Abstract
Minimum bactericidal concentrations (MBCs) for ciprofloxacin were significantly higher among 41 members of the H30 subclone within Escherichia coli sequence type 131 than among 48 other fluoroquinolone-resistant E. coli isolates. This MBC difference, which was not explained by ciprofloxacin MICs, gyrA, parC, and parE mutations, the presence of aac(6′)-Ib-cr, or organic solvent tolerance (a surrogate for efflux pump activity), conceivably could have promoted the pandemic emergence of the H30 sequence type 131 subclone.
TEXT
Escherichia coli is clonally diverse and increasingly antimicrobial resistant (1). The H30 subclone within E. coli sequence type 131 (ST131) has emerged inexplicably in many locales as the dominant E. coli lineage among human clinical isolates, including those resistant to fluoroquinolones or extended-spectrum cephalosporins (2–9).
We recently documented higher fluoroquinolone MICs for the ST131 H30 subclone (here, H30) isolates than for other fluoroquinolone-resistant E. coli isolates (10). Here, we assessed whether H30 isolates also have higher minimum bactericidal concentrations (MBCs) for ciprofloxacin and explored the associations between the ciprofloxacin MBCs, MICs, and fluoroquinolone resistance mechanisms.
Isolates.
The 89 fluoroquinolone-resistant (i.e., ciprofloxacin nonsusceptible) E. coli study isolates were from our study of fluoroquinolone MICs in relation to clonal background and resistance-associated traits (10). They included 41 H30 and 48 non-H30 E. coli isolates and were selected from larger source collections for diversity of gyrA and parC alleles, multilocus sequence types, and pulsed-field gel electrophoresis profiles (3, 7, 11, 12). The non-H30 group included 31 STs from 20 different ST complexes (as determined by eBURST software) and 4 non-H30 ST131 strains.
MICs and MBCs.
The ciprofloxacin MICs were determined by broth microdilution using standard methods (13), with doubling dilutions of ciprofloxacin (range, 0.5 to 2,048 mg/liter) in cation-adjusted Mueller-Hinton broth, plus turbidity-adjusted suspensions of each test isolate (0.5 McFarland standard; approximately 1 × 108 CFU/ml). The MIC for a given titration series was the lowest ciprofloxacin concentration yielding no visible growth after overnight incubation.
For MBC determination, aliquots from each overnight MIC dilution series underwent quantitative plating to antibiotic-free agar. The MBC was the lowest ciprofloxacin concentration that yielded a ≥99.9% decrease in viable counts (14).
For each isolate, the MIC and MBC were determined initially in duplicate. If the duplicates disagreed by >2-fold for any result, up to 5 total determinations were done. The geometric mean value was used.
Resistance-associated traits.
The presence of aac(6′)-Ib-cr (encoding a fluoroquinolone- and aminoglycoside-modifying enzyme) (15), mutations in the quinolone resistance-determining region (QRDR) of gyrA, parC, and parE (16, 17) and the organic solvent tolerance (OST) (a proxy for efflux pump activity) (18) were determined previously (10).
Statistical analysis.
Comparisons involving continuous variables were tested using the Mann-Whitney U test. The correlation was analyzed by simple regression.
Distribution of ciprofloxacin MBCs versus resistance-associated traits in H30 and non-H30 isolates.
The 89 study isolates exhibited a broad range of ciprofloxacin MBCs (Fig. 1). Compared with the non-H30 isolates, the H30 isolates had significantly higher MBCs (median, 4-fold; P < 0.001). For both MICs and MBCs, the four non-H30 ST131 strains resembled more closely the non-ST131 strains than the H30 strains (not shown).
FIG 1.
Ciprofloxacin minimum bactericidal concentration (MBC) in relation to H30 subclone status and resistance mechanisms. (A) H30 versus non-H30 isolates. (B) Subgroups based on H30 status and the presence of aac(6′)-Ib-cr (aac) and/or nonsynonymous mutations at parE458. The circles indicate individual isolates. The horizontal bars show group medians (wide bars) and the 25th and 75th percentiles (narrow bars). P values (two-tailed) are by the Mann-Whitney U test. NS, not significant.
