Abstract
GS-5806 is a small-molecule inhibitor of human respiratory syncytial virus fusion protein-mediated viral entry. During viral entry, the fusion protein undergoes major conformational changes, resulting in fusion of the viral envelope with the host cell membrane. This process is reproduced in vitro using a purified, truncated respiratory syncytial virus (RSV) fusion protein. GS-5806 blocked these conformational changes, suggesting a possible mechanism for antiviral activity.
TEXT
Respiratory syncytial virus (RSV) is an enveloped, single-stranded, negative-sense RNA virus that belongs to the Pneumovirinae subfamily of Paramyxoviridae (1). RSV infects the respiratory tract of infants, young children, and immunocompromised adults, causing severe disease (2–11). GS-5806 is a small-molecule inhibitor of RSV replication that is active against a diverse collection of RSV A and RSV B clinical isolates, with a mean 50% effective concentration (EC50) of 0.43 nM (12, 13). GS-5806 blocks RSV fusion (F) protein-mediated cell-cell fusion, and mutations that confer drug resistance map to the RSV F gene, suggesting that the target of GS-5806 is the RSV F protein. Viral-cell membrane coalescence mediated by paramyxovirus fusion proteins involves several proteins, such as an attachment protein, cell surface receptors, and other cellular components that trigger conformational changes in the fusion protein that catalyze fusion of the two membranes (14–16). In vitro, triggering of pre- to postfusion conformational changes of RSV F proteins can be achieved by lowering the ionic strength of the buffer or by increasing the temperature (17–19). The conformational changes expose the buried hydrophobic fusion peptides, which interact with fusion peptides of neighboring molecules to form rosette-like structures (see Fig. S2, top panel, in the supplemental material). These macromolecular structures are distinct and easily observed by electron microscopy (EM). The conformational changes can also be triggered in the presence of liposomes prepared in low-ionic-strength buffer. The RSV F protein triggered in the presence of liposomes inserts into the lipid bilayer presumably mediated by the fusion peptides. To evaluate the effects of GS-5806 on the pre- to postfusion conformational changes of RSV F, we expressed the extracellular domain of RSV F protein (ΔTM-RSV F) in HEK293 cells. The protein was stored in high-ionic-strength buffer (500 mM NaCl, 250 mM imidazole, 20 mM Tris, pH 8.0) to keep it in pretriggered conformation. On exposure to low-ionic-strength buffer (10 mM HEPES, pH 8.0) (see Fig. S1 in the supplemental material), ΔTM-RSV F formed rosettes or inserted into liposomes that were easily observable by EM (see Fig. S2, bottom panel, in the supplemental material). These experiments were used to measure the effect of GS-5806 on the conformational changes of ΔTM-RSV F protein (18).
ΔTM-RSV F protein was triggered in the presence of GS-5806 (5-fold molar excess over protein), an inactive analog of GS-5806 (GSC-1), or 0.1% dimethyl sulfoxide (DMSO). The number of rosettes observed in 6 to 8 random EM images was quantified by visual inspection. The average number of rosettes per image in the DMSO- (control), GSC-1-, and GS-5806-treated samples was 108, 106, and 23, respectively. The decrease in the number of rosettes formed in the presence of GS-5806 was significant compared to that with the GSC-1-treated (P < 0.002) or DMSO-treated (P < 0.002) samples (Fig. 1A). The inhibitory effect of GS-5806 was dose dependent, with fewer rosettes observed with increasing concentrations of GS-5806 (Fig. 1B).
FIG 1.
