Abstract
In the treatment of HIV infection, a combination of anti-HIV drugs is commonly used in highly active antiretroviral therapy (HAART). One such combination recommended for clinical therapy consists of the two HIV protease inhibitors atazanavir and ritonavir and the HIV nucleotide reverse transcriptase inhibitor tenofovir. The detection of plasma and cell drug concentrations provides an assessment of actual drug exposure and patient compliance. We thus developed a simple, efficient, and sensitive method to simultaneously extract and detect these three drugs in plasma and peripheral blood mononuclear cells. The use of a liquid-liquid extraction followed by protein precipitation provided a simple process, yielding a high recovery rate for all three drugs in plasma (>92%) and in cells (>86%). The liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay was able to detect 0.01, 0.25, and 2.5 pg (2, 50, and 500 pg/ml, respectively) in 5 μl for atazanavir, ritonavir, and tenofovir, respectively. Validation of the method exhibited high precision and accuracy. This method was subsequently applied to a primate study to determine the concentrations of all three drugs in both plasma and cell samples. This validated method can be useful for an evaluation of drug concentrations in biological samples in an efficient and sensitive manner.
INTRODUCTION
Since the introduction of highly active antiretroviral therapy (HAART) in the 1990s, the outcome in terms of life expectancy for people suffering from human immunodeficiency virus (HIV) has greatly improved due to the plasma virus load reduction to below-detectable levels (1, 2). ART treatment usually includes two or three anti-HIV drugs from different drug classes targeting different viral proteins in order to increase efficacy and lower the risk of the virus developing resistance to the treatment. A common drug combination is a nucleoside or nucleotide reverse transcriptase inhibitor (NRTI or NtRTI, respectively) with a nonnucleoside reverse transcriptase inhibitor (NNRTI) or with one or more protease inhibitors (PIs) (3).
One of the first-line therapies recommended for ART-naive people is the combination of atazanavir (ATZ), ritonavir (RTV), and tenofovir (TFV) (3). ATZ is a commonly used PI that exhibits 10- to 100-fold higher antiviral potency than that of other PIs, including nelfinavir or indinavir, and demonstrates a lower rate of viral drug resistance (4–9). ATZ is typically used in combination with RTV, another PI, which inhibits the cytochrome P450 3A isoenzyme (CYP3A) metabolism of ATZ, thereby boosting ATZ exposure. RTV has also been shown to inhibit drug efflux transport by P-glycoprotein (PgP) and therefore might enhance the cellular retention of other PIs (10). TFV is a nucleotide reverse transcriptase inhibitor (NtRTI) clinically administered as tenofovir disoproxil fumarate (TDF), an oral prodrug that increases absorption and is readily hydrolyzed to the TFV active form (11). While a number of bioanalytical assays have been developed for the three drugs, they are often time-consuming and have an increased overall resource burden. For example, high volumes of biological samples and different chromatographic assays may be required to detect the levels of the three drugs in samples collected from patients (12–14).
We previously reported a method using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to simultaneously detect three HIV drugs, lopinavir (LPV), RTV, and TFV, which have distinctly different hydrophobic and physiochemical properties (15). The availability of a simple, sensitive, selective, efficient, and validated assay has enabled reliable quality control in the production of long-acting anti-HIV combination drug nanoparticles containing LPV, RTV, and TFV and the in vivo pharmacokinetic analysis of the nanoparticles and their distribution to the lymphoid tissue and blood mononuclear cells (16).
Based on a similar strategy, the goal of this research was to determine whether a similar three-drug ART combinations containing ATZ, RTV, and TFV can be detected in biological samples based on the same LC-MS/MS assay. A key challenge that remains is the significant differences in the hydrophobicity of the PIs ATZ and RTV versus that of the water-soluble NtRTI TFV. This makes it challenging to effectively and simultaneously extract the three drugs from plasma and also to identify a chromatographic column matrix for the separation. The optimized assay procedure was validated to be able to extract all three compounds and simultaneously quantify the plasma and peripheral blood mononuclear cell drug concentrations with high efficiency, selectivity, and sensitivity.
MATERIALS AND METHODS
Chemicals.
The original standard samples of atazanavir (ATZ), ritonavir (RTV), and tenofovir (TFV) were kindly provided by National Institutes of Health (NIH) AIDS Research and Reference Reagent Program. Further samples were purchased from Waterstone Technology (Carmel, IN). Cyheptamide was obtained from Sigma-Aldrich (St. Louis, MO). Acetic acid (HAc) of glacial grade, trifluoroacetic acid (TFA), and dimethyl sulfoxide (DMSO) were purchased from J. T. Baker (Center Valley, PA). Water, acetonitrile, methanol (all of Optima grade), methylene chloride, and sodium bicarbonate (NaHCO3) were obtained from Fisher Scientific (Pittsburgh, PA). Tween 20 was purchased from Roche Diagnostics (Indianapolis, IN). Injection-grade 0.9% sodium chloride (NaCl) and sterile water were obtained from Baxter Healthcare (Deerfield, IL).
Instrumentation.
