Abstract
We aimed to describe blood plasma (BP) and seminal plasma (SP) pharmacokinetics of emtricitabine (FTC) in HIV-1-infected men, assess its penetration in the male genital tract, and evaluate its impact on seminal plasma HIV load (spVL) detection. Men from the EVARIST ANRS EP49 study receiving combined antiretroviral therapy with FTC and with suppressed BP viral load were included in the study. A total of 236 and 209 FTC BP and SP concentrations, respectively, were available. A population pharmacokinetic model was developed with Monolix 4.1.4. The impact of FTC seminal exposure on spVL detection was explored by receiver operating characteristic (ROC) curves and mixed-effects logistic regressions. FTC BP pharmacokinetics was described by a two-compartment model. The addition of an effect compartment with different input and output constants best described FTC SP pharmacokinetics. No covariates were found to explain the variability in SP. FTC exposures (area under the concentration-time curve from 0 to 24 h [AUC0–24]) were higher in SP than in BP (median AUC0–24, 38.04 and 12.95 mg · liter−1 · h, respectively). The median (range) SP-to-BP AUC0–24 ratio was 2.91 (0.84 to 10.08). Less than 1% of FTC AUC0–24 ratios were lower than 1. The impact of FTC SP AUC0–24 or FTC SP-to-BP AUC0–24 ratio on spVL detection was not significant (P = 0.943 or 0.893, respectively). This is the first population model describing FTC pharmacokinetics simultaneously in both BP and SP. FTC distributes well in the male genital tract with higher FTC concentrations in SP than in BP. FTC seminal plasma exposures were considered efficient in the majority of men.
INTRODUCTION
Approximately 35 million people are living with human immunodeficiency virus (HIV) worldwide, with 2.1 million new infections reported globally in 2013 (1). The sexual transmission of HIV remains the main route of transmission, with semen being one of the most important sources of HIV infections. Combined antiretroviral treatments (cART), used to treat HIV infection, resulted in a significant decrease in blood plasma (BP) HIV load (bpVL). Several studies have shown that low HIV-RNA levels in BP were associated with a reduced risk of HIV sexual transmission (2, 3), suggesting that HIV RNA levels in BP reflected HIV RNA levels in the genital tract. Other studies have reported that bpVL correlated with seminal plasma HIV load (spVL) in patients receiving cART (4–6). Thus, the Swiss Federal commission for HIV/AIDS has stated that an HIV-infected individual receiving effective and stable cART, with an undetectable bpVL (<40 copies · ml−1) for at least 6 months and without additional sexually transmitted diseases could not transmit HIV through sexual contact (7). On the other hand, several studies have demonstrated that, despite suppressed bpVL (<50 copies · ml−1), spVL could be higher and detectable, reflecting a genital shedding of HIV. The proportion of patients with undetectable bpVL but detectable HIV-RNA levels in seminal plasma (SP) ranged from 3 to 48% (8–11). The mean proportion of patients with this discordance was estimated at 10% (12, 13). A longitudinal study in HIV-1-infected men who have sex with men (MSM) has reported a 7.6% discordance (14).
The genital tract, and more especially semen, is known for viral replication (15). Genital shedding of HIV can be influenced by different factors related to patients, virus, and antiretroviral therapy. There are few studies evaluating the penetration of antiretroviral (ARV) drugs in the semen, and most of them were carried out on a small number of patients (16). ARV drug diffusion in semen usually is measured at a given time point by a ratio of SP-to-BP concentrations. This concentration ratio is highly variable depending on the time between drug intake and sampling. It has been suggested that an SP-to-BP exposure (area under the concentration-time curve [AUC]) ratio should be more representative of the variability of penetration in the male genital tract (16). Thus, Dumond et al. have reported AUC ratios varying from 1.48 to 2.97 for zidovudine (AZT) and 4.1 to 9.14 for lamivudine (3TC), while concentration ratios ranged from 1.9 to 91.4 and 1.9 to 30.5, respectively (17). The penetration of ARV drugs in the male genital tract has been reported to be highly variable between drugs but also between individuals (16). This high variability could explain the variability observed in spVL detection among individuals.
Emtricitabine (FTC) is a nucleosidic reverse transcriptase inhibitor approved for the treatment of HIV infection in adult patients (18). Although FTC is recommended in the first-line regimen and is used worldwide, only one study has investigated its penetration in the male genital tract (19). Patterson et al. have reported higher FTC seminal plasma AUC from 24 h to 14 days than those of blood plasma exposures (408 versus 83 ng · days · ml−1). The median SP-to-BP AUC ratio was estimated at 4.5. However, that study included a small number of subjects (n = 8) and reported an SP-to-BP AUC ratio for a single dose of FTC, which may not be representative of the FTC behavior in the seminal plasma during daily administration. Thus, the aims of this study were the following: (i) to describe FTC blood plasma and seminal plasma pharmacokinetics (PK) in a large population of HIV-1-infected men; (ii) to evaluate FTC penetration in the male genital tract by a seminal plasma-to-blood plasma exposure ratio; and (iii) to evaluate the impact of FTC seminal plasma exposure on the seminal plasma HIV load.
MATERIALS AND METHODS
Study.