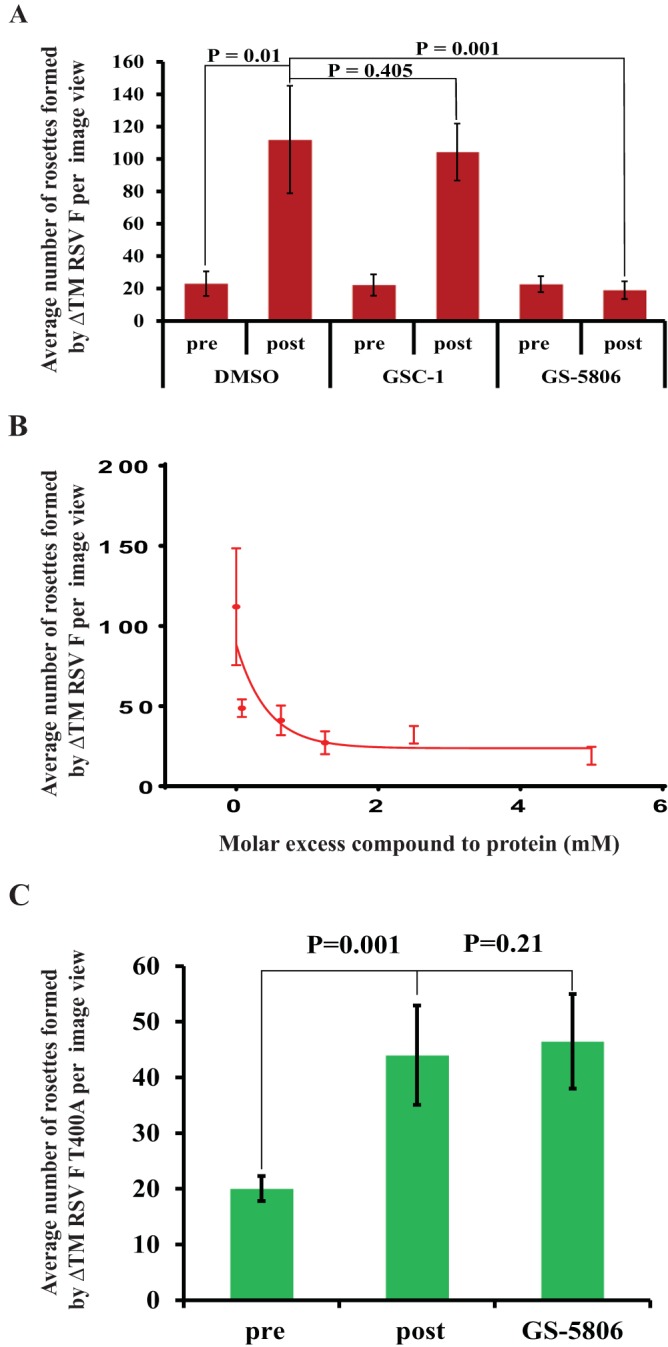
(A) GS-5806 inhibits pre- to posttriggered conformational changes of RSV F protein. Pre- to posttriggered conformational change was initiated by dialyzing ΔTM-RSV F protein overnight at 4°C in low-ionic-strength buffer (10 mM HEPES, pH 8.0) in the presence of a 5-fold molar excess of GS-5806, GSC-1 (an inactive analog), or DMSO (∼0.1%). The mean number of rosettes observed per grid view for different samples was calculated from at least 6 randomly selected EM images. The plot shows the mean values, with error bars representing the standard deviation. (B) The formation of rosettes in GS-5806-containing samples decreased in a dose-dependent manner. The mean number of rosettes observed per grid view for different samples containing various concentrations of GS-5806 was calculated from 6 to 10 randomly selected EM images and plotted as a function of GS-5806 concentration. The error bars represent the standard deviation of the mean values. (C) The ΔTM-RSV F protein containing the T400A amino acid change is associated with reduced susceptibility to GS-5806. The effects of GS-5806 on ΔTM-RSV F T400A protein rosette formation were evaluated. The mean number of rosettes observed per grid view for different samples was calculated from 6 randomly selected EM images. The plot shows the mean values, with error bars representing the standard deviation.
An RSV F resistance variant that contains a threonine-to-alanine amino acid change at position 400 of the RSV F protein was selected in vitro (12). ΔTM-RSV F T400A protein purified in the prefusion conformation was also triggered by low-ionic-strength buffer in a manner similar to that in the wild-type protein, but this process could not be inhibited by GS-5806. The numbers of rosettes formed in the presence of 0.1% DMSO (44 ± 9) and GS-5806 (46.6 ± 8) were similar (Fig. 1C), consistent with the reduced efficacy of GS-5806 observed in RSV variants expressing the T400A protein.
