Abstract
Artemisinin resistance in Plasmodium falciparum parasites in Southeast Asia is a major concern for malaria control. Its emergence at the China-Myanmar border, where there have been more than 3 decades of artemisinin use, has yet to be investigated. Here, we comprehensively evaluated the potential emergence of artemisinin resistance and antimalarial drug resistance status in P. falciparum using data and parasites from three previous efficacy studies in this region. These efficacy studies of dihydroartemisinin-piperaquine combination and artesunate monotherapy of uncomplicated falciparum malaria in 248 P. falciparum patients showed an overall 28-day adequate clinical and parasitological response of >95% and day 3 parasite-positive rates of 6.3 to 23.1%. Comparison of the 57 K13 sequences (24 and 33 from day 3 parasite-positive and -negative cases, respectively) identified nine point mutations in 38 (66.7%) samples, of which F446I (49.1%) and an N-terminal NN insertion (86.0%) were predominant. K13 propeller mutations collectively, the F446I mutation alone, and the NN insertion all were significantly associated with day 3 parasite positivity. Increased ring-stage survival determined using the ring-stage survival assay (RSA) was highly associated with the K13 mutant genotype. Day 3 parasite-positive isolates had ∼10 times higher ring survival rates than day 3 parasite-negative isolates. Divergent K13 mutations suggested independent evolution of artemisinin resistance. Taken together, this study confirmed multidrug resistance and emergence of artemisinin resistance in P. falciparum at the China-Myanmar border. RSA and K13 mutations are useful phenotypic and molecular markers for monitoring artemisinin resistance.
INTRODUCTION
Artemisinin (ART)-based combination therapies (ACTs) have played an indispensable role in reducing global malaria-associated mortality and morbidity. However, these achievements are threatened by the recent emergence of ART resistance in Plasmodium falciparum in the Greater Mekong Subregion (GMS) of Southeast Asia. Clinical ART resistance was first detected in western Cambodia (1–3) but is now detected in at least six areas of the GMS due to spread, independent emergence, or both (4–8). ART resistance is associated with a parasite clearance half-life of >5 h, whereas the normal value is ∼2 h (6, 7). Accurate determination of parasite clearance half-life requires monitoring parasitemia in patients' peripheral blood every 6 h (9). In practice, the proportion of patients remaining parasite positive 3 days after treatment with ART drugs is an alternative indicator for delayed parasite clearance (10). A working definition proposed by the WHO for suspected ART resistance uses “>10% of cases with parasites detectable on day 3 after treatment with an ACT.” The delayed-clearance phenotype cannot be measured using standard in vitro assays but corresponds to decreased susceptibility of ring-stage parasites to ARTs in an in vitro ring-stage survival assay (RSA) (11). However, this novel method has been applied to only a small number of field parasites from Cambodia with defined parasite clearance rates and to several genetically manipulated laboratory strains (11–14).
Recently, mutations in a kelch propeller gene (K13) on P. falciparum chromosome 13 (PF3D7_1343700) were associated with ART resistance in clinical parasites from Cambodia (12). Genetic manipulations conclusively showed that certain K13 propeller mutations conferred in vitro ART resistance (14, 15). Mechanistic studies indicated that resistant parasites upregulated the unfolded protein response pathway (16), and the K13 mutations probably mediated ART resistance via phosphatidylinositol-3-kinase signaling (17). A large multicenter clinical study further demonstrated that point mutations in the K13 propeller domain are collectively associated with the slowly clearing infections (6). Molecular surveillance showed that K13 mutations associated with ART resistance were restricted to certain areas of the GMS, with C580Y being the predominant mutation and approaching fixation in western Cambodia (12, 18–20), but such mutations were not identified in Africa (6, 19, 21–24). Even within the GMS, there is significant geographic heterogeneity in both the patterns of the K13 mutations and their prevalence (25–27), possibly reflecting different drug histories and evolutionary origins (28). To date, the correlations of K13 mutations with delayed parasite clearance have been demonstrated only in limited studies (6, 12, 18, 19).
In China's Yunnan province, ARTs have been used since the late 1970s (29), but clinical studies clearly documenting ART resistance in this region are lacking (5, 30, 31). Here, we aim to provide conclusive evidence of ART resistance in the China-Myanmar border area and to validate RSA and K13 for monitoring ART resistance. By performing correlation analysis between day 3 parasite positivity from clinical studies, RSA results with culture-adapted parasites, and K13 polymorphisms, we provide strong evidence indicating the emergence of ART resistance at the China-Myanmar border and validated RSA as a phenotypic measure and K13 as a promising molecular marker for monitoring ART resistance.
MATERIALS AND METHODS
Study design and participants.
We analyzed three efficacy studies of ART family drugs conducted previously in Kachin State of northeast Myanmar (97.56°E, 24.75°N) and adjacent Yingjiang County, Yunnan Province, China. Uncomplicated P. falciparum malaria was identified by microscopy after screening febrile patients attending local clinics (Table 1). The two studies conducted in 2008 and 2012 to 2013 were 2-day dihydroartemisinin-piperaquine (DP) treatment (30, 32), whereas the 2009-2010 study used 7-day artesunate (ATS) monotherapy (31). Patients with P. falciparum monoinfection and no signs of severe malaria were enrolled. Prior to treatment, 2 to 5 ml of venous blood was obtained from patients and cryopreserved in liquid nitrogen for laboratory culture adaption. Parasite density was determined as described earlier (32). After treatment, patients were followed up on days 1, 2, 3, 7, 14, and 28 (and 42 in 2012 to 2013). Informed consent/assent was obtained from all patients or legal guardians of participants prior to enrollment. All studies were approved by the ethical committees from the participating institutions and the local health department in Kachin.
TABLE 1.