The EVARIST ANRS EP 49 study's main objective was to evaluate the proportion of HIV-1-infected MSM with a fully suppressed bpVL (<50 copies · ml−1) and a detectable spVL (14). The secondary objective was to assess the biological and behavioral factors that could be associated with viral load dissociation between blood and semen. MSM older than 18 years, infected by HIV-1, receiving stable cART for at least 3 months, and having a suppressed bpVL (<50 copies · ml−1) for at least 6 months were eligible. This study included patients from 6 different clinical centers in Paris, France, and nearby suburbs (Hôpital Hôtel Dieu, Hôpital Bichat, Hôpital Bicêtre, Hôpital Foch, Hôpital Saint-Louis, and Hôpital Lariboisière). The EVARIST ANRS EP 49 study protocol was approved by the Ethics Committee of the Bicêtre Hospital. All patients have signed written informed consent forms.
Patients, treatment, and sampling.
Our study population included HIV-1-infected MSM from the EVARIST ANRS EP 49 study receiving an FTC-based cART. These patients received a daily 200-mg FTC dose. Blood and semen samples were obtained at two different visits (at inclusion and 1 month after) for various time intervals between FTC intake and sampling. Both bpVL (>10 copies · ml−1) and spVL (>100 copies · ml−1) quantifications were assessed during these visits. For each man, body weight (BW), age, serum creatinine (SCR), associated antiretroviral drugs, and spVL detection were recorded. Creatinine clearance (CLCR) was obtained using the Cockcroft and Gault formula. Whether or not the 48-h abstinence period before semen sampling was respected and the clinical symptoms of potential sexually transmitted infections (discomfort or difficulty in urination, genital lesion or ulceration, and herpes) also were recorded.
Analytical method.
FTC blood plasma and seminal plasma concentrations were determined in the Clinical Pharmacology Unit (Hôpital Cochin, Paris, France) using a validated high-performance liquid chromatography tandem mass spectrometry method (S. M. Illamola, E. Valade, D. Hirt, E. Dulioust, J. P. Wolf, and J. M. Tréluyer, submitted for publication). The lower limit of quantification (LLOQ) was 0.00625 mg · liter−1, and the calibration curve was constructed in spiked blank blood plasma and seminal plasma over a concentration range of 0.00625 to 2.0 mg · liter−1. In blood plasma, the levels of intra- and interassay accuracy were lower than 18.29% and 5.61%, whereas intra- and interassay precision values were lower than 19.85% and 5.63%, respectively. In seminal plasma, the levels of intra- and interassay accuracy were lower than 12.73% and 15.22%, while intra- and interassay precision values were lower than 10.88% and 4.17%, respectively. Serum creatinine was measured using the colorimetric method of the Jaffe reaction on a Cobas 8000 (Roche/Hitachi).
Population pharmacokinetic modeling strategy.
Data were analyzed by a population approach, using the nonlinear mixed-effect modeling software program Monolix (version 4.1.4; www.lixoft.eu). Parameters were estimated by computing the maximum likelihood estimator of the parameters, using the stochastic approximation expectation maximization (SAEM) algorithm combined with a Markov chain Monte Carlo (MCMC) procedure (20). The number of MCMC chains was fixed to five for all estimations. Standard errors were calculated by stochastic approximation and log-likelihood by appropriate sampling methods.
First, all of the FTC blood plasma concentrations were analyzed, and PK parameters were estimated. These parameters then were fixed in order to estimate seminal plasma PK parameters. In a final step, all of the BP and SP parameters were estimated.
(i) FTC blood plasma pharmacokinetic model.
Several structural models for FTC BP pharmacokinetics were investigated, i.e., one and two compartments with linear absorption and elimination. Models with estimated or fixed values for the absorption rate constant (ka) also were considered. Continuous covariates (CO), such as age, BW, SCR, and CLCR, were tested with the following equation, using apparent elimination clearance (CL/F) as an example: CL/F = θCL/F · (CO/median CO)βCO, where θCL/F is the typical value of apparent elimination clearance for a subject with the median covariate value and βCO is the estimated influential factor for the continuous covariate. Binary covariates, such as associated ARV drugs, were tested according to the equation CL/F = θCL/F · βCOCO, where CO = 0 for the reference value of CL/F, CO = 1 for the value of CL/F with the covariate, and βCO is the estimated influential factor for the binary covariate.
(ii) FTC seminal plasma pharmacokinetic model.
The FTC BP pharmacokinetic model was fixed, and several models were investigated to describe FTC SP concentrations: connecting another compartment to the central compartment by first-order or saturation (Michaelis Menten) processes, connecting an effect compartment of negligible volume to the central compartment by a saturation process or by a first-order process with the same constant (ke0) or different input and output constants (k1e and ke1), inserting a transit compartment between the effect and central compartments, and connecting an effect compartment to the peripheral compartment. The effect compartment was modeled as a virtual compartment of negligible volume, which does not modify the BP compartmental model.
Several error models (proportional, additive, and combined) were investigated to describe the residual variability (ε) in blood plasma and seminal plasma. Interindividual variabilities (IIV or η) were assumed to be exponential. The effect of patient covariates on pharmacokinetic parameters was tested as described above.