The high-performance liquid chromatography (HPLC) system was from Shimadzu (Columbia, MD) and consisted of two LC-20A pumps, a DGU-20A5 degasser, and an SIL-20AC HT autosampler. This was coupled with a 3200 QTRAP mass spectrometer from Applied Biosystems (Grand Island, NY) equipped with an electrospray ionization (ESI) TurboIonSpray source. The controlling and processing software used was Analyst (AB Sciex, Framingham, MA).
HPLC-MS/MS method.
The HPLC-MS/MS method was based on a previously published paper (14). In brief, the three compounds were separated on a Synergi Polar-RP column (100 by 2.0 mm, 4 μm) (Phenomenex, Torrance, CA) with a C8 guard column (4 by 2.0 mm) (Phenomenex). The mobile phase consisted of water for A and acetonitrile for B, both with 0.1% acetic acid added, and the gradient starting at 97/3 A/B to 0/100 over 4 min, as described previously (14). The flow rate was set to 0.35 ml/min, and the sample injection volume was 5 μl.
The analytes were monitored by multiple reaction monitoring (MRM) in positive mode using the following ion transitions: atazanavir, m/z 705.5→168.2; ritonavir, m/z 721.3→296.1; tenofovir, m/z 288.1→176.1; and IS, m/z 238.1→193.2. The detector parameters were as follows: curtain gas (N2), 30 lb/in2; ion spray voltage, 5 kV; temperature, 475°C; nebulizer gas (N2), 40 lb/in2; dry gas (N2), 40 lb/in2; and collision gas was set to medium.
Standard samples.
Standard samples were weighted independently in two separate batches for all three drugs and dissolved to form stock solutions at a concentration of 50 μg/ml. ATZ and RTV were kept in acetonitrile, while TFV was kept in 50/50 (vol/vol) water-acetonitrile. The stock solutions were kept at −20°C. Working standard solutions were diluted from the stock solutions to a concentration of 1 μg/ml in 50/50 (vol/vol) water-acetonitrile and kept at 4°C. One of the stock solutions of each drug was diluted to calibrate the assay, while the other was used for quality control (QC). The internal standard cyheptamide was prepared in a stock solution of 250 μg/ml in acetonitrile and kept at −20°C. A working solution of 1 μg/ml was diluted from the stock and kept at 4°C.
Eleven different samples in concentrations ranging from 1 ng/ml to 1,000 ng/ml were prepared in 90/10 (vol/vol) water-acetonitrile with 0.1% HAc for the method calibration. Quality controls were prepared at low, medium, and high concentrations (5, 50, and 750 ng/ml, respectively).
Sample preparation.
Preparation of the plasma samples was done according to a previously published procedure (15). A similar sample preparation procedure was used to detect drugs found in peripheral blood mononuclear cells (PBMCs). The PBMCs separated from primate whole blood were counted and aliquoted into Eppendorf tubes at 2 million cells per tube. The cell pellets were dissolved in 200 μl of 50/50 (vol/vol) methanol-H2O. These samples were subsequently extracted and processed in the same way as those for drugs in plasma samples. To generate a standard curve for the cells, samples were spiked at concentrations of 0.5, 1, 5, 10, 25, 50, and 100 ng/ml. This standard curve was used to evaluate linearity and estimate drug concentrations in PBMCs.
Validation.
The assay validation was based on Guidance for industry: Bioanalytical Method Validation, issued by the U.S. Food and Drug Administration (FDA) (17). The parameters evaluated were linearity, sensitivity, accuracy, precision, stability, selectivity, and recovery.
Linearity was evaluated in the range of 1 to 1,000 ng/ml for the three drugs. Eleven concentration points were injected in triplicate, and the back-calculated accuracy had to be within ±15% (±20% for the lowest point) for the standard curve to be accepted.
The sensitivity for each substance was determined by the limit of detection (LOD) and limit of quantification (LOQ) according to the protocols for determination of limits of detection and limits of quantification, issued by the Clinical and Laboratory Standards Institute (18).
To determine the intra- and interday precision and accuracy, QC samples were used at low, medium, and high concentrations (5, 50, and 750 ng/ml, respectively). The acceptable limit for both precision and accuracy was set to ±15% (±20% for the lowest concentration).
In order to assess the selectivity of the method, blank plasma samples were injected and evaluated for interference at the retention times for the analytes of interest.
Robustness was confirmed by running the same method on a second LC-MS/MS system consisting of the 1290 Infinity LC system from Agilent (Santa Clara, CA) and an API 4000 mass spectrometer from Applied Biosystems (Grand Island, NY).
The recovery was calculated as the extraction yield, comparing extracted standard samples with blank extracted plasma and PBMCs spiked with known concentrations of drug representing 100% at the final dissolving step.
Primate study.
In order to confirm and evaluate the analytical method, a study was carried out with nonhuman primates (pigtailed macaques [Macaca nemestrina]). The study was conducted under a protocol approved by the University of Washington Institutional Animal Care and Use Committee. Two primates were injected subcutaneously with suspensions of ATZ-RTV-TFV with a mole ratio of 10:5:15 (20:10.2:12.2 mg/kg of body weight). ATZ and RTV were suspended in 1:1 DMSO-water with 1% Tween 20, and TFV was injected separately in a solution containing 0.5% NaCl and 60 mM NaHCO3.