Demographic information of P. falciparum patients involved in three clinical efficacy studies of artemisinin drugs conducted between 2008 and 2013 at the China-Myanmar border
| Parameter | Value(s) for study: |
||
|---|---|---|---|
| 1 (2008; DPa) | 2 (2009–2010; artesunateb) | 3 (2012–2013; DPa) | |
| Patient data | |||
| Febrile patients screened (no.) | 1,073 | 1,095 | 9,044 |
| P. falciparum patients identified (no.) | 91 | 65 | 142 |
| P. falciparum patients recruited (no.) | 74 | 65 | 109 |
| Completed 28-day follow-up (no.) | 64 | 47 | 71 |
| Age (yr) | 2–60 | 3–58 | 1–60 |
| Male:female | 54:20 | 48:17 | 66:43 |
| Mean (range) day 0 asexual parasite density (/μl) | 16,666 (560–625,040) | 60,351 (1,004–657,818) | 3,905 (140–147,600) |
| Day 28 ACPR (%) | 100 | 95.9 (1 LCF, 1 LPF) | 100c |
| Parasite positivity,d n (%) | |||
| Day 1 | 21 (32.8) | 52 (80) | 32 (45.1) |
| Day 2 | 11 (17.2) | 27 (41.5) | 18 (25.4) |
| Day 3 | 4 (6.3) | 15 (23.1) | 5 (7.0) |
A concentration of ∼6 mg/kg of body weight of DHA in 4 doses in 2 days was used.
Artesunate was used at 16 mg/kg for 7 days.
Day 42 follow-up with 100% ACPR.
Parasite positivity rates were calculated using patients available on each date instead of patient numbers who completed the 28-day follow-up.
Laboratory procedures.
Thin and thick blood smears were stained with 2% Giemsa for 30 min and read by two experienced microscopists. Parasites on thick blood smears were counted against 200 leukocytes, and parasite density (number of parasites per microliter of blood) was calculated assuming 8,000 white blood cells per milliliter of peripheral blood. Parasite genomic DNA was isolated from blood spots on filter paper or cultured parasites using a QIAamp DNA minikit (Qiagen). Parasites were genotyped at three polymorphic antigenic markers, merozoite surface protein 1 (msp1), msp2, and glutamate-rich protein (glurp), as described previously, and only monoclonal infections were used for culture adaptation (33).
Parasite culture and drug assays.
For culture adaptation, frozen clinical samples were thawed in saline processing solutions, washed twice with RPMI 1640 medium, mixed with fresh type O+ human red blood cells (RBCs), and suspended at 5% hematocrit in complete medium supplemented with 6% AB human serum (33). The culture was maintained under an atmosphere of 90% N2, 5% O2, and 5% CO2. On average, parasites were continuously cultured for 3 to 4 weeks before drug sensitivities were measured.
Parasite cultures were assayed for their in vitro susceptibilities to eight antimalarial drugs, chloroquine (CQ), piperaquine (PPQ), mefloquine (MQ), quinine (QN), lumefantrine (LMF), pyronaridine (PND), artesunate (ATS), and dihydroartemisinin (DHA), using a SYBR green I-based assay (34). CQ, MQ, QN, and ART drugs were purchased from Sigma (St. Louis, MO); PPQ was from Chongqing Kangle Pharmaceutical Co. (Chongqing, China), while LMF and PND were from Kunming Pharmaceutical Co. (Kunming, Yunnan, China). The stock solutions of CQ and PND were prepared in distilled water, PPQ in 90% methanol and 10% 1 M hydrochloric acid, while other drugs were dissolved in dimethyl sulfoxide (DMSO). Cultures were synchronized by 5% d-sorbitol treatment (35), and ring-stage parasites were diluted with fresh complete medium to 1% hematocrit and 0.5% parasitemia. In vitro drug assays were performed in 96-well microtiter plates with serially diluted concentrations of each drug. Three technical repeats and two biological replications were performed for each drug concentration and each parasite isolate. For consistency, 3D7 was included throughout the study as an internal reference strain.
RSA was performed on culture-adapted parasite isolates by following the established procedure (11). Briefly, tightly synchronized young ring-stage parasites (0 to 3 h) were exposed to 700 nM DHA or 0.1% DMSO for 6 h, washed with RPMI 1640, and continuously cultured for 66 h, and then ∼10,000 RBCs on thin blood smears of the culture were assessed independently by two microscopists to count viable parasites. A third microscopist read the slides in cases of >20% discrepancy between the two readings. Survival rates were expressed as ratios of viable parasites in DHA-exposed and DMSO-exposed samples.
Sequencing of genes associated with drug resistance.
Polymorphisms in candidate genes associated with drug resistance were determined by PCR and sequencing using previously described protocols. These include two pfcrt fragments covering codons 72 to 76 and 220 and two pfmdr1 fragments including codons 86, 184, 1034, 1042, and 1246 (33, 36) and the pfmrp1 gene (37), pfdhfr fragments containing codons 51, 59, 108, and 164, pfdhps fragments containing codons 436, 437, 540, and 581 (36), a pfnhe1 fragment containing the ms4760 minisatellite (33), and the full-length K13 gene (27). The haplotype network was constructed by Network software using the median joining algorithm (38).
Statistical analyses.
Statistical analyses were performed using GraphPad Prism 6.0. The geometric mean 50% inhibitory concentration (IC50) and 95% confidence interval (CI) were calculated by fitting the drug response data to a sigmoid curve. Medians and interquartile ranges (IQR) were calculated if the data were not normally distributed. IC50s between the groups were compared by Mann-Whitney U test. Correlations between IC50s of drugs were determined using the Spearman's test. Mutation frequencies between two groups were compared using Fisher's exact test. Associations between assay results and mutations were investigated by multiple t tests.
Nucleotide sequence accession numbers.
K13 gene sequences reported in this study were deposited in GenBank under accession numbers KT328077 to KT328133.
RESULTS
Clinical efficacies of ART drugs.