The objective function value (OFV) was used to test different hypotheses regarding the structural model, the structure of the variance-covariance matrix for IIV, and residual variability models. The OFV also was used to test the covariate effect(s) on pharmacokinetic parameter(s). A covariate finally was retained in the model if its effect was biologically plausible, if the OFV was decreased by at least 3.84 (χ2 with 1 df; P < 0.05), and if it produced a reduction in the variability of the pharmacokinetic parameter (IIV).
Model evaluation.
For the evaluation of the goodness of fit, the following graphs were created for BP and SP: observed concentrations versus population predictions, weighted residuals versus time, and weighted residuals versus population predictions. Similar graphs with individual predictions were displayed.
For evaluation, a visual predictive check (VPC) was performed. The model was used to simulate 500 FTC concentrations for each sampling time. Simulated FTC concentrations and observed data were compared: the 5th, 50th, and 95th percentiles of observed data were overlaid on the 90% confidence interval (CI) of the 5th, 50th, and 95th simulated percentiles, and a visual inspection was performed.
FTC blood plasma and seminal plasma exposures.
Blood plasma and seminal plasma exposures (AUC from 0 to 24 h [AUC0–24]) were obtained by integrating analytical equations at steady state from 0 to 24 h for each patient using the estimated individual pharmacokinetic parameters. Distribution in the male genital tract was estimated by calculating SP-to-BP AUC0–24 ratios. The percentage of exposure ratios lower than 1 was calculated.
Impact of FTC exposures on seminal plasma HIV load.
In order to assess the association between SP AUC0–24 or SP-to-BP AUC0–24 ratio and the spVL, receiver operating characteristic (ROC) curve analyses were performed using R software. A 95% confidence interval on the area under the ROC curve was derived from a bootstrap method. Mixed-effect logistic regressions with random effects on individuals also were performed with R software (R package lme4; D. Bates, M. Maechler, B. Bolker, and S. Walker [http://cran.r-project.org/web/packages/lme4/index.html]) in order to evaluate the impact of FTC SP AUC0–24 and FTC SP-to-BP exposure ratios and potentially significant cutoffs derived from the ROC curve on the spVL detectability.
RESULTS
Demographic data.
Data from 122 and 117 men were available for blood plasma and seminal plasma analyses, respectively. Table 1 summarizes the patients' characteristics. For all patients, a daily 200-mg FTC dose was combined with tenofovir disoproxil fumarate (TDF). FTC and TDF were associated with one nonnucleoside reverse transcriptase inhibitor or one ritonavir-boosted protease inhibitor or raltegravir in 52, 29, or 14% of patients, respectively. A total of 95% of these men respected the 48-h abstinence period before semen sampling, and 14% had clinical symptoms of potential sexually transmitted infections.
TABLE 1.
Demographic characteristics of HIV-infected men receiving FTC (n = 122)
| Parametera | Value(s) |
||
|---|---|---|---|
| Median | Range | Proportion | |
| Age (yr) | 43 | 27–63 | |
| BW (kg) | 73 | 46–108 | |
| SCR (μmol · liter−1) | 78 | 28–113 | |
| CLCR (ml · min−1) | 113 | 67–368 | |
| Drug regimen | |||
| FTC+TDF+EFV | 56/122 | ||
| FTC+TDF+NVP | 7/122 | ||
| FTC+TDF+ETR | 1/122 | ||
| FTC+TDF+RAL | 17/122 | ||
| FTC+TDF+DRV/r | 16/122 | ||
| FTC+TDF+ATZ/r | 11/122 | ||
| FTC+TDF+LPV/r | 6/122 | ||
| FTC+TDF+IP/r | 3/122 | ||
| Other combination | 5/122 | ||
Abbreviations: BW, body weight; SCR, serum creatinine; CLCR, creatinine clearance; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate; EFV, efavirenz; NVP, nevirapine; ETR, etravirine; RAL, raltegravir; DRV, darunavir; ATZ, atazanavir; LPV, lopinavir; IP, other protease inhibitors; /r, combined with ritonavir.
Population pharmacokinetics.
A total of 236 FTC BP concentrations and 209 FTC SP concentrations at steady state were available for the analysis. One BP concentration was below the LLOQ and was set to half of the LLOQ value. A two-compartment model with first-order absorption and elimination best described the FTC blood plasma concentrations. An effect compartment connected to the central compartment with different input and output rate constants satisfactorily described the FTC SP concentrations (Fig. 1). Adding a transit compartment between central and effect compartments did not improve the fit for the SP concentrations. The absorption rate constant value (ka) could not be estimated. Thus, fixing ka to a previously reported value estimated in adults (0.53 h−1) improved the stability of the model (21). Figure 1 depicts the final pharmacokinetic model, whose parameters were the apparent elimination clearance (CL/F), central volume of distribution (Vc/F), intercompartmental clearance (Q/F), peripheral volume of distribution (Vp/F), BP-to-SP transfer rate constant (k1e), and SP elimination rate constant (ke1), with F being the unknown bioavailability. The differential equations corresponding to this model are the following.
| (1) |
| (2) |
| (3) |
| (4) |
where k12 = Q/Vc, k21 = Q/Vp, and k10 = CL/Vc; A(1) is the quantity of drug in the gut; A(2) is the quantity of drug in the central compartment; A(3) is the quantity of drug in the peripheral compartment; and Ae is the concentration of drug in the effect compartment.