The effect of GS-5806 on ΔTM-RSV F protein conformational change was further evaluated by a liposome binding experiment. In order to increase the chances of insertion into the lipid bilayer and to avoid rosette formation, the liposome concentration was kept high (∼8 mM, 3,000-fold excess relative to RSV F). During the triggering process, RSV F molecules inserted into a few liposomes rather than partitioning evenly across all of the liposomes. The number of liposomes containing ΔTM-RSV F protein molecules was quantified by inspection of seven randomly selected EM images for each experiment. Very few ΔTM-RSV F-inserted liposomes were observed in the GS-5806-treated sample compared to those observed in the DMSO- or GSC-1-treated samples (Fig. 2A). On average, 4% ± 3% of ΔTM-RSV F-containing liposomes were observed in the GS-5806-treated sample, whereas 13% ± 4% (DMSO) or 13% ± 6% (GSC-1) of ΔTM-RSV F-containing liposomes were observed in control samples. Interestingly, the average number of ΔTM-RSV F molecules per liposome in the GS-5806-treated sample was 7 ± 3, versus 25 ± 10 in the DMSO-treated or GSC-1-treated samples. Similarly, the number of liposomes with ΔTM-RSV F T400A molecules in GS-5806-treated samples was 8% ± 3%, versus those in the DMSO-treated (14% ± 3%) or GSC-1-treated (16% ± 2%) samples (Fig. 2B). Unlike ΔTM-RSV F, the average numbers of ΔTM-RSV F T400A molecules deposited per liposome (∼30) were similar for all three treatments. These observations are supported by liposome flotation experiments (see Fig. S3 in the supplemental material). Rosette formation and liposome association experiments show that GS-5806 interferes with the pre- to posttriggered conformational changes of ΔTM-RSV F protein, similar to the conformational changes inhibited by influenza virus entry inhibitor (19).
FIG 2.
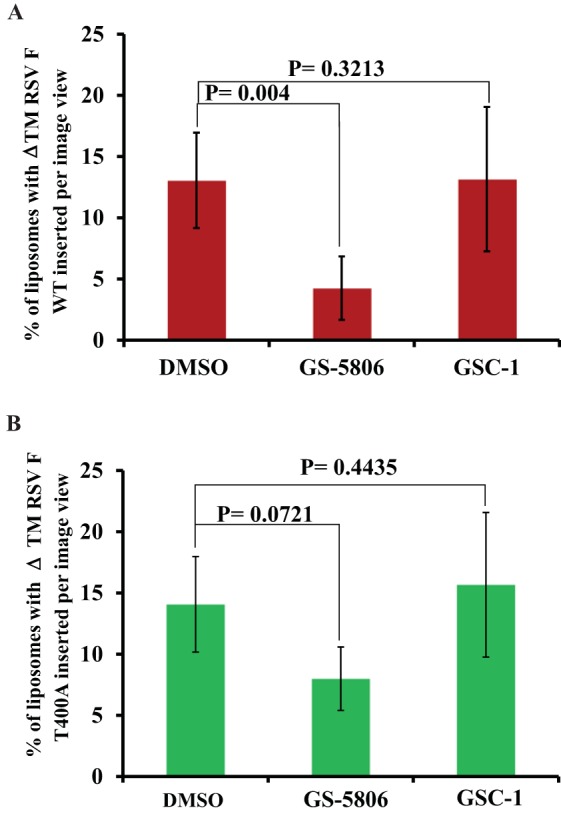
(A) GS-5806 inhibits the deposition of the ΔTM-RSV F protein into liposomes. The ΔTM-RSV F protein conformational change was triggered by mixing with liposomes prepared in low-ionic-strength buffer. The number of liposomes containing ΔTM-RSV F molecules in GS-5806-, GSC-1-, or DMSO-treated samples was quantified from 7 randomly selected EM images and represented in the plot as a percentage of the total liposomes in the sample. The error bars represent the standard deviations of the mean values. (B) GS-5806 does not affect the deposition of RSV F T400A protein on liposomes. The number of liposomes containing ΔTM RSV F T400A molecules in GS-5806-, GSC-1-, or DMSO-treated samples was quantified from 7 randomly selected EM images and represented in the plot as a percentage of the total liposomes in the sample. The error bars represent the standard deviations of the mean values.