We analyzed parasites from three recent efficacy studies conducted in 2008, 2009 to 2010, and 2012 to 2013 on ART drugs (ATS monotherapy and DP) in the China-Myanmar border area. Collectively, >11,000 febrile patients were screened, and 248 patients with single P. falciparum infections were enrolled. ART drugs remained highly efficacious in treating falciparum malaria with 28-day adequate clinical and parasitological responses (ACPR) above 95.9% (Table 1). Notably, DP retained 100% day 28 ACPR and 100% day 42 ACPR for the two DP studies, although the populations in both studies were relatively small. Further, the two DP studies also showed day 3 parasite-positive rates below 10% (6.3 and 7.0%, respectively). In contrast, the day 3 parasite-positive rate for the ATS study reached 23.1%. Interestingly, the one case with late clinical failure (LCF) (fever and parasitemia on day 20) and the one with late parasitological failure (LPF) (parasitemia on day 27) in the ATS monotherapy study were both day 3 parasite negative. When parasite density data from three trials were analyzed together, the day 0 parasite density was significantly higher in the day 3 parasite-positive group (P = 0.0114 by Mann-Whitney U test).
K13 mutations and day 3 parasite positivity.
To validate the predictability of K13 mutations for potential ART resistance, we selected 24 and 33 day 3 parasite-positive and -negative cases, respectively, from the three efficacy studies. The day 3 parasite-negative cases were chosen to match each of the day 3 parasite-positive cases from each of the efficacy studies. Sequencing 57 full-length K13 sequences identified 9 nonsynonymous mutations in 38 (66.7%) samples, of which 7 mutations were located in the propeller domain (i.e., after amino acid 440) (Table 2). The mutations are very distinctive from those found in other regions of the GMS. F446I was the predominant mutation, with 49.1% prevalence, whereas other mutations were at low frequencies (1.8 to 5.3%). Of the four mutations correlated with ART resistance in Cambodia, only R539T was detected in one isolate (1.8%). Besides point mutations, an NN insert between amino acids 136 and 137 in the 3D7 sequence was identified in 49 (86.0%) samples. There were significant differences in the prevalence of F446I mutation and NN insert between day 3 parasite-positive and -negative cases (P < 0.05 by Fisher's exact test) (Table 2). The total mutations in the propeller domain, the predominant F446I mutation, and the NN insert all were significantly associated with day 3 parasitemia in patients (odds ratios of 5.30, 4.86, and 16.33, respectively; P < 0.05). It is worth mentioning that the day 0 parasite in the LCF case had a wild-type K13 gene, whereas the day 0 parasite in the LPF case had both an NN insert and the F446I mutation in the K13 gene.
TABLE 2.
Prevalence of K13 gene mutations in P. falciparum clinical isolates from day 3 parasite-positive and -negative patients after treatment with artemisinin family drugs
| Mutation | Prevalence of mutations in day 3 samples (no. [%]) |
Odds ratio (95% CI) | P valueb | ||
|---|---|---|---|---|---|
| Parasite positive (n = 24) | Parasite negative (n = 33) | Total (n = 57) | |||
| NN insertiona | 24 (100.0) | 25 (75.8) | 49 (86.0) | 16.33 (0.9–298.7) | 0.0158 |
| E252Q | 0 | 1 (3.0) | 1 (1.8) | ||
| R255K | 0 | 1 (3.0) | 1 (1.8) | ||
| F446I | 17 (70.8) | 11 (33.3) | 28 (49.1) | 4.86 (1.6–15.2) | 0.0074 |
| N458Y | 0 | 1 (3.0) | 1 (1.8) | ||
| C469Y | 1 (4.2) | 0 | 1 (1.8) | ||
| R539T | 1 (4.2) | 0 | 1 (1.8) | ||
| P574L | 1 (4.2) | 2 (6.1) | 3 (5.3) | ||
| A676D | 0 | 1 (3.0) | 1 (1.8) | ||
| H719N | 0 | 1 (3.0) | 1 (1.8) | ||
| After 440 | 20 (83.3) | 16 (48.5) | 36 (63.2) | 5.3 (1.5–19.0) | 0.0116 |
Presence of NN insertion between amino acids 136 and 137.
Comparison between the two groups (Fisher's exact test).
Correlation of the ring-stage survival phenotype with day 3 parasitemia.
We culture adapted 44 clinical parasite isolates (19 from day 3 parasite-positive and 25 from day 3 parasite-negative cases) from the three efficacy studies and measured their ring survival by RSA. Ring survival rates of these parasites varied drastically, ranging from 0 to 61.1% (Table 3 and Fig. 1). Remarkably, the median percent ring survival rate of the day 3 parasite-positive group (5.0%) was ∼10 times greater than that of the day 3 parasite-negative group (0.5%) (P < 0.0001) (Table 3), indicating that parasites having higher ring survival rates tended to persist through day 3 in vivo. Despite this general consistency, three parasite isolates in the day 3 parasite-negative group had ring survival rates (7.4%, 21.6%, and 61.1%) radically different from those of the rest of the samples in this group (Fig. 1). K13 sequencing revealed that these three parasites all carried a mutation in the K13 propeller domain (N458Y in one isolate and F446I in two isolates), indicating that RSA results and K13 genotypes were more compatible with each other. Furthermore, parasites with K13 propeller mutations had an ∼8-fold higher ring-stage survival rate than wild-type parasites (P = 0.0032 by Mann-Whitney U test) (Fig. 1). Similarly, parasites with the 446I mutation also had a significantly higher ring survival rate than the F446 parasites (P = 0.0326 by Mann-Whitney U test) (see Fig. S1 in the supplemental material). Interestingly, the K13 NN insert also was significantly associated with increased ring survival (P = 0.0046 by Mann-Whitney U test).
TABLE 3.