To identify the mechanistic correlates, the MBCs were compared statistically with the fluoroquinolone resistance mechanisms. All 41 H30 strains carried gyrA and parC QRDR mutations, and 10 of these additionally carried aac(6′)-Ib-cr. None carried a parE458 mutation. In contrast, of the 48 non-H30 strains, 27 carried gyrA and parC QRDR mutations only, 21 carried a parE458 mutation (in addition to gyrA and parC QRDR mutations, except in 1 strain), and 4 carried aac(6′)-Ib-cr (3 with gyrA and parC mutations only and 1 with gyrA, parC, and parE458 mutations).
aac(6′)-Ib-cr was associated with significantly higher MBCs among the H30 isolates (P = 0.04), and a similar trend was exhibited among the non-H30 isolates (Fig. 1B). Among the aac(6′)-Ib-cr-positive isolates, the MBCs did not differ significantly between the H30 and non-H30 isolates. Among the aac(6′)-Ib-cr-negative non-H30 isolates, the presence of a parE458 mutation was also associated with a higher MBC (P = 0.04). Nonetheless, among the aac(6′)-Ib-cr-negative isolates, the MBCs still were significantly higher among the H30 than the non-H30 isolates, whether the latter lacked or contained parE458 mutations (P = 0.004 and P < 0.001, respectively).
Thus, even in the absence of aac(6′)-Ib-cr, H30 strains have significantly higher MBCs than non-H30 strains, despite lacking parE458 mutations. This indicates a possible MBC-determining role for the unique combination of QRDR mutations and/or other factors in H30 strains.
Correlation between MBCs and MICs in H30 and non-H30 strains.
For more closely matched comparisons of MICs and MBCs in H30 versus non-H30 isolates, analyses were restricted to the 55 isolates (24 H30 and 31 non-H30) with QRDR replacement mutations involving only gyrA and parC (not parE458) and without aac(6′)-Ib-cr. Within this population, in each subset (H30 and non-H30), MBCs and MICs were correlated only moderately (Fig. 2). Interestingly, however, although the two regression curves were nearly parallel, the H30 curve was shifted significantly upward to higher MBCs. This shows that at a given MIC, the MBCs tended to be much higher among the H30 than the non-H30 isolates. An even weaker correlation between the MBCs and MICs was observed when outliers with very low MICs/MBCs were excluded (data not shown).
FIG 2.
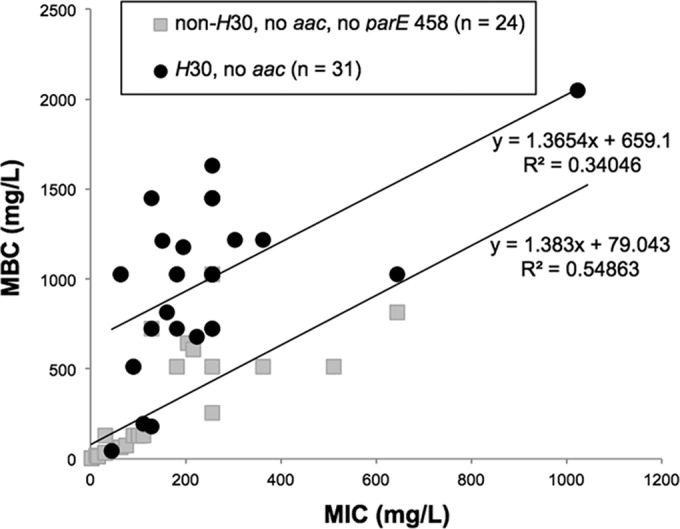
Ciprofloxacin minimum bactericidal concentrations (MBCs) in relation to the MICs among 89 ciprofloxacin-resistant Escherichia coli isolates. H30 subclone members and non-H30 isolates are shown separately. Isolates containing aac(6′)-Ib-cr (aac) and/or nonsynonymous mutations at parE458 were excluded.
The OST score was associated positively with the MIC, both overall and among the H30 and non-H30 isolates separately (Table 1). However, it was associated with the MBC only among the non-H30 isolates.
TABLE 1.
P values for associations of MICs and MBCs with organic solvent tolerance among ciprofloxacin-resistant Escherichia coli isolates
| Parameter |
P valueb |
||
|---|---|---|---|
| Total (n = 89) | Non-H30 (n = 48) | H30 (n = 41) | |
| MIC | 0.006 | 0.007 | 0.01 |
| MBCa | NS | 0.04 | NS |
MBC, minimum bactericidal concentration.
P values (by Pearson correlation) are shown where the P value is <0.05. NS, not significant (P ≥ 0.05).
Thus, although there were modest correlations between the MBCs and MICs, the sizeable MBC differences between the H30 and non-H30 isolates could not be explained by their MIC differences, indicating that the MBC is a separate phenotype from the MIC.
Comment.
We found that, compared with fluoroquinolone-resistant non-H30 isolates, the fluoroquinolone-resistant H30 isolates typically have approximately 4-fold higher MBCs that are not explained by their corresponding MICs. This identifies a novel fitness mechanism that may underlie the striking epidemiological success of the ST131 H30 lineage.
