive of the newer SCr-based (KDIGObin) criteria. To our knowledge, this is the first study to evaluate the role of UrNGAL in the early diagnosis of AmB-induced AKI.
In studies of AKI after cardiac surgery, the UrNGAL level starts to rise as early as 1 to 2 h after cardiopulmonary bypass. In studies of contrast-induced nephropathy, the UrNGAL level has been measured anywhere from 2 to 24 h after contrast administration. The best timing of urine collection in studies of antimicrobial agent-related nephrotoxicity is not known. Gaspari et al. measured the UrNGAL level at 1 and 4 h and 1, 2, 3, 7, and 15 days after cisplatin administration and found that UrNGAL levels started to rise only after the first day (31). Considering their findings, we chose to measure the UrNGAL level at the baseline and daily after the initiation of AmB.
When looking at the entire group, the UrNGAL level on day 5 was associated with an AUC of 0.68 (95% CI, 0.41 to 0.95) for the diagnosis of AKI (according to the KDIGObin criterion). A level of UrNGAL of ≥0.37 ng/ml on day 5 was associated with a sensitivity of 81%, a specificity of 50%, and an accuracy of 73% for the detection of AKI. In individual case analyses, we chose an UrNGAL level cutoff of ≥2.54 ng/ml as the criterion for AKI. In these analyses, this UrNGAL criterion was associated with a sensitivity of 64.7%, a specificity of 57.1%, a positive predictive value of 78.6%, a negative predictive value of 40.0%, and an accuracy of 62.5%. This diagnostic performance is somewhat inferior to that of studies of UrNGAL in other settings. In 2008, Coca et al. (35) systematically reviewed the literature and encountered four studies of good quality that investigated the role of UrNGAL as an early biomarker of AKI. Two of these studies were in the setting of cardiac surgery (10, 11), one was in critically ill children (36), and was one after renal transplantation (37). The sensitivity of the UrNGAL level for the early detection of AKI varied from 73% (11) to 100% (10), the specificity varied from 72% (36) to 98% (10), and the AUC varied from 78% (11) to 99.8% (10). Only one of these studies looked at the ability of the UrNGAL level to predict AKI severity and did not find a strong association (36). Similarly, we did not find an association between AKI severity and UrNGAL levels in the present study.
UrNGAL levels tended to be higher in users of AmB deoxycholate than in users of liposomal AmB, even when controlling for AKI status. When we analyzed the data by AmB preparation subgroup, we found a better diagnostic performance of the UrNGAL level for the detection of AKI in patients receiving AmB deoxycholate than in those receiving liposomal AmB.
In 2009, Haase and coworkers also conducted a systematic review and meta-analysis of the use of the NGAL level for the diagnosis of AKI (9). When looking at individual studies, the authors found that the UrNGAL cutoffs used for prediction of AKI varied widely, differing more than 50 times from the lowest (36) to the highest (38) reported values. In analyses of the pooled diagnostic and prognostic accuracy of the NGAL level, these authors found cutoffs that were almost 3 times higher in studies of cardiac surgery than in studies of contrast-induced nephropathy. This suggests that the context in which the renal injury occurs might have an impact on UrNGAL levels. The UrNGAL levels that we found in our study are similar to those reported by Gaspari et al. in recipients of cisplatin (31) and by Zappitelli et al. in critically ill children (36) but much lower than those encountered in all studies of cardiac surgery. There are no similar studies with AmB for comparison. Perhaps the less intense renal injury caused by drug nephrotoxicity was responsible for the overall low levels of UrNGAL. The lower UrNGAL levels found in recipients of liposomal AmB than in recipients of AmB deoxycholate likely reflects the better safety profile of the liposomal AmB preparation.