In vitro IC50 values and ring-stage survival of culture-adapted P. falciparum from day 3 parasite-positive and -negative patients
| Druga | Parasite positive (n = 19) |
Parasite negative (n = 25) |
P valueb | Total (n = 44) |
3D7 (mean) | P valuec | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Mean (95% CI) | Range | Median (IQR) | Mean (95% CI) | Range | Median (IQR) | Mean (95% CI) | Range | ||||
| DHARSA | 5.0 (2.2–7.7) | 1.0–19.8 | 0.5 (0–1.9) | 0–61.1 | <0.0001 | 2.0 (0.1–6.1) | 0–61.1 | 0 | <0.0001 | |||
| ATS | 1.6 (1.2–2.2) | 1.1–4.8 | 2.3 (1.6–3.2) | 1.1–6.3 | 0.0155 | 1.8 (1.4–2.8) | 1.1–6.3 | 1.7 | 0.0040 | |||
| DHA | 2.2 (1.8–3.0) | 0.8–6.4 | 2.1 (1.6–2.7) | 1.1–3.6 | 0.3996 | 2.2 (1.6–2.8) | 0.8–6.4 | 1.5 | <0.0001 | |||
| PND | 9.1 (7.0–11.8) | 4.5–34.8 | 7.2 (6.0–8.4) | 5.1–14.5 | 0.0642 | 7.6 (6.3–9.8) | 4.5–34.8 | 4.1 | <0.0001 | |||
| CQ | 1,567.0 (1,155.0–1,980.0) | 478.8–3,278.0 | 1,606.0 (1,118.0–2,094.0) | 296.2–4,097.0 | 0.6386 | 1,589.0 (1,277.0–1,901.0) | 296.2–4,097 | 17.6 | <0.0001 | |||
| PPQ | 23.8 (18.9–28.8) | 8.9–46.4 | 20.0 (17.3–22.7) | 10.5–34.5 | 0.1305 | 21.7 (19.1–24.3) | 8.9–46.4 | 14.7 | <0.0001 | |||
| MQ | 34.1 (21.5–46.7) | 2.0–93.8 | 23.0 (19.0–27.0) | 7.1–44.7 | 0.2346 | 28.0 (22.0–34.0) | 2.0–93.8 | 17.2 | 0.0008 | |||
| QN | 47.2 (38.4–55.9) | 21.1–79.2 | 85.1 (57.0–113.2) | 28.3–207.2 | 0.0563 | 68.1 (51.5–84.7) | 21.1–207.2 | 15.1 | <0.0001 | |||
| LMF | 4.8 (3.0–6.6) | 1.4–12.5 | 9.0 (6.4–11.6) | 1.4–19.6 | 0.0247 | 7.1 (5.4–8.8) | 1.4–19.6 | 5.2 | 0.0310 | |||
DHARSA was performed by exposing tightly synchronized young ring-stage parasites (0 to 3 h) to a bolus DHA treatment for 6 h and measuring parasite survival (as percentages) 66 h later. The rest of the assays measuring IC50 values (in nM) were the standard drug assay where parasites were exposed to continuous drugs for 72 h, and parasite growth was measured by SYBR green I fluorescence.
Mann-Whitney U test.
Student's t test.
FIG 1.
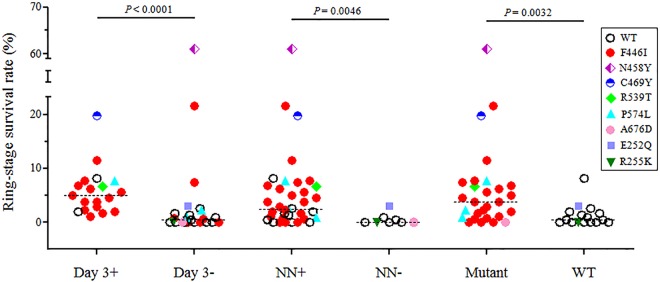
Comparison of ring-stage parasite survival rates with in vivo parasite clearance and K13 genotypes. Ring-stage survival rates of the culture-adapted parasites measured by the RSA were compared between day 3 parasite-positive and -negative samples, between parasites with and without the NN insertion at the N terminus, and between parasites with and without mutations in the K13 propeller domain. Wild-type (WT) parasites are shown as open circles, whereas parasites carrying K13 mutations are color coded. Statistical analysis was performed using a Mann-Whitney U test, and P values are shown.
In vitro sensitivities of parasites to eight antimalarial drugs.
The IC50s of eight antimalarials were determined for 44 culture-adapted parasites and 3D7. All parasites were highly resistant to CQ, with IC50s exceeding 296 nM (Table 3). The ranges of IC50s to PPQ, MQ, and QN were relatively wide, whereas the median or mean IC50s to LMF, PND, DHA, and ATS were below 10 nM. Nonetheless, the median or mean IC50s of the field isolates to all tested drugs were significantly higher than those of 3D7 (P < 0.0001 by Student's t test). Pairwise comparison showed that there were highly significant, positive correlations between ATS and DHA and between ATS and LMF (P < 0.005 by Spearman's test) (Fig. 2). In addition, positive correlations also were found between the RSA result and MQ and between ATS and QN (P < 0.05). In comparison, significant, negative correlations were noticed between ATS and PND, LMF and PND, and LMF and MQ (P < 0.05). Surprisingly, the ring-stage survival rates and IC50s to ARTs did not show any significant correlation.
FIG 2.