Several RSV entry inhibitors representing diverse chemical classes (VP-14637, TMC-353121, and BMS-433771) have been reported in the literature (20–23). In the presence of these inhibitors (5-fold molar excess over protein), the number of rosettes formed was reduced by 2- to 5-fold compared to those with the DMSO control (see Fig. S4 in the supplemental material). The binding sites for two of these entry inhibitors, TMC-353121 and BMS-433771, were identified by X-ray crystallography and chemical cross-linking methods and found to be close to the six-helix bundle of the RSV F protein (24, 25). However, GS-5806 did not influence the formation of six-helix bundles when isolated peptides were mixed in the presence of GS-5806 (see Fig. S5A in the supplemental material). In addition, differential scanning calorimetry, isothermal titration calorimetry, and circular dichroism did not detect direct interaction of GS-5806 with isolated six-helix bundles (see Fig. S5B in the supplemental material). These results suggest that GS-5806 does not interact with the isolated six-helix bundle the same as TMC-353121 or BMS-433771 compounds but still interferes with the transition of the RSV F protein from the pre- to posttriggered conformation to elicit that it is antiviral activity.
Supplementary Material
ACKNOWLEDGMENTS
We extend our sincere thanks to Chris Rhodes for evaluation of EM grids, Jason Perry for help with model building, Albert Liclican for help with figures, and Hyock Joo Kwon for suggestions and critical review of the manuscript.
All authors except J.W. are employees of Gilead Sciences, Inc., and receive regular compensation according to the company employment policies. J.W. has performed contract work for Gilead Sciences, Inc.
D.S. performed protein purification, characterization, and assays; analyzed the data; and wrote the manuscript. W.X. expressed RSV F proteins in HEK293 cells, purified protein, prepared samples for assays, and analyzed data. A.N.-M. prepared samples for liposome assays and analyzed data. J.S.W. performed EM analysis. M.H. cloned RSV F genes. K.M.B. performed mass spectrometry analysis of the RSV F variants. M.P. performed cell culture experiments with the compounds. R.J. helped to prepare the manuscript. D.S. prepared compounds used in the assays. X.L., R.J., R.M., and R.S. provided scientific insights and critical comments.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00761-15.
REFERENCES
- 1.Collins PL, Crowe JEJ. 2007. Respiratory syncytial virus and metapneumovirus, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. 2000. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr 137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, Zhu Y, Grijalva CG, Prill MM, Iwane MK. 2013. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero JR. 2003. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J 22:S46–S54. doi: 10.1097/01.inf.0000053885.34703.84. [DOI] [PubMed] [Google Scholar]
- 7.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 8.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. 2012. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr Infect Dis J 31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 9.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 10.Lee N, Lui GC, Wong KT, Li TC, Tse EC, Chan JY, Yu J, Wong SS, Choi KW, Wong RY, Ngai KL, Hui DS, Chan PK. 2013. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 57:1069–1077. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- 11.Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. 2012. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis 206:56–62. doi: 10.1093/infdis/jis309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perron M, Stray K, Kinkade A, Theodore D, Lee G, Eisenberg E, Gilbert BE, Jordan R, Piedra PA, Mackman RL, Cihlar T. 2014. GS-5806 inhibits a broad range of respiratory syncytial virus clinical isolates via a blockade of the virus fusion process. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackman RL, Sangi M, Sperandio D, Parrish JP, Eisenberg E, Perron M, Hui H, Zhang L, Siegel D, Yang H, Saunders O, Boojamra C, Lee G, Samuel D, Babaoglu K, Carey A, Gilbert BE, Piedra PA, Strickley R, Iwata Q, Hayes J, Stray K, Kinkade A, Theodore D, Jordan R, Desai M, Cihlar T. 2015. Discovery of an oral respiratory syncytial virus (RSV) fusion inhibitor (GS-5806) and clinical proof of concept in a human RSV challenge study. J Med Chem 58:1630–1643. doi: 10.1021/jm5017768. [DOI] [PubMed] [Google Scholar]
