LETTER
Debio 1452, formerly called AFN-1252 (Fig. 1), is an investigational FabI inhibitor that has demonstrated potent activity against Staphylococcus aureus (1–5). Currently, Debio 1450 (prodrug of Debio 1452) is in clinical development by the Debiopharm Group (6, 7). Debio 1452 selectively inhibits bacterial enoyl-acyl carrier protein (enoyl-ACP) reductase, an essential step in the elongation cycle of bacterial fatty acid biosynthesis. Among the four known enzyme forms of enoyl-ACP (FabI, FabK, FabL and FabV), FabI is present in S. aureus, Staphylococcus epidermidis, and other staphylococci (1). This narrow target for Debio 1452 is beneficial to minimize the effect on associated normal bacterial flora, thus reducing potential adverse events. In addition, the Debio 1452 targeted spectrum of activity exhibits a low rate of single-step mutational resistance and cross-resistance to other antibacterial classes. A multilaboratory study conforming to the Clinical Laboratory and Standards Institute (CLSI) quality control (QC) study design (8) was performed to establish broth microdilution QC ranges for S. aureus ATCC 29213 to assist clinical microbiology laboratories in monitoring the activity of this investigational compound during further clinical trials.
FIG 1.
Chemical structure of Debio 1452.
Eight laboratories (seven are required by CLSI M23-A3 guidelines) were used in this broth microdilution QC study (8). These laboratories were experienced microbiology facilities and each followed the CLSI procedure for broth microdilution MIC testing (9). The sites participating were Wheaton Franciscan Laboratory, Wauwatosa, WI (E. Munson); JMI Laboratories, North Liberty, IA (R. N. Jones); ThermoFisher Scientific, Cleveland, OH (C. Knapp); University of Alberta, Edmonton, Alberta, Canada (R. Rennie); University of Washington, Seattle, WA (S. Swanzy); Cleveland Clinic Foundation, Cleveland, OH (G. Hall/G. Procop); Massachusetts General Hospital, Boston, MA (M. J. Ferraro); and Duke University Medical Center, Durham, NC (S. Mirrett).
Reference frozen-form broth microdilution panels were prepared by ThermoFisher Scientific according to good manufacturing practice (GMP) guidelines and shipped frozen to all participating sites. Panels contained four lots of cation-adjusted Mueller-Hinton broth (CAMHB; Oxoid, Hampshire, United Kingdom; BBL, Sparks, MD, USA; and Difco [two lots], Detroit, MI, USA). Rifampin was utilized as the control agent (9, 10). Each laboratory tested 10 replicates of S. aureus ATCC 29213, and colony counts of the inoculum density were performed on drug-free agar medium and resulted in the following acceptable average count: 3.4 × 105 CFU/ml (range, 0.3 × 105 to 7.3 × 105 CFU/ml). Results from the study were analyzed by methods found in the CLSI document (8) and the Range Finder statistical program (11).
The Debio 1452 broth microdilution MIC QC results across four CAMHB lots from all eight laboratories are summarized in Fig. 2. The modal MICs were 0.008 μg/ml with all results ranging from 0.004 to 0.03 μg/ml (only one occurrence). No significant variations were noted among CAMHB lots (modes at 0.004 [1 lot] or 0.008 μg/ml [3 lots]) or for the study sites with modal occurrences at 0.008 μg/ml for four participants, as well as one and three occurrences at 0.015 and 0.004 μg/ml, respectively. The geometric mean of all S. aureus ATCC 29213 MIC values was 0.007 μg/ml.
FIG 2.
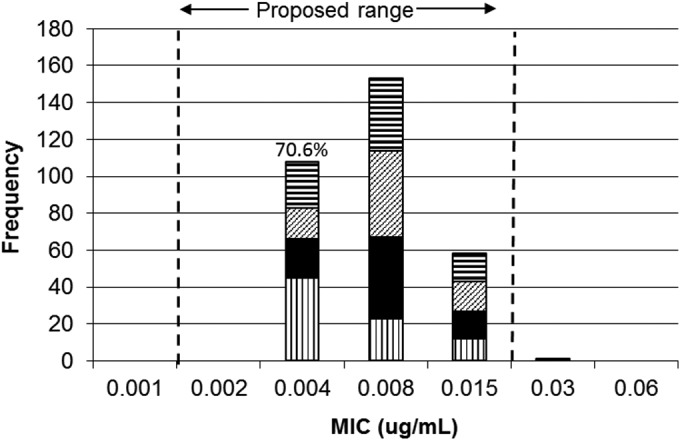
Debio 1452 MIC distributions for the S. aureus ATCC 29213 quality control (QC) strain. The patterns indicate, from top to bottom, medium lot A (horizontal lines), medium lot B (diagonal lines), medium lot C (black), and medium lot D (vertical lines).