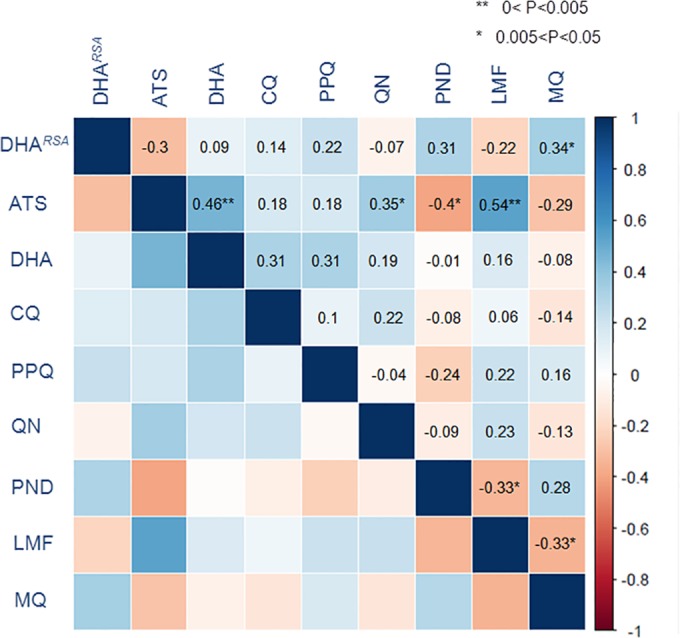
Correlations between IC50s of cultured parasite isolates to eight antimalarial drugs. The correlations between IC50s were analyzed by Spearman's test. The degree of correlation between IC50s of two drugs is color coded, and coefficients are shown in the upper diagonal. One and two asterisks show the significance levels. Except for DHARSA, which is the result from the RSA, susceptibilities (IC50s) to the rest of the drugs were measured using the SYBR green I-based assay. ATS, artesunate; DHA, dihydroartemisinin; CQ, chloroquine; PPQ, piperaquine; QN, quinine; PND, pyronaridine; LMF, lumefantrine; MQ, mefloquine.
The IC50s for CQ, PPQ, MQ, QN, PND, and DHA were not significantly different between day 3 parasite-positive and -negative isolates (Table 3). However, parasites from day 3 parasite-negative cases had higher IC50s for ATS and LMF than those from day 3 parasite-positive cases (P < 0.05 by Mann-Whitney U test).
Molecular polymorphisms of other genes associated with drug resistance.
Polymorphisms in pfcrt, pfdhfr, pfdhps, pfmdr1, pfmrp1, and pfnhe-1 were genotyped and are shown in Table 4 (also see Table S1 in the supplemental material). All parasites carried CVIET at positions 72 to 76 of pfcrt, and both 76T and 220S reached fixation (see Table S1). Similarly, C59R, S108N, and I164L in pfdhfr and A437G and K540E/N in pfdhps approached fixation. The quintuple mutants with pfdhfr (N51I, C59R, and S108N) and pfdhps (A437G and K540E) exceeded 50%, indicating high resistance to antifolates. The frequencies of the seven SNPs in pfmrp1 ranged from 13.2% to 71.1%, with mutations H191Y, S437A, and I876V occurring in >50% isolates. Parasites harboring I876V exhibited higher IC50s to most of the tested drugs, whereas those with H191Y, S437A, and H785N had decreased susceptibilities to DHA, ATS, CQ, PPQ, and LMF (see Fig. S1). Sequencing pfnhe-1 found six ms4760 alleles (39), with allele 7 prevailing in both day 3 parasite-positive and -negative groups (>50%) (see Fig. S2). In agreement with our previous study (33), parasites with higher R1 (DNNND) copy numbers displayed reduced sensitivity to QN, whereas an increased R2 (NHNDNHNNDDD) copy number was significantly associated with increased QN susceptibility (P = 0.0199). Interestingly, the NN insert and F446I mutation in the K13 gene were associated with significantly increased sensitivity to QN (P < 0.05) (see Fig. S1 in the supplemental material). Except for the prevalent K13 mutations (NN insert and F446I), none of the mutations in other genotyped genes showed significant differences between day 3 parasite-positive and -negative groups (see Table S1).
TABLE 4.
Comparison of frequencies of major haplotypes of five genes associated with drug resistance between day 3 parasite-positive and -negative isolates
| Gene | Haplotypea | Presence of parasite (no. [%]) on day 3 |
P valueb | |
|---|---|---|---|---|
| Positive | Negative | |||
| pfdhfr | NRNL | 2 (11.8) | 7 (33.3) | 0.1484 |
| IRNL | 13 (76.5) | 12 (57.1) | 0.3068 | |
| pfdhps | AGEA | 10 (58.8) | 10 (47.6) | 0.5318 |
| SGEG | 1 (5.9) | 5 (23.8) | 0.1965 | |
| pfmrp1 | HNSHITF | 6 (35.3) | 2 (9.5) | 0.1066 |
| YSAHITF | 1 (5.9) | 4 (19.0) | 0.3551 | |
| YNAHVTF | 1 (5.9) | 5 (23.8) | 0.1965 | |
| YNANVMI | 2 (11.8) | 0 (0) | 0.1935 | |
| YNANVMI | 2 (11.8) | 0 (0) | 0.1935 | |
| pfmdr1 | NYN | 10 (58.8) | 15 (71.4) | 0.5017 |
| NFN | 7 (41.2) | 6 (28.6) | 0.5017 | |
| pfnhe-1 | 6 | 2 (11.8) | 5 (23.8) | 0.4267 |
| 7 | 12 (70.6) | 11 (52.4) | 0.3264 | |
Point mutations are shown in boldface.
Comparison between the two groups was done by Fisher's exact test.