FIG 1.
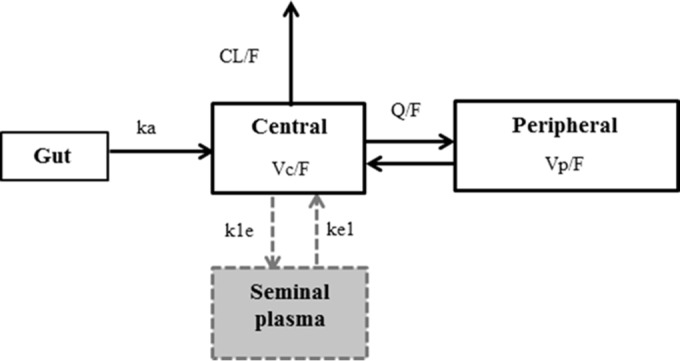
Population pharmacokinetic model of emtricitabine in blood plasma and seminal plasma. The parameters of this model are the absorption rate constant (ka), apparent elimination clearance (CL/F), central volume of distribution (Vc/F), intercompartmental clearance (Q/F), peripheral volume of distribution (Vp/F), blood plasma-to-seminal plasma transfer (k1e), and seminal plasma elimination rate constants (ke1), with F being unknown bioavailability.
The analytical solution for the effect compartment was the following:
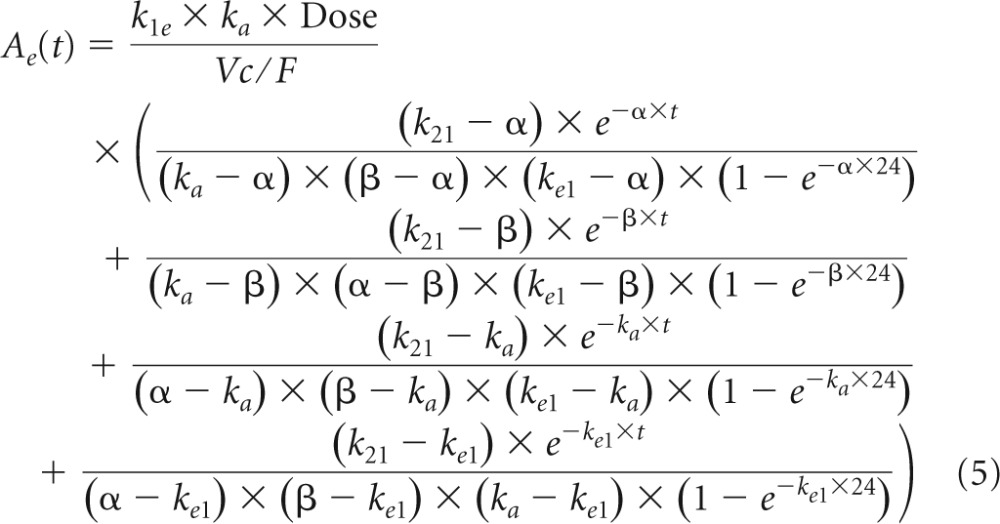 |
where β=1/2× [k12+k21+k10 − ] and α = (k21 × k10)/β. Residual variabilities for both blood plasma and seminal plasma were best described by proportional error models. Interindividual variabilities were significant for CL/F and the BP-to-SP transfer rate constant (k1e). For FTC blood plasma modeling, the most significant decrease in OFV was obtained by adding the effect of CLCR on CL/F. After the inclusion of CLCR on CL/F in the blood plasma model, the addition of other covariates did not further improve the model. Thus, the final covariate submodel for FTC blood plasma PK was CL/F = 14.8 · (CLCR/113)0.178, where 14.8 liter · h−1 denotes the typical value of CL/F for a patient with a median CLCR value of 113 ml · min−1. For FTC seminal plasma modeling, adding the effects of covariates on blood plasma to the seminal plasma transfer rate constant (k1e) did not decrease significantly the OFV. Thus, no covariates were found to explain the interindividual variability on k1e. Table 2 summarizes the final population pharmacokinetic estimates for BP and SP models, including the relative standard errors.
TABLE 2.
FTC population pharmacokinetic parameters for blood plasma and seminal plasmaa
| Parameter | Value |
|
|---|---|---|
| Estimate | RSE (%) | |
| Structural model | ||
| ka (h−1) | 0.53* | |
| CL/F (liter · h−1) | 14.8 | 4 |
| Vc/F (liter) | 51.6 | 11 |
| Q/F (liter · h−1) | 8.19 | 26 |
| Vp/F (liter) | 106 | 44 |
| βCLCR on CL/F | 0.178 | 35 |
| k1e (h−1) | 0.341 | 28 |
| ke1 (h−1) | 0.113 | 27 |
| Statistical model | ||
| ω CL/F | 0.255 | 12 |
| ω k1e | 0.533 | 10 |
| σplasma | 0.339 | 6 |
| σseminal plasma | 0.357 | 7 |
Abbreviations: RSE, relative standard error; ka, absorption rate constant; CL/F, apparent elimination clearance; Vc/F, apparent central volume of distribution; Q/F, apparent intercompartmental clearance; Vp/F, apparent peripheral volume of distribution; βCLCR on CL/F, an influential factor of creatinine clearance on CL/F; k1e, blood plasma to seminal plasma transfer rate constant; ke1, seminal plasma elimination rate constant; ω, interindividual variability estimates; σplasma, residual variability estimate for plasma concentrations; σseminal plasma, residual variability estimate for seminal plasma concentrations. The asterisk indicates a fixed value.