Venous blood was collected at the following time points: 0 (predose), 0.5, 1, 3, 5, 8, and 24 h. The samples were collected in K2-EDTA collection tubes and immediately put on ice. The blood was centrifuged for 10 min at 1,200 rpm, separating the plasma, which was transferred to cryovials and stored at −80°C until analysis. PBMCs were isolated by a density gradient method (19).
RESULTS
With a previously reported chromatographic support matrix and drug elution conditions (15), we were able to detect all three drugs, ATZ, RTV, and TFV, in one step. A typical LC-MS/MS chromatogram is shown in Fig. 1. TFV, which showed the weakest binding to the column compared to that of the other compounds, eluted first at 2.51 min (±0.6 min). TFV was followed by cyheptamide (internal standard [IS]) at 4.85 min (±0.02 min), ATZ at 4.96 min (±0.05 min), and RTV at 5.06 min (±0.05 min). While separated by different mass transitions, there is still a possibility of quenching similarly eluting compounds. Thus, we have investigated signal quenching at low, medium, and high concentrations and found that signal quenching, if any, was negligible.
FIG 1.
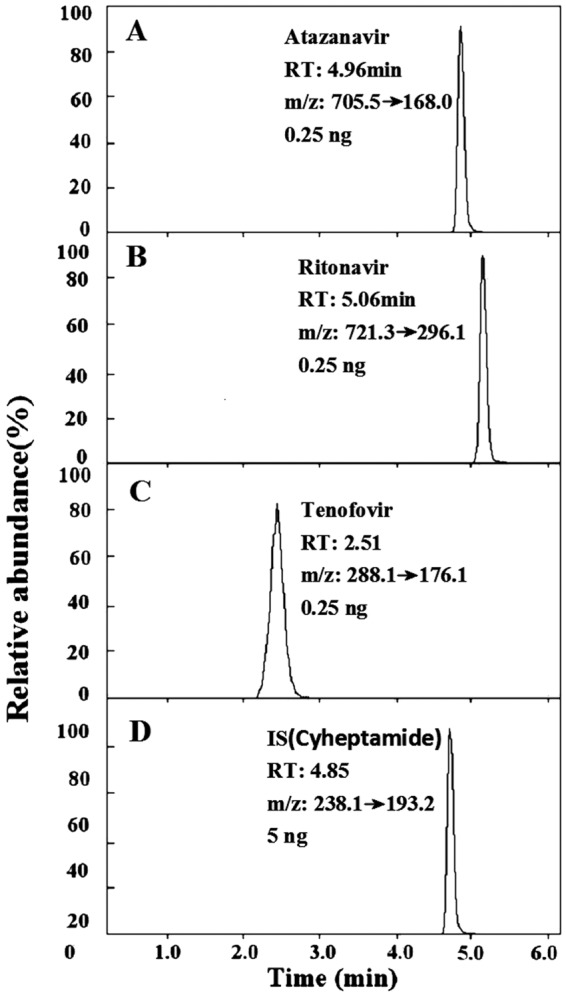
Chromatograms of the drugs atazanavir (A), ritonavir (B), tenofovir (C), and IS (cyheptamide) (D). RT, retention time.
In accordance with a previously reported approach (15), we evaluated the combinations of liquid-liquid extraction for the two hydrophobic PIs, ATZ and RTV, followed by plasma protein precipitation for the recovery of the hydrophilic TFV. We found that the combination detailed in the method gave a high recovery rate of all three compounds in a reproducible manner within a time frame of 1 h.
These optimized conditions were used for all subsequent validation studies and the primate pharmacokinetic study.
Assay validation.
To determine the assay linearity, we evaluated ATZ, RTV, and TFV either alone or in combination based on the peak area comparison to the internal standard. We found that the assay is linear over the range of 1 to 1,000 ng/ml, with correlation coefficients (r2) of ≥0.99 for all three compounds (Fig. 2).
FIG 2.
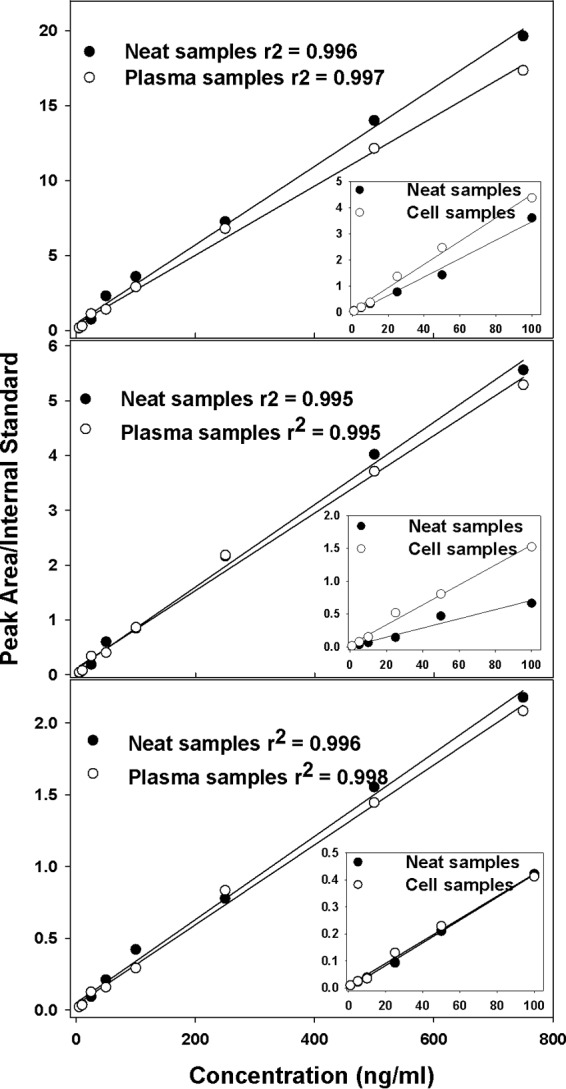
Extracted plasma and cell samples from primates compared to neat (undiluted) samples of atazanavir (A), ritonavir (B), and tenofovir (C). The data are presented as mean values from 6 repetitions with linear square regression lines. The main curve in each panel represents plasma-extracted samples versus neat samples, while the inset represents cell-extracted samples versus neat samples.