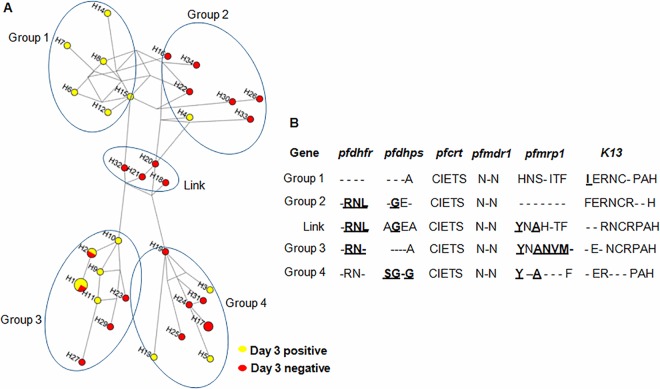
The SNPs in the seven genotyped drug resistance genes gave rise to 34 haplotypes in the 38 parasite isolates (see Fig. S3 in the supplemental material), demonstrating high-level genetic diversity in this region. Four and two samples showed haplotypes 1 and 2, respectively, while each of the remaining isolates was unique (see Fig. S3). It is noteworthy that parasites with haplotypes 13 and 25 showed reduced sensitivities to all tested drugs. To examine the phylogenetic relationship of the parasites, a haplotype network was generated based on SNPs observed in the seven genes. Four different branches of the network (groups 1 to 4) were connected through a linker group (Fig. 3A). Day 3 parasite-positive isolates appeared to be polyphyletic and were distributed across the entire network, suggesting independent origins of resistant mutations in different genes on diverse backgrounds. However, we did observe some clustering of parasite isolates based on day 3 parasitemia. Six haplotypes within group 1 contained only day 3 parasite-positive isolates and were separated from group 2, which primarily contained day 3 parasite-negative isolates. This haplotype grouping was strongly influenced by the F446I mutation, whereas all other mutations possessed common alleles between groups 1 and 2 (Fig. 3B). This suggests that some parasites with the F446I mutation shared a common origin, possibly resulting from a recent spread of parasites carrying the F446I mutation. In comparison, groups 3 and 4 were separated mostly based on mutations in pfmrp1 and pfdhps.
FIG 3.
Median-joining haplotype network of P. falciparum isolates harboring mutations in six drug resistance-associated genes. (A) The haplotype network was constructed for P. falciparum isolates using 34 haplotypes obtained from amino acid changes observed in pfdhfr, pfdhps, pfcrt, pfmdr1, pfmrp1, and K13. The size of each circle corresponds to the number of samples sharing the same haplotype, and the length of an edge is proportional to the number of variations between two haplotypes. Four branches of the network are circled and named groups 1, 2, 3, and 4, and the connecting group is named Link. (B) Consensus sequence corresponding to each group. Point mutations are in boldface and underlined, with the locations of mutations being 51/59/108/164 in pfdhfr, 436/437/540/581 in pfdhps, 72/74/75/76/220 in pfcrt, 86/184/1042 in pfmdr1, 191/325/437/785/876/1007/1390 in pfmrp1, and 446/252/255/458/469/539/574/676/719 in K13. Dashes indicate the presence of both alleles.
DISCUSSION
In the China-Myanmar border area where ART family drugs have the longest history of deployment, ART resistance has not been confirmed. Our studies showed 100% efficacies with DP (30, 32), compared to one LCF and one LPF out of 65 patients enrolled in ATS monotherapy (31). Consistently, <10% of patients receiving DP treatment had parasitemia by day 3, but such patients in the ATS study reached over 20%, suggesting that increased efficacies in DP treatment relied on the partner drug PPQ. ART resistance manifested as delayed parasite clearance rate is due largely to prolonged and dormant ring stages (13, 40). This resistance phenotype cannot be captured with the conventional drug assays measuring IC50s. Indeed, none of the eight drugs tested showed significantly higher IC50s in the day 3 parasitemic group. In contrast, RSA with 44 culture-adapted clinical isolates showed a >10-fold increase in ring survival in the day 3 parasitemic group. The suitability of K13 gene polymorphisms as a more convenient and accurate marker for rapid epidemiological assessment of ART resistance was first evaluated in a multicenter study (6). In our study, patients with parasites carrying K13 propeller mutations had >5 times odds to remain parasitemic on day 3 after ART treatment. Moreover, parasites with these K13 mutations also had ∼8 times higher levels of ring-stage survival than those with wild-type K13. Furthermore, K13 propeller mutations could even resolve the discrepancy between the RSA result and day 3 parasitemia data. Altogether, our study supports the use of RSA and K13 genotyping as complementary methods for epidemiological assessment of ART resistance.
At the China-Myanmar border, parasites with the K13 mutations have reached 66.7%. F446I is the most prevalent point mutation in this area (26, 27), and it is also prevalent in northern Myanmar (25). In southwestern China, this mutation was detected in significantly more isolates in day 3 parasite-positive groups and was associated with delayed parasite clearance (26). The distinct patterns of K13 mutations in the GMS suggest their independent emergence, which might reflect different drug histories. Haplotype network analysis also indicated a potential spread of parasites carrying the F446I allele in this region. Another point mutation, N458Y, detected earlier in Cambodia and Myanmar (6, 12, 25), was significantly associated with prolonged parasite clearance half-lives in clinical studies (6). Remarkably, a single isolate from our samples with N458Y showed a >60% ring-stage survival rate, whereas the median was 3.77% in all parasites carrying K13 propeller mutations. While most K13 genotyping studies so far focused on the propeller domain, our analysis of the full-length K13 sequences led to another intriguing finding. An NN insert in the N terminus of K13 protein was present in all day 3 parasite-positive isolates and was associated with increased ring survival. Since F446I was detected only in parasites carrying the NN insert, it is possible that the NN insert acts cooperatively with F446I in conferring ART resistance or is the result of hitchhiking with the spread of F446I. In Cambodia, the increased prevalence of K13 propeller mutations, especially C580Y, closely mirrored the emergence and spread of ART resistance (12). In parallel, significant increases in the prevalence of K13 propeller mutations in the last 7 to 8 years also suggest the emergence of ART resistance at the China-Myanmar border (27).