Our study has certain limitations. Since we measured UrNGAL levels approximately 24 h after each dose of AmB, we cannot rule out the possibility of an early UrNGAL peak. Although we were able to detect a significant difference in the mean time to AKI by the use of the UrNGAL criterion versus the SCr criterion, our study might have been underpowered for other comparisons, especially when looking at subgroups of AmB preparations. Moreover, our time to AKI comparisons considered only paired matches of concordant cases (true positives), which is not a scenario that would be encountered in the real world. Similar to any study of urine biomarkers, the use of the SCr level as the gold standard is in itself a limitation. Could an elevation of the UrNGAL level in patients without AKI by the SCr criterion (patients with false-positive AKI) represent subclinical AKI? Some experts currently recommend that, in the right clinical setting, renal tubular injury biomarkers be used to diagnose AKI even in the absence of elevations in SCr levels or reductions in urine output, as required by the RIFLE and AKIN criteria (39). On the other hand, elevation of SCr levels with low UrNGAL levels (false-negative AKI) could be due to a pre-renal state without overt tubular injury. Indeed, a significant component of AmB nephrotoxicity is due to direct renal vasoconstriction (40–42). Finally, recent data suggest that pyuria is an important potential confounder when measuring UrNGAL levels (43). We did not have urinalysis data for all patients, but our sample was comprised mostly of young men, in whom urinary tract infections would be uncommon. Additionally, no patients complained of urinary symptoms or had fever or leukocytosis.
In summary, we found that determination of the UrNGAL level was able to significantly shorten the time to detection of AmB-induced AKI, even compared to the use of the most sensitive SCr-based criteria. The diagnostic performance of the UrNGAL level against the SCr-based criterion was moderate when looking at the entire group but excellent in the AmB deoxycholate subgroup. Finally, UrNGAL levels were higher in recipients of AmB deoxycholate than in those of liposomal AmB.
In our recent retrospective study, we showed that the increase in sensitivity of the KDIGObin criterion for the detection of AmB-induced AKI was accompanied by a loss of specificity and the ability to predict hard outcomes, such as intensive care unit admission or mortality (34). Therefore, future studies should be conducted to evaluate if a UrNGAL-oriented treatment strategy will result in a reduction in the incidence of AmB-induced AKI (as defined by SCr-based criteria), as well as reductions in lengths of hospital stay and costs. When facing a significant elevation in the UrNGAL level prior to an elevation in the SCr level, the physician could institute one (or a combination) of several measures, such as switch from an AmB deoxycholate preparation to a liposomal AmB preparation, change the treatment regimen (reduce the dose, switch the dosing to alternate days, or temporarily discontinue AmB), or try volume expansion with intravenous 0.9% sodium chloride. We believe that our findings with UrNGAL in AmB-induced AKI in leishmaniasis patients could serve as a basis for the investigation of this and other urine biomarkers for the early detection of drug nephrotoxicity in various clinical settings.
ACKNOWLEDGMENTS
Paulo Novis Rocha conducted this project and wrote the manuscript while attending the Weill Cornell Graduate School of Medical Sciences' Master of Science Program in Clinical Epidemiology and Health Services Research and was supported by grant number 5 U2R TW006885 from the National Institutes of Health (NIH), Fogarty International Center. This study was supported by the Brazilian National Research Council (CNPq/MCT) and by NIH (grant number AI 030639).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
We thank Andrea Santos Magalhães (Immunology Service, Hospital Universitário Professor Edgard Santos) for helping with the packing and shipment of urine samples, Roberta Facioli (Science Pro Laboratories) for performing the UrNGAL assays, Ricardo Davi Couto and Rita Magalhães (Central Laboratory, Hospital Universitário Professor Edgard Santos) for performing SCr and electrolyte as well as urine creatinine assays, Warren Johnson, Mary Charlson, and Carol Mancuso as well as the entire faculty and student body of Weill Cornell Graduate School of Medical Sciences' Master of Science Program in Clinical Epidemiology and Health Services Research for their inestimable feedback during all steps of this work, Antonio Alberto Lopes and Reinaldo Martinelli (Nephrology Service, Hospital Universitário Professor Edgard Santos) for sharing the −80°C freezer, and all residents, interns, and students who helped us collect and store the urine samples.