Our findings suggest that even when inhibited by ciprofloxacin, H30 strains are more likely than other fluoroquinolone-resistant E. coli isolates to survive and regrow once the ciprofloxacin concentrations drop below the MIC. This tolerance phenomenon may contribute to the H30 strain-associated clinical scenario of same-strain recurrent urinary tract infection, with the causative organism disappearing during ciprofloxacin therapy and then reemerging after treatment completion (19, 20; J. R. Johnson, unpublished data).
Regarding mechanisms, our data suggest that aac(6′)-Ib-cr may differentially raise the MBC, which could explain why aac(6′)-Ib-cr is being selected in strains with preexisting QRDR mutations (10). Additionally, parE458 replacement mutations were associated with increased MBCs. However, neither aac(6′)-Ib-cr carriage nor parE458 (absent in H30 strains) (10) could explain the H30 versus non-H30 MBC gap. Likewise, the OST, which appeared to have less impact on the MBC than on the MIC, was associated with MBCs only marginally (non-H30 isolates) or not at all (total population and H30 isolates). Thus, to the extent that OST reflects the net efflux pump activity, efflux pumps also are unlikely to underlie the higher MBCs of the H30 isolates. As such, the mechanism(s) for higher MBCs among H30 isolates remains undefined.
Antimicrobial tolerance, which was studied initially mostly in relation to beta-lactam agents and staphylococci, conceivably could result from technical artifacts (21). Our findings are unlikely to represent technical artifacts, since we noted strong associations of the MBCs with the H30 subclone, independently from the MICs, when the H30 and non-H30 isolates were tested in parallel. Studies of quinolone tolerance in E. coli (22–25) have identified SOS-regulated genes (25), hipA (24), and defects in DNA repair (22) as possible contributors; these warrant future assessment in H30 isolates.
The study limitations include the small numbers in certain subgroups, the reliance on the OST as a proxy for efflux pump activity (versus measuring expression of multiple specific pumps), and the absence of attention to porin expression or to MBCs and the MIC/MBC discrepancy for drugs other than ciprofloxacin. The strengths include the well-characterized study population and the analysis of resistance mechanisms in relation to MBCs.
In summary, we newly documented higher ciprofloxacin MBCs for members of the H30 subclone of ST131, compared with those for other fluoroquinolone-resistant E. coli isolates. These phenotypes could not be explained by differences in the MICs, gyrA, parC, and parE mutations, or OST or the presence of aac(6′)-Ib-cr. Our findings identify a selectable phenotype that may have contributed to the emergence and pandemic spread of H30.
ACKNOWLEDGMENTS
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, grants 1 I01 CX000192 01 (to J.R.J.) and NIH R01 AI106007 (to E.V.S.).
J.R.J. has received grants and/or consultancies from Actavis, ICET, Jannsen/Crucell, Merck, Syntiron, and Tetraphase. Additionally, J.R.J., L.B.P., V.T., and E.V.S. have submitted patent applications pertaining to tests for specific E. coli strains. The other authors declare no conflicts of interest.
REFERENCES
- 1.Manges AR, Tabor H, Tellis P, Vincent C, Tellier P-P. 2008. Endemic and epidemic lineages of Escherichia coli that cause urinary tract infections. Emerg Infect Dis 14:1575–1583. doi: 10.3201/eid1410.080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolas-Chanoine M, Bertrand X, Madec J-Y. 2014. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddel K, Rogers P, Qin X, Butler-Wu S, Price LB, Aziz M, Nicolas-Chanoine M, Debroy C, Robicsek A, Hansen G, Urban C, Platell JL, Trott DJ, Zhanel G, Weissman SJ, Cookson B, Fang F, Limaye AP, Scholes D, Chattopadhyay S, Hooper DC, Sokurenko E. 2013. Abrupt emergence of a single dominant multi-drug-resistant strain of Escherichia coli. J Infect Dis 207:919–928. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petty NK, Ben Zakour N, Stanton-Cook M, Skippington E, Totsika M, Forde B, Phan M-D, Moriela DG, Peters K, Davies M, Rogers BA, Dougand G, Rodriguez-Baño J, Pascual A, Pitout J, Upton M, Paterson DL, Walsh TR, Schembri MA, Beatson SA. 2014. Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci U S A 111:5694−5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson DA, Roberts SA, Paterson DL, Sidjabat H, Silvey A, Masters J, Rice M, Freeman JT. 2012. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin Infect Dis 54:1406–1412. doi: 10.1093/cid/cis194. [DOI] [PubMed] [Google Scholar]