Both in vitro drug assay and genotyping of seven genes associated with drug resistance suggested the presence of multidrug-resistant parasites at the China-Myanmar border. Although standard in vitro assays of the 44 culture-adapted parasites did not detect significant differences in sensitivity between day 3 parasite-positive and -negative parasite groups to most drugs, 34.2% of the parasites showed higher IC50s than 3D7 to all drugs tested. In addition, the range of IC50s to several drugs was relatively wide, indicating the presence of parasite isolates with potential resistance to these drugs. Positive correlation was detected between several aminoalcohol drugs, suggesting a shared mechanism of resistance. It is noteworthy that the lack of correlation between the DHA RSA and ATS IC50 results is arguably counterintuitive, but it highlights the radical difference in the principles of the two assays. This study provided further support for the use of in vitro RSA to capture the in vivo delayed parasite clearance phenotype. Despite reduced P. falciparum prevalence in recent years, parasites still displayed extraordinarily high haplotype diversity at the seven genes analyzed. Genotypes of resistance genes also indicated high resistance to 4-aminoquinolines, antifolates, and quinine. In particular, 44.7% of parasites harbored haplotypes suggestive of resistance to multiple drugs. Thus, the evidence presented here showing the emergence of ART resistance is worrisome. Since day 3 parasitemia as a proxy measure of parasite clearance rate is imprecise and can be influenced by factors such as the initial parasite densities, host immunity, and drug pharmacokinetics in individual patients, the validation of a phenotypic assay (RSA) and K13 polymorphisms as predictive markers of ART resistance offers more convenient surveillance tools for monitoring drug resistance in P. falciparum.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U19 AI089672; to L.C.), a Yunnan provincial project, Yunnan, China (2013HA026), the National Science Foundation of China (U1202226 and 31260508; to Z.Y.), and Yunnan Province, China (2014YNPHXT05 and 2014FB005; to Y.W.).
We thank the patients from the villages and camps for participating and providing blood samples.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01255-15.
REFERENCES
- 1.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat Province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis 12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hien TT, Thuy-Nhien NT, Phu NH, Boni MF, Thanh NV, Nha-Ca NT, Thai LE, Thai HCQ, Toi PV, Thuan PD, Long LE, Dong LE, Merson TL, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. 2012. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malar J 11:355. doi: 10.1186/1475-2875-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bustos MD, Wongsrichanalai C, Delacollette C, Burkholder B. 2013. Monitoring antimalarial drug efficacy in the Greater Mekong Subregion: an overview of in vivo results from 2008 to 2010. Southeast Asian J Trop Med Public Health 44(Suppl 1):S201–S230. [PubMed] [Google Scholar]
- 6.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ, Tracking Resistance to Artemisinin Collaboration (TRAC). 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NP, White NJ, Anderson TJ, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH, Lindegardh N, Tarning J, Imwong M, Jacob CG, Rasmussen C, Perin J, Ringwald P, Nyunt MM. 2013. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One 8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegg JA, Guerin PJ, Nosten F, Ashley EA, Phyo AP, Dondorp AM, Fairhurst RM, Socheat D, Borrmann S, Bjorkman A, Martensson A, Mayxay M, Newton PN, Bethell D, Se Y, Noedl H, Diakite M, Djimde AA, Hien TT, White NJ, Stepniewska K. 2013. Optimal sampling designs for estimation of Plasmodium falciparum clearance rates in patients treated with artemisinin derivatives. Malar J 12:411. doi: 10.1186/1475-2875-12-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepniewska K, Ashley E, Lee SJ, Anstey N, Barnes KI, Binh TQ, D'Alessandro U, Day NP, de Vries PJ, Dorsey G, Guthmann JP, Mayxay M, Newton PN, Olliaro P, Osorio L, Price RN, Rowland M, Smithuis F, Taylor WR, Nosten F, White NJ. 2010. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis 201:570–579. doi: 10.1086/650301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WRJ, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 2013. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. 2013. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother 57:914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straimer J, Gnadig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, Dacheux M, Khim N, Zhang L, Lam S, Gregory PD, Urnov FD, Mercereau-Puijalon O, Benoit-Vical F, Fairhurst RM, Menard D, Fidock DA. 2015. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347:428–431. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghorbal MGM, Macpherson CR, Martins RM, Scherf A, Lopez-Rubio JJ. 2014. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol 32:819–821. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 16.Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, Chotivanich K, Imwong M, Pukrittayakamee S, Dhorda M, Nguon C, Lim P, Amaratunga C, Suon S, Hien TT, Htut Y, Faiz MA, Onyamboko MA, Mayxay M, Newton PN, Tripura R, Woodrow CJ, Miotto O, Kwiatkowski DP, Nosten F, Day NP, Preiser PR, White NJ, Dondorp AM, Fairhurst RM, Bozdech Z. 2015. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science 347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio JJ, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K. 2015. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520:683–687. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyunt MH, Hlaing T, Oo HW, Tin-Oo LL, Phway HP, Wang B, Zaw NN, Han SS, Tun T, San KK, Kyaw MP, Han ET. 2015. Molecular assessment of artemisinin resistance markers, polymorphisms in the K13 propeller, and a multidrug-resistance gene in the eastern and western border areas of Myanmar. Clin Infect Dis 60:1208–1215. doi: 10.1093/cid/ciu1160. [DOI] [PubMed] [Google Scholar]