The sensitivity is expressed as the limit of detection (LOD) and limit of quantification (LOQ). The LOD for this assay was determined to be 1, 25, and 250 pg/ml and the LOQ was 2, 50, and 500 pg/ml for ATZ, RTV, and TFV, respectively, based on the 5-μl injection volume. The on-column sensitivity of these data translates to an LOD of 0.005, 0.125, and 1.25 pg and an LOQ of 0.01, 0.25, and 2.5 pg for ATZ, RTV, and TFV, respectively. The LOD is defined as the mean response in blank samples (n = 6) + 1.645 × standard deviation (SD). This calculated value was confirmed by injection of standards (n = 6). The LOQ was set to where the coefficient of variation percentage (%CV) of 6 injected samples was ≤20%.
The results of intra- and interday precision and accuracy validation at low, medium, and high concentrations (5, 50, and 750 ng/ml, respectively) of ATZ, RTV, and TFV are listed in Table 1. The intraday accuracies ranged from 97.9% to 103.4% for all three compounds, while the interday accuracies ranged from 98.8% to 104.2%. The precision (CV) was <5% for both inter- and intraday comparisons. Thus, the assay is precise, accurate, and reproducible.
TABLE 1.
Intra- and interday precision and accuracy of atazanavir, ritonavir, and tenofovir in neat samples at indicated concentrations
| Parameter | Data for drugs by dose (ng/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atazanavir |
Ritonavir |
Tenofovir |
|||||||
| 5 | 50 | 750 | 5 | 50 | 750 | 5 | 50 | 750 | |
| Intra-assay comparison (n = 6) | |||||||||
| Avg concn (ng/ml) | 5.0 | 49.0 | 759.8 | 5.0 | 51.7 | 754.9 | 5.0 | 49.8 | 752.0 |
| SD | 0.1 | 0.9 | 7.4 | 0.1 | 1.7 | 12.0 | 0.1 | 2.1 | 13.6 |
| %CV (precision) | 1.0 | 1.9 | 1.0 | 1.7 | 3.3 | 1.6 | 1.6 | 4.3 | 1.8 |
| Accuracy % | 100.4 | 97.9 | 101.3 | 99.8 | 103.4 | 100.7 | 100.1 | 99.5 | 100.3 |
| Interassay comparison (n = 6) | |||||||||
| Avg concn (ng/ml) | 5.04 | 48.6 | 753.5 | 5.0 | 52.1 | 760.5 | 5.1 | 51.5 | 740.9 |
| SD | 0.1 | 0.8 | 12.3 | 0.1 | 2.1 | 10.4 | 0.0 | 2.0 | 7.6 |
| %CV (precision) | 2.0 | 1.7 | 1.6 | 2.3 | 4.0 | 1.4 | 0.8 | 3.9 | 1.0 |
| Accuracy % | 100.8 | 101.5 | 100.5 | 100.7 | 104.2 | 101.4 | 101.1 | 103.0 | 98.8 |
In order to ensure that the signals detected in the mass spectrometer were from the compounds of interest only and not from interfering residues in the biological samples, we investigated the selectivity of the assay. From our mass scan data, no interferences were found at the retention times for each compound based on the data collected with blank plasma samples. These data validate the assay selectivity for the three drugs.
We next determined the variation from run to run in the detection of drugs extracted from plasma. The results of extraction recovery, expressed as percentages for ATZ, RTV, or TFV at three concentrations, 5, 50, and 750 ng/ml, are presented in Table 2. The recovery percentages were calculated based on comparisons between mock- and plasma-extracted drugs. With this approach, we found that all three drugs showed >92.10% (range, 92.10% to 101.55%) recovery, with a %CV of <8.86%.
TABLE 2.