A total of 99.7% of S. aureus ATCC 29213 MIC results were included in the proposed Debio 1452 QC range of 0.002 to 0.015 μg/ml. The number of MIC values at 0.004 μg/ml represented 70.6% of the number of modal MIC results (0.008 μg/ml); therefore, a 4-dilution range was calculated as instructed in the CLSI M23-A3 document (8). Data from the study were also analyzed by the Range Finder program (11), and that statistical method confirmed the proposed range, without detecting any significant outlier laboratories. All rifampin control MIC results were within the published CLSI range (10), validating technical standards for the reference method (9). The proposed Debio 1452 QC range with S. aureus ATCC 29213 of 0.002 to 0.015 μg/ml was approved by the CLSI Subcommittee on Antimicrobial Susceptibility Testing (CLSI January 2011 meeting minutes).
As methicillin-resistant S. aureus continues to cause serious clinical infections with associated mortality and morbidity, the search for new antimicrobials has become vital (12, 13). Such new agents should have enhanced activity and potency against these multidrug-resistant staphylococci and possess novel targets, where possible. Debio 1452 is such a new agent directed against the FabI target found in staphylococci (MIC90, 0.008 to 0.12 μg/ml) (1). These MIC quality assurance guidelines (Fig. 1) from a multilaboratory investigation provide initial Debio 1452 QC ranges which can be applied as parameters for routine susceptibility testing when utilizing the reference broth microdilution method (9, 10) and as this new bacterial FabI inhibitor is being developed for oral and intravenous treatment of acute bacterial skin and skin structure infections (7).
ACKNOWLEDGMENTS
This study was sponsored by an educational/research grant from Affinium Pharmaceuticals Inc. (Toronto, Canada).
J. E. Ross, R. K. Flamm, and R. N. Jones are employees of JMI Laboratories, which received grant funds to study Debio 1452, and were paid consultants to Debiopharm International SA in connection with the development of the manuscript.
We thank the personnel and directors of the eight contributing laboratories for their excellent support of this protocol.
REFERENCES
- 1.Flamm RK, Rhomberg P, Kaplan N, Jones RN, Farrell DJ. 2015. Activity of Debio1452, a FabI inhibitor with potent activity against Staphylococcus aureus and coagulase-negative Staphylococcus spp., including multidrug-resistant strains. Antimicrob Agents Chemother 59:2583–2587. doi: 10.1128/AAC.05119-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan N, Albert M, Awrey D, Bardouniotis E, Berman J, Clarke T, Dorsey M, Hafkin B, Ramnauth J, Romanov V, Schmid MB, Thalakada R, Yethon J, Pauls HW. 2012. Mode of action, in vitro activity, and in vivo efficacy of AFN-1252, a selective antistaphylococcal FabI inhibitor. Antimicrob Agents Chemother 56:5865–5874. doi: 10.1128/AAC.01411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan N, Awrey D, Bardouniotis E, Berman J, Yethon J, Pauls HW, Hafkin B. 2013. In vitro activity (MICs and rate of kill) of AFN-1252, a novel FabI inhibitor, in the presence of serum and in combination with other antibiotics. J Chemother 25:18–25. doi: 10.1179/1973947812Y.0000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlowsky JA, Kaplan N, Hafkin B, Hoban DJ, Zhanel GG. 2009. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcus-specific spectrum of activity. Antimicrob Agents Chemother 53:3544–3548. doi: 10.1128/AAC.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlowsky JA, Laing NM, Baudry T, Kaplan N, Vaughan D, Hoban DJ, Zhanel GG. 2007. In vitro activity of API-1252, a novel FabI inhibitor, against clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 51:1580–1581. doi: 10.1128/AAC.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy B, Kaplan N, Hafkin B. 2013. Treatment of ABSSSI due to Staphylococcus with AFN-12520000 in a phase 2a clinical trial resulted in high rates of response at day 3 and at the test of cure, abstr L-206. Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO, 10 to 13 September 2013. [Google Scholar]
- 7.Hafkin B, Kaplan N. 2014. Safety, tolerability and pharmacokinetics in healthy subjects of single intravenous doses of the staphylococcal-specific antibiotic Debio 1450, abstr F-985. Abstr 54th Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2008. M23-A3. Development of in vitro susceptibility testing criteria and quality control parameters, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2015. M100-S25. Performance standards for antimicrobial susceptibility testing, 25th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Turnidge J, Bordash G. 2007. Statistical methods for establishing quality control ranges for antibacterial agents in Clinical and Laboratory Standards Institute susceptibility testing. Antimicrob Agents Chemother 51:2483–2488. doi: 10.1128/AAC.01457-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher H, Miller LG, Razonable RR. 2010. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 51(Suppl 2):S183–S197. doi: 10.1086/653519. [DOI] [PubMed] [Google Scholar]
- 13.Gould IM, David MZ, Esposito S, Garau J, Lina G, Mazzei T, Peters G. 2012. New insights into methicillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int J Antimicrob Agents 39:96–104. doi: 10.1016/j.ijantimicag.2011.09.028. [DOI] [PubMed] [Google Scholar]