Model evaluation.
As shown in Table 2, all of the model parameters were well estimated, with small relative standard errors (RSE). The visual predictive check of the final model (Fig. 2) confirmed that the predictions matched the observed concentrations for BP and SP and that variability was well estimated.
FIG 2.
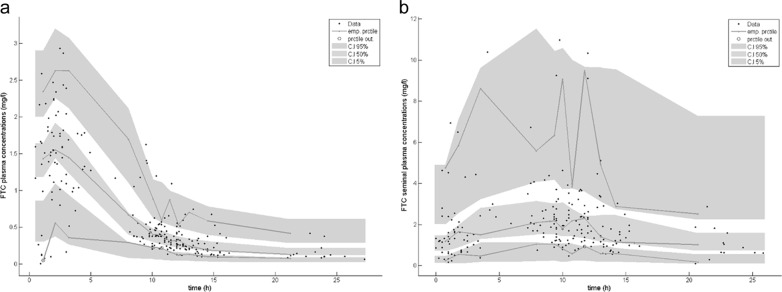
Evaluation of the final model by visual predictive check (VPC). We performed a comparison between the 5th, 50th, and 95th percentiles (prctile) of observed data (lines), the 90% confidence interval of simulated percentiles (areas), and the observed data (circles) for FTC blood plasma concentrations (a) and FTC seminal plasma concentrations (b).
FTC blood plasma and seminal plasma exposures.
Figure 3 displays FTC blood plasma and seminal plasma concentration time courses estimated using our model. FTC seminal plasma concentrations were higher than blood plasma concentrations, with median residual concentrations (range) estimated at 0.850 (0.258 to 3.687) mg · liter−1 for SP and 0.136 (0.057 to 0.459) mg · liter−1 for BP. Data corresponding to FTC blood plasma and seminal plasma AUC0–24, as well as SP-to-BP exposure ratios, are summarized in Table 3. The median FTC blood plasma AUC0–24 was 12.95 (8.36 to 25.13) mg · liter−1 · h, the median FTC seminal plasma AUC0–24 was 38.04 (13.40 to 148.18) mg · liter−1 · h, and the median SP-to-BP AUC0–24 ratio was 2.91 (0.84 to 10.08). The FTC distribution in the male genital tract was variable, with an SP-to-BP AUC0–24 ratio coefficient of variation (CV) of 54.7%. Only 2 SP-to-BP AUC0–24 ratios among 209 were lower than 1 (0.96% of ratios). Thus, FTC seminal plasma AUC0–24 values were higher than blood plasma AUC0–24s for more than 99% of patients.
FIG 3.
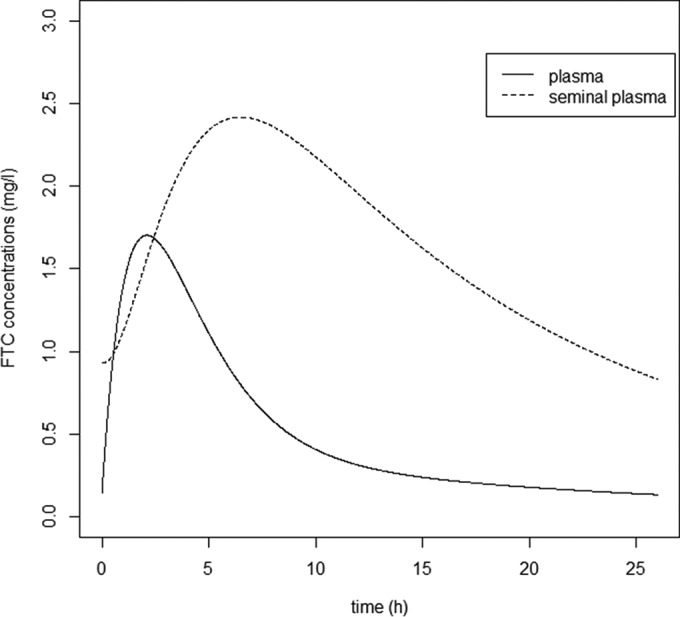
Evolution of FTC plasma concentrations (solid line) and FTC seminal plasma concentrations (dashed line) at steady state versus time.
TABLE 3.
FTC BP and seminal plasma SP exposures for a daily 200-mg dose
| AUC0–24 model | Sample size (no. of concn available) | Median (range; mg · liter−1 · h) |
|---|---|---|
| BP | 236 | 12.95 (8.36–25.13) |
| SP | 209 | 38.04 (13.40–148.18) |
| SP-to-BP ratio | 209 | 2.91 (0.84–10.08) |
Impact of FTC exposures on seminal plasma HIV RNA load.