Plasma extraction efficiency of atazanavir, ritonavir, and tenofovira
| Parameter | Data for drugs by dose (ng/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atazanavir |
Ritonavir |
Tenofovir |
|||||||
| 5 | 50 | 750 | 5 | 50 | 750 | 5 | 50 | 750 | |
| Calculated concn (ng/ml) for run: | |||||||||
| 1 | 4.85 | 42.12 | 785.60 | 5.17 | 49.41 | 784.15 | 4.81 | 48.73 | 682.82 |
| 2 | 4.55 | 44.92 | 746.24 | 4.99 | 51.50 | 739.56 | 5.27 | 51.45 | 758.56 |
| 3 | 4.76 | 50.71 | 762.47 | 5.35 | 54.27 | 750.69 | 5.32 | 44.00 | 746.56 |
| 4 | 4.13 | 48.52 | 767.89 | 4.79 | 43.10 | 758.97 | 4.42 | 47.86 | 761.30 |
| 5 | 4.65 | 46.24 | 738.54 | 5.25 | 44.65 | 724.27 | 4.42 | 44.27 | 728.28 |
| 6 | 4.71 | 49.27 | 760.28 | 4.92 | 46.36 | 727.40 | 4.78 | 48.99 | 756.36 |
| Avg concn (ng/ml) | 4.61 | 46.96 | 760.17 | 5.08 | 48.21 | 747.51 | 4.84 | 47.55 | 738.98 |
| SD | 0.26 | 3.16 | 16.58 | 0.21 | 4.27 | 22.32 | 0.39 | 2.90 | 30.03 |
| %CV | 5.56 | 6.73 | 2.18 | 4.21 | 8.86 | 2.99 | 8.16 | 6.10 | 4.06 |
| % recovery | 92.10 | 93.93 | 101.36 | 101.55 | 96.42 | 99.67 | 96.76 | 95.10 | 98.53 |
Values are given as calculated concentrations, with spiked extracted blank plasma representing 100%.
Next, we determined the extraction recovery of the drugs from PBMC samples, with the results shown in Table 3. As for the plasma extractions, the results are expressed as percentages and calculated from comparisons between mock- and cell-extracted drugs. The concentrations investigated were 0.5, 5, and 10 ng/106 cells, with a recovery of >86.10% and a %CV of <8.33%.
TABLE 3.
Cell extraction efficiency of atazanavir, ritonavir, and tenofovir
| Parameter | Data for drugs by concentration (ng/106 cells) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atazanavir |
Ritonavir |
Tenofovir |
|||||||
| 0.5 | 5 | 10 | 0.5 | 5 | 10 | 0.5 | 5 | 10 | |
| Calculated concn (ng/106 cells) for run: | |||||||||
| 1 | 0.47 | 4.63 | 8.62 | 0.43 | 4.11 | 9.12 | 0.49 | 4.61 | 8.50 |
| 2 | 0.49 | 4.76 | 8.71 | 0.45 | 4.01 | 8.67 | 0.51 | 4.65 | 9.72 |
| 3 | 0.55 | 4.83 | 9.22 | 0.48 | 4.06 | 8.87 | 0.50 | 4.64 | 8.76 |
| 4 | 0.58 | 4.61 | 8.60 | 0.44 | 4.47 | 8.77 | 0.50 | 4.77 | 8.83 |
| 5 | 0.51 | 4.71 | 8.97 | 0.49 | 4.60 | 9.06 | 0.50 | 4.84 | 8.63 |
| 6 | 0.57 | 4.90 | 8.79 | 0.44 | 4.57 | 9.09 | 0.51 | 4.96 | 8.89 |
| Avg concn (ng/106 cells) | 0.53 | 4.74 | 8.81 | 0.46 | 4.31 | 8.93 | 0.50 | 4.74 | 8.89 |
| SD | 0.04 | 0.11 | 0.25 | 0.02 | 0.27 | 0.18 | 0.01 | 0.14 | 0.43 |
| %CV | 8.33 | 2.32 | 2.78 | 5.22 | 6.35 | 2.07 | 0.91 | 2.88 | 4.86 |
| % recovery | 105.63 | 94.80 | 88.09 | 91.43 | 86.10 | 89.30 | 100.57 | 94.89 | 88.89 |
Values are given as calculated concentrations, with spiked extracted blank plasma representing 100%.
We also compared both plasma samples and PBMC samples with neat standard samples, and the resulting curves are shown in Fig. 2. In patients, the expected concentrations in cell samples are much lower than those in plasma; therefore, the standard curve generated for cells has a lower range. While the slope of the curve may vary due to matrix effects, the linearity and sensitivity were suitable for this assay procedure to detect all three drugs in plasma and in cells (PBMCs).
The robustness of the method was confirmed by running the method on a second system, and the results showed satisfying comparable results for the peak area divided by the internal standard for the two systems. The results in calculated concentrations are shown in Table 4.
TABLE 4.
System comparisons for robustness validation
| Systema | Data for drugs by dose (ng/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atazanavir |
Ritonavir |
Tenofovir |
|||||||
| 5 | 50 | 750 | 5 | 50 | 750 | 5 | 50 | 750 | |
| 1 | 5.0 | 49.0 | 759.8 | 5.0 | 51.7 | 754.9 | 5.0 | 49.8 | 752.0 |
| 2 | 5.2 | 53.1 | 753.7 | 5.1 | 49.1 | 755.9 | 5.0 | 47.5 | 768.0 |
n = 6 for the system comparison.
With this sensitive and validated assay with good plasma and cell extraction recovery values, we proceeded to measure time course plasma ATZ, RTV, and TFV concentrations in primates dosed subcutaneously with these three drugs in combination.
Time course plasma drug concentrations of ATZ, RTV, and TFV in primates.