- 6.Colpan A, Johnston B, Porter S, Clabots C, Anway R, Thao L, Kuskowski MA, Tchesnokova V, Sokurenko EV, Johnson JR, VICTORY (Veterans Influence of Clonal Types on Resistance: Year 2011) Investigators . 2013. Escherichia coli sequence type 131 (ST131) as an emergent multidrug-resistant pathogen among U.S. veterans. Clin Infect Dis 57:1256–1265. doi: 10.1093/cid/cit503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. 2010. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis 51:286–294. doi: 10.1086/653932. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JR, Johnston B, Clabots C, Kuskowski MA, Pendyala S, Debroy C, Nowicki B, Rice J. 2010. Escherichia coli sequence type ST131 as an emerging fluoroquinolone-resistant uropathogen among renal transplant recipients. Antimicrob Agents Chemother 54:546–550. doi: 10.1128/AAC.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. 2013. Escherichia coli sequence type ST131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol 34:361−369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JR, Johnston B, Kuskowski MA, Sokurenko EV, Tchesnokova V. 2015. Intensity and mechanisms of fluoroquinolone resistance within the H30 and H30Rx subclones of Escherichia coli sequence type 131 versus other fluoroquinolone-resistant E. coli. Antimicrob Agents Chemother 59:4471−4480. doi: 10.1128/AAC.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of ESBL-producing Escherichia coli ST131 is driven by a single highly virulent subclone, H30-Rx. mBio 6:e00377-13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JR, Nicolas-Chanoine M, Debroy C, Castanheira M, Robiscek A, Hansen G, Weissman SJ, Urban C, Platell JL, Trott DJ, Zhanel GG, Clabots C, Johnston BD, Kuskowski MA, the MASTER Investigators. 2012. Comparison of Escherichia coli sequence type ST131 pulsotypes by epidemiologic traits, 1967-2009. Emerg Infect Dis 18:598–607. doi: 10.3201/eid1804.111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Swenson JM, Hindler JA, Peterson LR. 1995. Special tests for detecting antibacterial resistance, p 1356−1367. In Tenover FC, Jorgensen JH, Sahm DF (ed), Manual of clinical microbiology, 6th ed ASM Press, Washington, DC. [Google Scholar]
- 15.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 16.Morgan-Linnell SK, Boyd LB, Steffen D, Zechiedrich L. 2009. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob Agents Chemother 53:235–241. doi: 10.1128/AAC.00665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorlozano A, Gutierrez J, Jimenez A, de Dios Luna J, Martínez JL. 2007. Contribution of a new mutation in parE to quinolone resistance in extended-spectrum-β-lactamase-producing Escherichia coli isolates. J Clin Microbiol 45:2740–2742. doi: 10.1128/JCM.01093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oethinger M, Kern WV, Goldman JD, Levy SB. 1998. Association of organic solvent tolerance and fluoroquinolone resistance in clinical isolates of Escherichia coli. J Antimicrob Agents Chemother 41:111–114. doi: 10.1093/jac/41.1.111. [DOI] [PubMed] [Google Scholar]
- 19.Assimacopoulos A, Johnston B, Clabots C, Johnson JR. 2012. Post-prostate biopsy infection with Escherichia coli ST131 leading to epididymo-orchitis and meningitis caused by Gram-negative bacilli. J Clin Microbiol 50:4157–4159. doi: 10.1128/JCM.02026-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ender PT, Gajanana D, Johnston B, Clabots C, Tamarkin FJ, Johnson JR. 2009. Transmission of extended-spectrum beta-lactamase-producing Escherichia coli (sequence type ST131) between a father and daughter resulting in septic shock and emphysematous pyelonephritis. J Clin Microbiol 47:3780–3782. doi: 10.1128/JCM.01361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenknecht FD. 1986. Bacterial tolerance to antimicrobial agents. Clin Microbiol Newslett 8:72–74. doi: 10.1016/0196-4399(86)90016-4. [DOI] [Google Scholar]
- 22.Wolfson JS, Hooper DC, Shih DJ, McHugh GL, Swartz MN. 1989. Isolation and characterization of an Escherichia coli strain exhibiting partial tolerance to quinolones. Antimicrob Agents Chemother 33:705–709. doi: 10.1128/AAC.33.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiuff C, Zappala RM, Regoes RR, Garner KN, Baquero F, Levin BR. 2005. Phenotypic tolerance: antibiotic enrichment of noninherited resistance in bacterial populations. Antimicrob Agents Chemother 49:1483–1494. doi: 10.1128/AAC.49.4.1483-1494.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falla TJ, Chopra I. 1998. Joint tolerance to β-lactam and fluoroquinolone antibiotics in Escherichia coli results from overexpression of hipA. Antimicrob Agents Chemother 42:3282–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theodore A, Lewis K, Vulic M. 2013. Tolerance of Escherichia coli to fluoroquinolone antibiotics depends on specific components of the SOS response pathway. Genetics 195:1265–1276. doi: 10.1534/genetics.113.152306. [DOI] [PMC free article] [PubMed] [Google Scholar]