- 19.Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, Fukuda MM, Hien TT, Mayxay M, Noedl H, Nosten F, Kyaw MP, Nhien NT, Imwong M, Bethell D, Se Y, Lon C, Tyner SD, Saunders DL, Ariey F, Mercereau-Puijalon O, Menard D, Newton PN, Khanthavong M, Hongvanthong B, Starzengruber P, Fuehrer HP, Swoboda P, Khan WA, Phyo AP, Nyunt MM, Nyunt MH, Brown TS, Adams M, Pepin CS, Bailey J, Tan JC, Ferdig MT, Clark TG, Miotto O, MacInnis B, Kwiatkowski DP, White NJ, Ringwald P, Plowe CV. 2015. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis 211:670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thriemer K, Hong NV, Rosanas-Urgell A, Phuc BQ, Ha DM, Pockele E, Guetens P, Van NV, Duong TT, Amambua-Ngwa A, D'Alessandro U, Erhart A. 2014. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 58:7049–7055. doi: 10.1128/AAC.02746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conrad MD, Bigira V, Kapisi J, Muhindo M, Kamya MR, Havlir DV, Dorsey G, Rosenthal PJ. 2014. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS One 9:e105690. doi: 10.1371/journal.pone.0105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, Mumba D, Kekre M, Yavo W, Mead D, Bouyou-Akotet M, Apinjoh T, Golassa L, Randrianarivelojosia M, Andagalu B, Maiga-Ascofare O, Amambua-Ngwa A, Tindana P, Ghansah A, MacInnis B, Kwiatkowski D, Djimde AA. 2014. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis 211:1352–1355. doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, Tagbor H, Williams J, Bojang K, Njie F, Desai M, Kariuki S, Gutman J, Mathanga DP, Martensson A, Ngasala B, Conrad MD, Rosenthal PJ, Tshefu AK, Moormann AM, Vulule JM, Doumbo OK, ter Kuile FO, Meshnick SR, Bailey JA, Juliano JJ. 2015. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis 211:680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torrentino-Madamet M, Fall B, Benoit N, Camara C, Amalvict R, Fall M, Dionne P, Fall KB, Nakoulima A, Diatta B, Dieme Y, Menard D, Wade B, Pradines B. 2014. Limited polymorphisms in K13 gene in Plasmodium falciparum isolates from Dakar, Senegal in 2012-2013. Malar J 13:472. doi: 10.1186/1475-2875-13-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, Lin K, Kyaw MP, Plewes K, Faiz MA, Dhorda M, Cheah PY, Pukrittayakamee S, Ashley EA, Anderson TJ, Nair S, McDew-White M, Flegg JA, Grist EP, Guerin P, Maude RJ, Smithuis F, Dondorp AM, Day NP, Nosten F, White NJ, Woodrow CJ. 2015. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis 15:415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang F, Takala-Harrison S, Jacob CG, Liu H, Sun X, Yang H, Nyunt MM, Adams M, Zhou S, Xiao Z, Ringwald P, Bustos MD, Tnag L, Plowe CV. 24 April 2015. A single mutation in K13 predominates in Southern China and is associated with delayed clearance of Plasmodium falciparum following artemisinin treatment. J Infect Dis doi: 10.1093/infdis/jiv249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Shrestha S, Li X, Miao J, Yuan L, Cabrera M, Grube C, Yang Z, Cui L. 2015. Prevalence of K13-propeller polymorphisms in Plasmodium falciparum from China-Myanmar border in 2007-2012. Malar J 14:168. doi: 10.1186/s12936-015-0672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talundzic E, Okoth SA, Congpuong K, Plucinski MM, Morton L, Goldman IF, Kachur PS, Wongsrichanalai C, Satimai W, Barnwell JW, Udhayakumar V. 2015. Selection and spread of artemisinin-resistant alleles in Thailand prior to the global artemisinin resistance containment campaign. PLoS Pathog 11:e1004789. doi: 10.1371/journal.ppat.1004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui L, Su XZ. 2009. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther 7:999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Zhang Z, Wang J, Deng Y, Yang Y, Lasi J, Sun X, Wang H. 2011. Therapeutic efficacy and safety of compound dihydroartemisinin/piperaquine for uncomplicated Plasmodium falciparum infection in Laiza city of Myanmar bordering on China. Chin J Parasitol Parasit Dis 29:372–375. [PubMed] [Google Scholar]
- 31.Huang F, Tang L, Yang H, Zhou S, Sun X, Liu H. 2012. Therapeutic efficacy of artesunate in the treatment of uncomplicated Plasmodium falciparum malaria and anti-malarial, drug-resistance marker polymorphisms in populations near the China-Myanmar border. Malar J 11:278. doi: 10.1186/1475-2875-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Yang Z, Yuan L, Zhou G, Lee M-C, Fan Q, Xiao Y, Cao Y, Yan G, Cui L. 2015. Clinical efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria at the China-Myanmar border. Am J Trop Med Hyg 93:577–583. doi: 10.4269/ajtmh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng H, Zhang R, Yang H, Fan Q, Su X, Miao J, Cui L, Yang Z. 2010. In vitro sensitivity of Plasmodium falciparum clinical isolates from the China-Myanmar border area to quinine and association with polymorphism in the Na+/H+ exchanger. Antimicrob Agents Chemother 54:4306–4313. doi: 10.1128/AAC.00321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Li C, Miao M, Zhang Z, Sun X, Meng H, Li J, Fan Q, Cui L. 2011. Multidrug-resistant genotypes of Plasmodium falciparum, Myanmar. Emerg Infect Dis 17:498–501. doi: 10.3201/eid1703.100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta B, Xu S, Wang Z, Sun L, Miao J, Cui L, Yang Z. 2014. Plasmodium falciparum multidrug resistance protein 1 (pfmrp1) gene and its association with in vitro drug susceptibility of parasite isolates from north-east Myanmar. J Antimicrob Chemother 69:2110–2117. doi: 10.1093/jac/dku125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandelt HJ, Forster P, Rohl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 39.Menard D, Andriantsoanirina V, Khim N, Ratsimbasoa A, Witkowski B, Benedet C, Canier L, Mercereau-Puijalon O, Durand R. 2013. Global analysis of Plasmodium falciparum Na(+)/H(+) exchanger (pfnhe-1) allele polymorphism and its usefulness as a marker of in vitro resistance to quinine. Int J Parasitol Drugs Drug Resist 3:8–19. doi: 10.1016/j.ijpddr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hott A, Casandra D, Sparks KN, Morton LC, Castanares GG, Rutter A, Kyle DE. 2015. Artemisinin-resistant Plasmodium falciparum parasites exhibit altered patterns of development in infected erythrocytes. Antimicrob Agents Chemother 59:3156–3167. doi: 10.1128/AAC.00197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.