To evaluate the capability of this assay, we evaluated time course plasma drug concentrations of two primates (pigtailed macaques [M. nemestrina]) injected subcutaneously with ATZ, RTV, and TFV (20:10.2:12.2 mg/kg, respectively). As shown in Fig. 3, all three drugs were detectable within a few minutes after administration. While the peak plasma drug concentrations varied from 160 ng/ml for ATZ to 10 μg/ml for TFV, all three drugs readily declined within the first 24 h to low but still-detectable levels. In addition, the drug levels extracted from plasma were reproducible, as the variations between two extractions and three LC-MS/MS runs were small. Collectively, these data indicate that this one-step assay to determine plasma drug concentrations of the three drugs ATZ, RTV, and TFV is reproducible and sensitive for plasma drug analysis.
FIG 3.
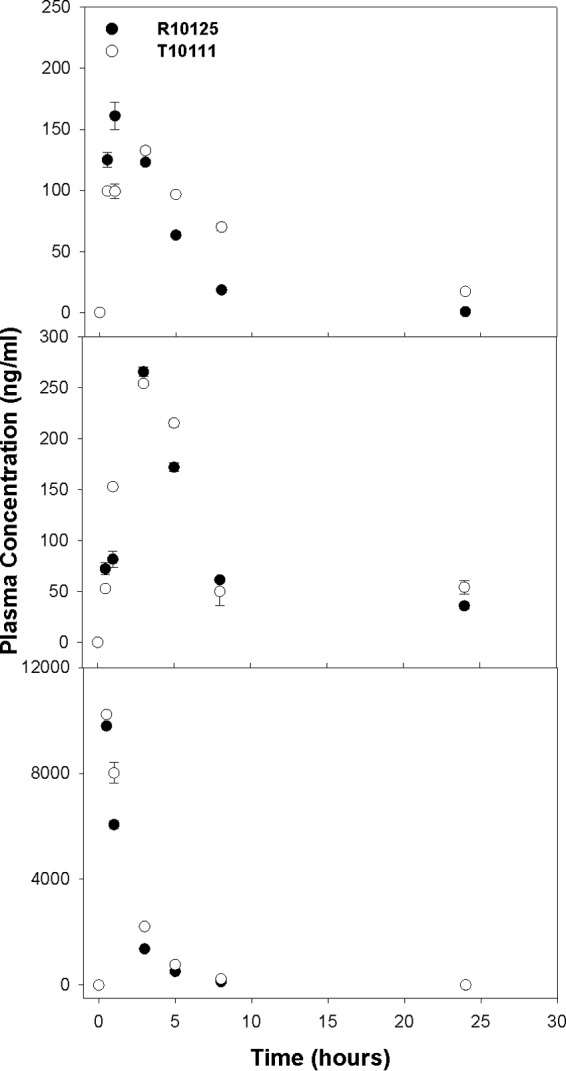
Time course plasma concentrations of atazanavir (top), ritonavir (middle), and tenofovir (bottom) in two primates (M. nemestrina). The two primates were subcutaneously administered atazanavir (20 mg/kg), ritonavir (10.2 mg/kg), and tenofovir (12.2 mg/kg). Plasma samples were extracted with the validated assay, as described in the text, and were analyzed using LC-MS/MS. The data were analyzed for each drug at specific time points and are presented as the means ± SD (n = 6). The symbols represent the samples collected from two different primates.
To evaluate intracellular drug concentrations, drug levels in PBMCs isolated from the blood sample were measured at indicated time points (Table 5). Over 8 h after administration of ATZ, RTV, and TFV, all three drugs were detectable in PBMCs isolated from primates. These data demonstrate that the assay can be used in clinical settings to detect the three drugs in cells and in plasma.
TABLE 5.
Intracellular drug concentrations in mononuclear cells isolated from the whole blood of primates dosed subcutaneously with atazanavir (20 mg/kg), ritonavir (10.2 mg/kg), and tenofovir (12.2 mg/kg)
| Drug or parameter | Dataa for drugs by time (h) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atazanavir |
Ritonavir |
Tenofovir |
||||||||||
| 0 | 1 | 5 | 8 | 0 | 1 | 5 | 8 | 0 | 1 | 5 | 8 | |
| R10125 | 0.0 | 151.0 | 166.3 | 65.4 | 0.0 | 124.5 | 544.2 | 366.4 | 0.0 | 3,523.5 | 838.5 | 338.3 |
| T10111 | 0.0 | 132.7 | 228.5 | 153.4 | 0.0 | 106.4 | 1,012.7 | 745.1 | 0.0 | 4,689.4 | 660.9 | 377.5 |
| Avg | 0.0 | 141.8 | 197.4 | 109.4 | 0.0 | 115.4 | 778.4 | 555.8 | 0.0 | 4,106.4 | 749.7 | 357.9 |
| SD | 0.0 | 9.1 | 31.1 | 44.0 | 0.0 | 9.1 | 234.3 | 189.4 | 0.0 | 583.0 | 88.8 | 19.6 |
Concentration (picograms per million cells).