Seminal plasma HIV load was measured in 116 patients. The spVL was detectable (>100 copies · ml−1) for 16 patients among 116 (13.8%) and for 18 samples among 207 (8.7%). Two patients had detectable spVL for the two visits, 12 patients had detectable spVL for one of the two visits, and 2 patients with detectable spVL attended only one visit. Figure 4 shows FTC seminal plasma AUC0–24 and SP-to-BP AUC0–24 ratios versus spVL detectability. Median SP AUC0–24 and SP-to-BP AUC0–24 ratios were slightly lower for patients with detectable spVL than for patients with undetectable spVL (36.83 versus 38.57 mg · liter−1 · h for SP AUC0–24, 2.60 versus 2.91 for SP-to-BP exposure ratios) but did not reach significance (P = 0.96 and P = 0.51, respectively, by Wilcoxon signed-rank test). In the same way, ROC curve analyses showed no association between spVL detectability and both SP AUC0–24 and SP-to-BP AUC0–24 ratio. The values corresponding to the areas under these ROC curves (95% CI) were relatively low and did not achieve significance: 0.503 (0.376 to 0.631) and 0.547 (0.406 to 0.688), respectively. Using mixed-effects logistic regressions with random effects on individuals, the impact of FTC seminal plasma AUC0–24 and SP-to-BP AUC0–24 ratio on spVL detectability was not significant (P = 0.943 and 0.893, respectively). The influence of FTC seminal plasma AUC0–24 and SP-to-BP AUC0–24 ratio on the spVL detectability remained nonsignificant after adjusting for confounding factors in the multivariate logistic regression. The controlled variables were clinical symptoms of potential sexually transmitted infections and herpes infection, previously described as influencing HIV shedding in the male genital tract (10).
FIG 4.
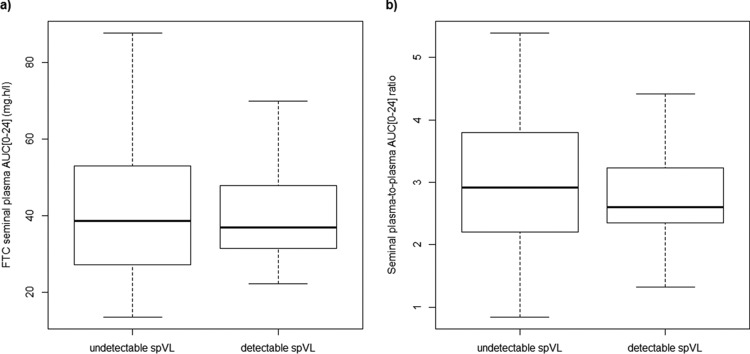
FTC seminal plasma AUC0–24 (a) and seminal plasma-to-blood plasma AUC0–24 ratios (b) versus seminal plasma HIV load (spVL) detectability.
DISCUSSION
FTC blood plasma and seminal plasma pharmacokinetics were satisfactorily described by a two-compartment model, including an effect of CLCR on CL/F connected to an effect compartment with two different input and output constants. The influence of renal function on FTC blood plasma pharmacokinetics has been described previously and supports the inclusion of the effect of CLCR on CL/F in our model (21). The final model was evaluated by visual predictive check, and parameters such as blood plasma AUC0–24 or CL/F were close to previously published values for adults (22–24). A previous study has reported the pharmacokinetic modeling of several ARV drugs in the female genital tract (25). However, to our knowledge, this is the first population pharmacokinetic modeling approach that describes the distribution of an ARV drug in the male genital tract.
FTC seminal plasma concentrations were higher than FTC blood plasma concentrations, with median FTC seminal plasma concentrations at 24 h (C24) of 0.850 mg · liter−1, compared to a median C24 of 0.136 mg · liter−1 in blood plasma. Only one study has reported a median FTC seminal plasma C24 of 0.253 mg · liter−1 (19). However, that study focused on the pharmacokinetics of a single dose of FTC. Thus, the discrepancy between our median SP C24 value (0.850 mg · liter−1) and the C24 value of Patterson et al. (0.253 mg · liter−1) may come from an accumulation of FTC in the male genital tract following repeated administrations (19). Thanks to the population model developed, we were able to estimate individual FTC seminal plasma and blood plasma AUC0–24. The median AUC0–24 was 38.04 mg · liter−1 · h in seminal plasma, whereas the FTC median blood plasma AUC0–24 was 12.95 mg · liter−1 · h. The median SP-to-BP exposure ratio was estimated to be 2.91 (25th to 75th percentile, 2.20 to 3.78). One previous study has reported a slightly higher SP-to-BP exposure ratio of 4.5 (25th to 75th percentile, 3.3 to 6.1). However, that study included a small number of men (n = 8) and reported SP-to-BP ratios of AUC from 24 h to 14 days (19). Thus, that study excluded the 0- to 24-h period from the analysis and only focused on the final phase of decay for a single dose of FTC.
In our study, less than 1% of SP-to-BP AUC0–24 ratios were less than 1. Thus, FTC seminal plasma AUC0–24 were higher than blood plasma AUC0–24 in a large majority of patients, even in a very substantial population (n = 117 men).