- 14.Ebata SN, Cote MJ, Kang CY, Dimock K. 1991. The fusion and hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus 3 are both required for fusion. Virology 183:437–441. doi: 10.1016/0042-6822(91)90162-5. [DOI] [PubMed] [Google Scholar]
- 15.Hu XL, Ray R, Compans RW. 1992. Functional interactions between the fusion protein and hemagglutinin-neuraminidase of human parainfluenza viruses. J Virol 66:1528–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng R, Wang Z, Mirza AM, Iorio RM. 1995. Localization of a domain on the paramyxovirus attachment protein required for the promotion of cellular fusion by its homologous fusion protein spike. Virology 209:457–469. doi: 10.1006/viro.1995.1278. [DOI] [PubMed] [Google Scholar]
- 17.Kohn WD, Kay CM, Hodges RS. 1997. Salt effects on protein stability: two-stranded alpha-helical coiled-coils containing inter- or intrahelical ion pairs. J Mol Biol 267:1039–1052. doi: 10.1006/jmbi.1997.0930. [DOI] [PubMed] [Google Scholar]
- 18.Chaiwatpongsakorn S, Epand RF, Collins PL, Epand RM, Peeples ME. 2011. Soluble respiratory syncytial virus fusion protein in the fully cleaved, pretriggered state is triggered by exposure to low-molarity buffer. J Virol 85:3968–3977. doi: 10.1128/JVI.01813-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cianci C, Yu KL, Dischino DD, Harte W, Deshpande M, Luo G, Colonno RJ, Meanwell NA, Krystal M. 1999. pH-dependent changes in photoaffinity labeling patterns of the H1 influenza virus hemagglutinin by using an inhibitor of viral fusion. J Virol 73:1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKimm-Breschkin J. 2000. VP-14637 ViroPharma. Curr Opin Investig Drugs 1:425–427. [PubMed] [Google Scholar]
- 21.Bonfanti JF, Roymans D. 2009. Prospects for the development of fusion inhibitors to treat human respiratory syncytial virus infection. Curr Opin Drug Discov Devel 12:479–487. [PubMed] [Google Scholar]
- 22.Sin N, Yu KL, Combrink K, Venables B, Wang XA, Gulgeze HB, Civiell RL, Kadow KF, Cianci C, Clark J, Genovese E, Voss S, Lamb L, Medina I, Yang Z, Zadjura L, Huang S, Wu DD, Gao Q, Krystal M, Meanwell NA. 2005. Discovery of BMS-433771, a respiratory syncytial virus fusion inhibitor. Abstr Papers Am Chem Soc 230:U2607–U2608. [Google Scholar]
- 23.Yan D, Lee S, Thakkar VD, Luo M, Moore ML, Plemper RK. 2014. Cross-resistance mechanism of respiratory syncytial virus against structurally diverse entry inhibitors. Proc Natl Acad Sci U S A 111:E3441–E3449. doi: 10.1073/pnas.1405198111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roymans D, De Bondt HL, Arnoult E, Geluykens P, Gevers T, Van Ginderen M, Verheyen N, Kim H, Willebrords R, Bonfanti JF, Bruinzeel W, Cummings MD, van Vlijmen H, Andries K. 2010. Binding of a potent small-molecule inhibitor of six-helix bundle formation requires interactions with both heptad-repeats of the RSV fusion protein. Proc Natl Acad Sci U S A 107:308–313. doi: 10.1073/pnas.0910108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cianci C, Langley DR, Dischino DD, Sun Y, Yu KL, Stanley A, Roach J, Li Z, Dalterio R, Colonno R, Meanwell NA, Krystal M. 2004. Targeting a binding pocket within the trimer-of-hairpins: small-molecule inhibition of viral fusion. Proc Natl Acad Sci U S A 101:15046–15051. doi: 10.1073/pnas.0406696101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