REFERENCES
- 1.Bellomo R, Kellum JA, Ronco C. 2012. Acute kidney injury. Lancet 380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Thadhani R, Pascual M, Bonventre JV. 1996. Acute renal failure. N Engl J Med 334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 3.Venkataraman R, Kellum JA. 2007. Prevention of acute renal failure. Chest 131:300–308. doi: 10.1378/chest.06-1246. [DOI] [PubMed] [Google Scholar]
- 4.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. 2004. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. 2012. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 7.Kellum JA, Lameire N. 2013. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siew ED, Ware LB, Ikizler TA. 2011. Biological markers of acute kidney injury. J Am Soc Nephrol 22:810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 9.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. 2009. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. 2005. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 11.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. 2006. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. 2009. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4:873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S. 2006. Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol 26:287–292. doi: 10.1159/000093961. [DOI] [PubMed] [Google Scholar]
- 14.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. 2008. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 108:c176–c181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 15.Shaker OG, El-Shehaby A, El-Khatib M. 2010. Early diagnostic markers for contrast nephropathy in patients undergoing coronary angiography. Angiology 61:731–736. doi: 10.1177/0003319710373093. [DOI] [PubMed] [Google Scholar]
- 16.Lacquaniti A, Buemi F, Lupica R, Giardina C, Murè G, Arena A, Visalli C, Baldari S, Aloisi C, Buemi M. 2013. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology 267:86–93. doi: 10.1148/radiol.12120578. [DOI] [PubMed] [Google Scholar]
- 17.Alharazy SM, Kong N, Saidin R, Gafor AHA, Maskon O, Mohd M, Zakaria SZS. 2014. Neutrophil gelatinase-associated lipocalin as an early marker of contrast-induced nephropathy after coronary angiography. Angiology 65:216–223. doi: 10.1177/0003319712474947. [DOI] [PubMed] [Google Scholar]
- 18.Bagshaw SM, Bennett M, Haase M, Haase-Fielitz A, Egi M, Morimatsu H, D'Amico G, Goldsmith D, Devarajan P, Bellomo R. 2010. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med 36:452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 19.Kümpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D, Faulhaber-Walter R, Kielstein JT. 2010. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care 14:R9. doi: 10.1186/cc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB, Gunnerson K, Milzman D, Gaieski DF, Goyal M, Cairns CB, Kupfer K, Lee S-W, Rivers EP. 2010. The diagnostic accuracy of plasma neutrophil gelatinase-associated lipocalin in the prediction of acute kidney injury in emergency department patients with suspected sepsis. Ann Emerg Med 56:52–59.e1. doi: 10.1016/j.annemergmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 21.De Geus HRH, Bakker J, Lesaffre EMEH, le Noble JLML. 2011. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med 183:907–914. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- 22.Westenfelder C. 2011. Earlier diagnosis of acute kidney injury awaits effective therapy. Kidney Int 79:1159–1161. doi: 10.1038/ki.2011.19. [DOI] [PubMed] [Google Scholar]
- 23.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu Y-Z, Thompson KL, Goering PL, Vidal J-M, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, et al. 2010. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol 28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 24.Waring WS, Moonie A. 2011. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol (Phila) 49:720–728. doi: 10.3109/15563650.2011.615319. [DOI] [PubMed] [Google Scholar]
- 25.Burt D, Crowell SJ, Ackley DC, Magee TV, Aubrecht J. 2014. Application of emerging biomarkers of acute kidney injury in development of kidney-sparing polypeptide-based antibiotics. Drug Chem Toxicol 37:204–212. doi: 10.3109/01480545.2013.834360. [DOI] [PubMed] [Google Scholar]