FTC distribution in the male genital tract was variable, with a 53.3% interindividual variability estimated on the BP-to-SP transfer rate constant (k1e). However, no effects of any covariate (including other antiretroviral drugs) were found to explain this interindividual variability on k1e. The blood-testis barrier is mainly composed of Sertoli cells and prevents the entry of xenobiotic compounds into seminiferous tubules. Robillard et al. have studied the expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells. The expression of P-glycoprotein (Pgp) and multidrug resistance-associated proteins MRP1 and MRP4 was confirmed in human Sertoli cells (26). These transporters could be responsible for limitation or variation in the penetration of antiretroviral drugs in the male genital tract. FTC is eliminated mainly by glomerular filtration combined with active tubular secretion involving multidrug resistance-associated protein MRP1 (27) and organic cation transporters (OCT) (28). Thus, as FTC has been described to be a substrate of MRP1 and as MRP1 has been shown to be expressed in the blood-testis barrier, variability in activity/expression or genetic polymorphisms of this efflux transporter could lead to variability in FTC penetration in the genital tract.
Despite undetectable bpVL (<10 copies · ml−1), spVL (>100 copies · ml−1) was detectable in 18 samples among 207 (8.7%). This discordance between BP HIV-RNA level and spVL was close to the percentage reported in the literature, with the mean proportion of patients with this discordance being estimated at 10% among studies (12, 13). No influence of FTC seminal plasma AUC0–24 or SP-to-BP AUC0–24 ratio was found on the detection of spVL using mixed-effect logistic regression (P = 0.943 and 0.893, respectively). Sexually transmitted diseases and genital inflammation have been associated with seminal plasma HIV shedding in ART-naive men and also in men on cART (10). However, despite adjusting for these factors, the influence of FTC seminal plasma AUC0–24 or SP-to-BP AUC0–24 ratio remained nonsignificant. This could be explained by efficient FTC distribution as determined by seminal plasma AUC0–24 in a large majority of patients. In fact, blood plasma HIV load was undetectable for all men involved in the study, and 91% of FTC blood plasma AUC0–24 values were higher than 10 mg · h−1 · liter, the typical exposure associated with a maximal anti-HIV activity (23). Although the FTC distribution in the male genital tract has been described to be variable (CV of the SP-to-BP AUC0–24 ratio of 54.7%), FTC seminal plasma AUC0–24 values were higher than the efficient FTC blood plasma AUC0–24 values in 100% of men. The nonsignificance of the influence of FTC seminal plasma AUC0–24 or SP-to-BP AUC0–24 ratio also could be explained by a lack of data on the phosphorylation of FTC into its active moiety or the predominant effect of the seminal plasma exposures of the other concomitant ARV drugs. Seminal plasma viral load plays a major role in the risk of HIV-1 sexual transmission. To investigate potential associations between antiretroviral drug levels and viral load in seminal plasma remains an important issue to be addressed.
In conclusion, our study presents the first population model describing FTC pharmacokinetics in blood plasma and seminal plasma in a large population of HIV-1-infected MSM. FTC seminal plasma concentrations were higher than FTC blood plasma concentrations. FTC accumulates in the seminal plasma, and the median SP-to-BP AUC0–24 ratio was estimated at 2.91. In our study, a majority of men had efficient FTC seminal plasma exposures.
ACKNOWLEDGMENTS
We acknowledge the French National Agency for Research on AIDS and Viral Hepatitis (ANRS) for sponsoring the EVARIST ANRS-EP 49 study. We also acknowledge SIDACTION for the financial support of this work.
We greatly thank all of the patients and staff of the HIV clinical centers who participated in the study.
REFERENCES
- 1.UNAIDS. 2014. Global AIDS epidemic facts and figures. UNAIDS, Geneva, Switzerland: http://www.unaids.org/en/resources/documents/2014/20140716_FactSheet_en.pdf. [Google Scholar]
- 2.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 342:921–929. [DOI] [PubMed] [Google Scholar]
- 3.Attia S, Egger M, Müller M, Zwahlen M, Low N. 2009. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 4.Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, Boggian K, Cohen MS, Fiscus SA, Eron JJ. 2000. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS 14:117–121. [DOI] [PubMed] [Google Scholar]
- 5.Chan DJ, McNally L, Batterham M, Smith DE. 2008. Relationship between HIV-RNA load in blood and semen in antiretroviral-naïve and experienced men and effect of asymptomatic sexually transmissible infections. Curr HIV Res 6:138–142. doi: 10.2174/157016208783885074. [DOI] [PubMed] [Google Scholar]
- 6.Chan DJ, Ray JE, McNally L, Batterham M, Smith DE. 2008. Correlation between HIV-1 RNA load in blood and seminal plasma depending on antiretroviral treatment status, regimen and penetration of semen by antiretroviral drugs. Curr HIV Res 6:477–484. doi: 10.2174/157016208785861177. [DOI] [PubMed] [Google Scholar]