DISCUSSION
Although various anti-HIV drug assays have been reported to successfully detect multiple HIV protease inhibitors (PIs) or reverse transcriptase inhibitors in separate assays, it is more challenging to simultaneously detect both classes of drugs on one column. As with most PIs, ATZ and RTV are very hydrophobic, while nucleoside and nucleotide analogue reverse transcriptase inhibitor drugs, such as TFV, tend to be hydrophilic under physiologic conditions (pH 7). Leveraging the ability of a column matrix and tandem mass-spectrometry (MS/MS) technique to separate two PIs, ATZ and RTV, and an NtRTI, TFV, we have successfully developed a single-step assay with a simplified extraction procedure to measure the concentrations of all three compounds in a single biological sample. The final assay was validated for plasma and PBMC samples and proven to be reliable and reproducible with high accuracy, precision, and extraction recovery. Also, by running the assay on an additional LC-MS/MS system, we observed satisfactory robustness.
Methods for the detection of antiretroviral agents mostly use LC-MS/MS, which can offer high sensitivity and selectivity, which are required for pharmacokinetic and therapeutic drug monitoring (TDM) studies. Two methods were previously described for the determination of >10 antiretroviral drugs for routine TDM (20, 21). The simultaneous detection of different classes of antiretroviral drugs (including hydrophobic and hydrophilic compounds) can save labor, time, and sample volumes. However, in the present paper, we have shown some limitations, including recovery percentage and sensitivity, which should be addressed.
According to the guidelines developed by the Clinical and Laboratory Standards Institute (18), a minimum of three concentrations in the range of expected concentrations is needed for method validation, including accuracy, precision, and recovery experiments. However, in the study by Jung et al. (20), the QC samples used for the recovery and matrix effects experiments were selected at two concentrations, 10 and 100 ng/ml, making the range of the assay narrow and not representative compared to the reported linearity range (1 to 500 ng/ml). In the research of Djerada et al. (21), the concentrations of the QC samples were 63, 2,000, and 4,000 ng/ml for ATZ, 25, 200, and 400 ng/ml for TFV, and 25, 800, and 1,600 ng/ml for RTV. All of these may be too high to get an accurate and sensitive measurement of the low concentrations of drugs in biological samples. In this report, we evaluated accuracy, precision, and extraction recovery by using QC samples at concentrations of 5, 50, and 750 ng/ml for each of the three drugs, which better represents the real drug concentrations in plasma and PBMC samples.
The simplified extraction strategy also provides a significant improvement in assay throughput for this method. A liquid-liquid extraction (LLE), followed by a protein precipitation step, offers excellent recovery for hydrophobic drugs in the LLE phase, and the protein precipitation step can recover most TFV. This offers a higher extraction recovery rate of tenofovir of >93.93%. This value is significantly higher than the tenofovir extraction efficiency reported by Jung et al. and Djerada et al. (about 70%) (20, 21).
The sensitivity of all three drugs in our assay has also improved over that of reported methods. The LOQ of ATZ, RTV, and TFV has been improved by 2,500-fold, 100-fold, and 10-fold, respectively, compared to that reported by Jung et al. (20). It also has been improved by 8,750-fold, 156-fold, and 12-fold, respectively, in a comparison with that in the study of Djerada et al. (21).
The improvements discussed above enable us to detect consistently lower concentrations in plasma and cell samples. Robustness and extraction efficiency combined with time reduction could be critically important factors in clinical settings, particularly in situations in which plasma drug concentrations might vary due to induced drug metabolism or multifaceted disease conditions. We have also shown in a primate study that our method is reliable and consistent, even for very low drug concentrations at extended time points.
In cells, TFV is first transformed into monophosphate and then further to its active form, diphosphate. When subjected to our method for cell extraction, the metabolites are hydrolyzed back to TFV, and the concentration we measure is the combined amount of the three forms. We previously reported various approaches to stabilize the nucleoside drug azidothymidine (AZT) phosphorylated metabolites AZT monophosphate, diphosphate, and triphosphate (22). We have successfully separated and detected all three phosphorylated TFV metabolites in a single run using LC-MS/MS, and with some adjustments to the previously published assay for AZT, we are currently working on stabilizing the TFV metabolites from biological samples. These studies are beyond the scope of this report and a subject of our current investigation.
In our assay, we focused on detecting two protease inhibitors, ATZ and RTV, plus the NtRTI TFV, since this three-drug combination is recommended in the most recent HIV/AIDS treatment guideline as a key HAART combination (3). With some modifications, this one-step clinical assay could be developed for other PI-and-NRTI/NtRTI drug combinations, such as darunavir and emtricitabine plus TFV. However, such studies are a part of our future investigation.
In summary, using a combination of liquid extraction and protein precipitation and single chromatography column separation, we have successfully developed a one-step LC-MS/MS assay to detect three analytes, ATZ, RTV, and TFV, in a single sample. This assay was validated to be reproducible, with outstanding extraction efficiency, for two hydrophobic drugs, ATZ and RTV, and a hydrophilic drug, TFV. This validated assay could be used to evaluate plasma drug concentration in a sensitive, specific, and reproducible manner with good precision and consistency.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI-077390, AI077390-S1, AI-077390-S2, AI-077390-S3, and 1UL1-RR025014.
We thank Cuiling Shu for processing the blood and plasma samples and the primate center staff members Michael Gough and Drew May for their assistance in dosing and collecting blood samples.
We declare no conflicts of interest.