- 26.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. 2003. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14:2534–2543. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 27.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. 2004. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol 24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 28.Kondo C, Aoki M, Yamamoto E, Tonomura Y, Ikeda M, Kaneto M, Yamate J, Torii M, Uehara T. 2012. Predictive genomic biomarkers for drug-induced nephrotoxicity in mice. J Toxicol Sci 37:723–737. doi: 10.2131/jts.37.723. [DOI] [PubMed] [Google Scholar]
- 29.Ghlissi Z, Hakim A, Mnif H, Ayadi FM, Zeghal K, Rebai T, Sahnoun Z. 2013. Evaluation of colistin nephrotoxicity administered at different doses in the rat model. Ren Fail 35:1130–1135. doi: 10.3109/0886022X.2013.815091. [DOI] [PubMed] [Google Scholar]
- 30.Luo Q, Chen M, Sun F, Chen Z, Li M, Zeng W, Gong L, Cheng A, Peng X, Fang J, Tang L, Geng Y. 2014. KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem 397:53–60. doi: 10.1007/s11010-014-2171-7. [DOI] [PubMed] [Google Scholar]
- 31.Gaspari F, Cravedi P, Mandalà M, Perico N, de Leon FR, Stucchi N, Ferrari S, Labianca R, Remuzzi G, Ruggenenti P. 2010. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron Clin Pract 115:c154–c160. doi: 10.1159/000312879. [DOI] [PubMed] [Google Scholar]
- 32.Park H-D, Seo J-Y, Lee S-Y. 2012. The relationship between serum neutrophil gelatinase-associated lipocalin and renal function in patients with vancomycin treatment. Ann Clin Lab Sci 42:7–13. [PubMed] [Google Scholar]
- 33.Shavit L, Manilov R, Wiener-Well Y, Algur N, Slotki I. 2013. Urinary neutrophil gelatinase-associated lipocalin for early detection of acute kidney injury in geriatric patients with urinary tract infection treated by colistin. Clin Nephrol 80:405–416. doi: 10.5414/CN107974. [DOI] [PubMed] [Google Scholar]
- 34.Rocha PN, Kobayashi CD, de Carvalho Almeida L, de Oliveira Dos Reis C, Santos BM, Glesby MJ. 2015. Incidence, predictors, and impact on hospital mortality of amphotericin B nephrotoxicity defined using newer acute kidney injury diagnostic criteria. Antimicrob Agents Chemother 59:4759–4769. doi: 10.1128/AAC.00525-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coca SG, Yalavarthy R, Concato J, Parikh CR. 2008. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int 73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 36.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. 2007. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care 11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P. 2006. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6:1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 38.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, Kasza KE, Connor MFO, Konczal DJ, Trevino S, Devarajan P, Murray PT. 2008. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int 74:1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCullough PA, Shaw AD, Haase M, Bouchard J, Waikar SS, Siew ED, Murray PT, Mehta RL, Ronco C. 2013. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the Tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol 182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 40.Heidemann HT, Gerkens JF, Jackson EK, Branch RA. 1983. Effect of aminophylline on renal vasoconstriction produced by amphotericin B in the rat. Naunyn Schmiedebergs Arch Pharmacol 324:148–152. doi: 10.1007/BF00497021. [DOI] [PubMed] [Google Scholar]
- 41.Sabra R, Branch RA. 1991. Mechanisms of amphotericin B-induced decrease in glomerular filtration rate in rats. Antimicrob Agents Chemother 35:2509–2514. doi: 10.1128/AAC.35.12.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawaya BP, Weihprecht H, Campbell WR, Lorenz JN, Webb RC, Briggs JP, Schnermann J. 1991. Direct vasoconstriction as a possible cause for amphotericin B-induced nephrotoxicity in rats. J Clin Invest 87:2097–2107. doi: 10.1172/JCI115240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schinstock CA, Semret MH, Wagner SJ, Borland TM, Bryant SC, Kashani KB, Larson TS, Lieske JC. 2013. Urinalysis is more specific and urinary neutrophil gelatinase-associated lipocalin is more sensitive for early detection of acute kidney injury. Nephrol Dial Transplant 28:1175–1185. doi: 10.1093/ndt/gfs127. [DOI] [PubMed] [Google Scholar]