- 7.Vernazza P, Hirschel B, Bernasconi E, Flepp M. 2008. HIV-positive individuals without additional sexually transmitted diseases (STD) and on effective anti-retroviral therapy are sexually non-infectious. Bull Médecins Suisses 89:165–169. [Google Scholar]
- 8.Halfon P, Giorgetti C, Khiri H, Pénaranda G, Terriou P, Porcu-Buisson G, Chabert-Orsini V. 2010. Semen may harbor HIV despite effective HAART: another piece in the puzzle. PLoS One 5:e10569. doi: 10.1371/journal.pone.0010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert-Niclot S, Tubiana R, Beaudoux C, Lefebvre G, Caby F, Bonmarchand M, Naouri M, Schubert B, Dommergues M, Calvez V, Flandre P, Poirot C, Marcelin A-G. 2012. Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002-2011 survey. AIDS 26:971–975. doi: 10.1097/QAD.0b013e328352ae09. [DOI] [PubMed] [Google Scholar]
- 10.Politch JA, Mayer KH, Welles SL, O'Brien WX, Xu C, Bowman FP, Anderson DJ. 2012. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS 26:1535–1543. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth PM, Kovacs C, Kemal KS, Jones RB, Raboud JM, Pilon R, la Porte C, Ostrowski M, Loutfy M, Burger H, Weiser B, Kaul R. 2009. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS 23:2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- 12.Houzet L, Matusali G, Dejucq-Rainsford N. 2014. Origins of HIV-infected leukocytes and virions in semen. J Infect Dis 210(Suppl 3):S622–S630. doi: 10.1093/infdis/jiu328. [DOI] [PubMed] [Google Scholar]
- 13.Taylor S, Davies S. 2010. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS 5:335–343. doi: 10.1097/COH.0b013e32833a0b69. [DOI] [PubMed] [Google Scholar]
- 14.Ghosn J, Leruez-Ville M, Blanche J, Delobelle A, Beaudoux C, Mascard L, Lecuyer H, Canestri A, Landman R, Zucman D, Ponscarme D, Rami A, Viard J-P, Spire B, Rouzioux C, Costagliola D, Suzan-Monti M, Evarist–ANRS EP 49 Study Group. 2014. HIV-1 DNA levels in peripheral blood mononuclear cells and cannabis use are associated with intermittent HIV shedding in semen of men who have sex with men on successful antiretroviral regimens. Clin Infect Dis 58:1763–1770. doi: 10.1093/cid/ciu187. [DOI] [PubMed] [Google Scholar]
- 15.Svicher V, Ceccherini-Silberstein F, Antinori A, Aquaro S, Perno CF. 2014. Understanding HIV compartments and reservoirs. Curr HIV/AIDS Rep 11:186–194. doi: 10.1007/s11904-014-0207-y. [DOI] [PubMed] [Google Scholar]
- 16.Else LJ, Taylor S, Back DJ, Khoo SH. 2011. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 16:1149–1167. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 17.Dumond JB, Reddy YS, Troiani L, Rodriguez JF, Bridges AS, Fiscus SA, Yuen GJ, Cohen MS, Kashuba ADM. 2008. Differential extracellular and intracellular concentrations of zidovudine and lamivudine in semen and plasma of HIV-1-infected men. J Acquir Immun Defic Syndr 48:156–162. doi: 10.1097/QAI.0b013e31816de21e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. 2013. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 19.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, Cohen MS, Kashuba ADM. 2011. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med 3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn E, Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1038. doi: 10.1016/j.csda.2004.07.002. [DOI] [Google Scholar]
- 21.Valade E, Tréluyer J-M, Bouazza N, Ghosn J, Foissac F, Benaboud S, Fauchet F, Viard J-P, Urien S, Hirt D. 2014. Population pharmacokinetics of emtricitabine in HIV-1-infected adult patients. Antimicrob Agents Chemother 58:2256–2261. doi: 10.1128/AAC.02058-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blum MR, Chittick GE, Begley JA, Zong J. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol 47:751–759. doi: 10.1177/0091270007300951. [DOI] [PubMed] [Google Scholar]
- 23.Wang LH, Begley J, St Claire RL III, Harris J, Wakeford C, Rousseau FS. 2004. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retrovir 20:1173–1182. doi: 10.1089/aid.2004.20.1173. [DOI] [PubMed] [Google Scholar]
- 24.Zong J, Chittick GE, Wang LH, Hui J, Begley JA, Blum MR. 2007. Pharmacokinetic evaluation of emtricitabine in combination with other nucleoside antivirals in healthy volunteers. J Clin Pharmacol 47:877–889. doi: 10.1177/0091270007300808. [DOI] [PubMed] [Google Scholar]
- 25.Dumond JB, Nicol MR, Kendrick RN, Garonzik SM, Patterson KB, Cohen MS, Forrest A, Kashuba ADM. 2012. Pharmacokinetic modelling of efavirenz, atazanavir, lamivudine and tenofovir in the female genital tract of HIV-infected pre-menopausal women. Clin Pharmacokinet 51:809–822. doi: 10.1007/s40262-012-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robillard KR, Hoque T, Bendayan R. 2012. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther 340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- 27.Bousquet L, Pruvost A, Didier N, Farinotti R, Mabondzo A. 2008. Emtricitabine: inhibitor and substrate of multidrug resistance associated protein. Eur J Pharm Sci 35:247–256. doi: 10.1016/j.ejps.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani-Freshwater T, Taft DR. 2008. Renal excretion of emtricitabine I: effects of organic anion, organic cation, and nucleoside transport inhibitors on emtricitabine excretion. J Pharm Sci 97:5401–5410. doi: 10.1002/jps.21370. [DOI] [PubMed] [Google Scholar]