REFERENCES
- 1.Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Ashman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Yeni P. 2006. Update on HAART in HIV. J Hepatol 44:100–103. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services. 2012. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. AIDSinfo, Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services, Rockville, MD: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 4.Pérez-Elías MJ. 2007. Atazanavir: simplicity and convenience in different scenarios. Expert Opin Pharmacother 8:689–700. doi: 10.1517/14656566.8.5.689. [DOI] [PubMed] [Google Scholar]
- 5.Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Thiry A, McGrath D, CASTLE Study Team . 2008. Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet 372:646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 6.Cohen C, Nieto-Cisneros L, Zala C, Fessel WJ, Gonzalez-Garcia J, Gladysz A, McGovern R, Adler E, McLaren C, BMS AI424-043 Study Group . 2005. Comparison of atazanavir with lopinavir/ritonavir in patients with prior protease inhibitor failure: a randomized multinational trial. Curr Med Res Opin 21:1683–1692. doi: 10.1185/030079905X65439. [DOI] [PubMed] [Google Scholar]
- 7.Mills AM, Nelson M, Jayaweera D, Ruxrungtham K, Cassetti I, Girard PM, Workman C, Dierynck I, Sekar V, Abeele CV, Lavreys L. 2009. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS 23:1679–1688. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 8.Madruga JV, Berger D, McMurchie M, Suter F, Banhegyi D, Ruxrungtham K, Norris D, Lefebvre E, de Bethune MP, Tomaka F, De Pauw M, Vangeneugden T, Spinosa-Guzman S, TITAN Study Group . 2007. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet 370:49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang PG, Wei JS, Kim G, Chang M, El-Shourbagy T. 2006. Validation and application of a high-performance liquid chromatography-tandem mass spectrometric method for simultaneous quantification of lopinavir and ritonavir in human plasma using semi-automated 96-well liquid-liquid extraction. J Chromatogr A 1130:302–307. doi: 10.1016/j.chroma.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Holmstock N, Annaert P, Augustijns P. 2012. Boosting of HIV protease inhibitors by ritonavir in the intestine: the relative role of cytochrome P450 and P-glycoprotein inhibition based on Caco-2 monolayers versus in situ intestinal perfusion in mice. Drug Metab Dispos 40:1473–1477. doi: 10.1124/dmd.112.044677. [DOI] [PubMed] [Google Scholar]
- 11.Kearney BP, Flaherty JF, Shah J. 2004. Tenofovir disoproxil fumarate. Clin Pharmacokinet 43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 12.Rouzes A, Berthoin K, Xuereb F, Djabarouti S, Pellegrin I, Pellergrin JL, Coupet AC, Augagneur S, Budzinski H, Saux MC, Breilh D. 2004. Simultaneous determination of the antiretroviral agents: amprenavir, lopinavir, ritonavir, saquinavir and efavirenz in human peripheral blood mononuclear cells by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 813:209–216. doi: 10.1016/j.jchromb.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Temghare GA, Shetye SS, Joshi SS. 2009. Rapid and sensitive method for quantitative determination of lopinavir and ritonavir in human plasma by liquid chromatography-tandem mass spectrometry. E J Chem 6:223–230. doi: 10.1155/2009/709478. [DOI] [Google Scholar]
- 14.Ehrhardt M, Möck M, Haefeli WE, Mikus G, Burhenne J. 2007. Monitoring of lopinavir and ritonavir in peripheral blood mononuclear cells, plasma and ultrafiltrate using a selective and highly sensitive LC/MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci 850:249–258. doi: 10.1016/j.jchromb.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Koehn J, Ho RJY. 2014. Novel liquid chromatography-tandem mass spectrometry method for simultaneous detection of anti-HIV drugs lopinavir, ritonavir, and tenofovir in plasma. Antimicrob Agents Chemother 58:2675–2680. doi: 10.1128/AAC.02748-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeling JP, Koehn J, Shu C, Sun J, Ho RJY. 2014. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS 28:2625–2627. doi: 10.1097/QAD.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Health and Human Services, U.S. Food and Drug Administration. 2001. Guidance for industry, bioanalytical method validation. Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Rockville, MD: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2004. Protocols for determination of limits of detection and limits of quantification, approved guideline. CLSI document EP17-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Böyum A. 1968. Isolation of mononuclear cells from human blood. Isolation of monuclear [sic] cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97:77–89. [PubMed] [Google Scholar]
- 20.Jung BH, Rezek NL, Bridges AS, Corbett AH, Kashuba ADM. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 21:1095–1104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 21.Djerada Z, Feliu C, Tournois C, Vautier D, Binet L, Robinet A, Marty H, Gozalo C, Lamiable D, Millart H. 2013. Validation of a fast method for quantitative analysis of elvitegravir, raltegravir, maraviroc, etravirine, tenofovir, boceprevir and 10 other antiretroviral agents in human plasma samples with a new UPLC-MS/MS technology. J Pharm Biomed Anal 86:100–111. doi: 10.1016/j.jpba.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Qian M, Bui T, Ho RJ, Unadkat JD. 1994. Metabolism of 3′-azido-3′-deoxythymidine (AZT) in human placental trophoblasts and Hofbauer cells. Biochem Pharmacol 48:383–389. doi: 10.1016/0006-2952(94)90111-2. [DOI] [PubMed] [Google Scholar]


