Abstract
This report is the first published case of cerebral melioidosis in the western hemisphere. In this paper the authors review the literature on neurological melioidosis and its presentation and treatment in endemic areas, describe the clinical course of this unique case of a presentation of the disease with cranial abscess in the US, review the pathological and radiological findings associated with this seminal case, and put forth recommendations for recognizing and treating possible future instances of the disease within the western hemisphere.
Keywords: melioidosis, cerebral melioidosis, Burkholderia pseudomallei, neurological melioidosis, neurosurgery, infection
Melioidosis is the clinical syndrome that results from infection with the gram-negative bacteria Burkholderia pseudomallei—an endemic water and soil pathogen in eastern Asia and northern Australia. In these areas, the pathogen usually causes pneumonia; primary CNS involvement is exceedingly rare. Given the rarity of the disease, fewer than 50 case reports of cerebral melioidosis have been published in the worldwide literature over the last 50 years. Fewer than 5 of those cases have been due to disease presentations secondary to cranial abscesses.10 To our knowledge, we report the first case of cerebral melioidosis in the western hemisphere. We detail this unique presentation of the disease and review the literature on cerebral melioidosis, concluding with recommendations for its neurosurgical and medical management within the developed world. Although it is extremely rare, the recognition and treatment of this entity is pertinent in an era of seamless global travel.
Case Report
History
The patient is a 58-year-old man who grew up in Cambodia and had immigrated to the US in 1987; he has since traveled back to Cambodia on several occasions. The patient is HIV negative and was generally in good health before hospitalization, although he had lost approximately 15 pounds unintentionally over the course of the year prior to presentation. He was in his normal state of health when he left for a recreational trip to Battambang and Phnom Penh, Cambodia. He reported no contact with sick people, although he did admit to an episode of extramarital intercourse with a second Cambodian wife with whom he had an ongoing relationship. He developed a sudden onset of left-sided weakness, left-sided lip tingling, and severe headache, which was followed by a profound somnolence that prompted admission to a local hospital. Initial CT scans demonstrated mild ischemic changes in the right lentiform nucleus, and the patient was transferred to a major medical center in Thailand for further treatment.
Initial Examination
There the patient was noted to be febrile to 101.6°F, with concomitant left-sided hemiplegia, rightward tongue deviation, bilateral ptosis, mild neck stiffness, and bibasilar pulmonary rales. Urine and sputum cultures grew Escherichia coli, chest radiographs demonstrated right perihilar ground-glass opacities, and results of LP were consistent with bacterial meningitis, with a WBC count of 350 cells/μl in the CSF. A repeat CT scan of the head demonstrated obstructive hydrocephalus and 2 areas with ill-defined hypodense lesions in the left cerebellar hemisphere and right basal ganglia. An MRI study of the brain was performed that demonstrated extensive multifocal infiltrative lesions with patchy irregular rim enhancement along the upper cervical cord, right midbrain, bilateral basal ganglia, and corona radiata.
Initial Diagnosis and Treatment
The patient received a diagnosis of nonspecific brain abscess, urinary tract infection, and pneumonia, and he was placed on 2 g meropenem delivered intravenously every 8 hours, after which he defervesced. An LP was performed that demonstrated an elevated WBC count of 350 cells/μl (88% lymphocytes and 12% neutrophils), a glucose level of 69 mg/dl, and elevated protein. Blood and CSF cultures showed no organisms on Gram staining and demonstrated no growth in aerobic, anaerobic, and fungal media; Ziehl-Neelsen stain for AFB and India ink stain for Cryptococcus species were negative; and PCR for Mycobacterium tuberculosis was negative. Sputum and urine grew an extended-spectrum β-lactamase–producing E. coli.
Biopsy Procedure
The patient underwent a stereotactic brain biopsy 8 days after initial presentation; the material obtained demonstrated no growth on culture medium, no organisms on Gram staining, and was also negative for M. tuberculosis on PCR and AFB testing. After it was decided that the original biopsy procedure might not have sampled affected brain, the patient was taken back 2 days after the original biopsy for a suboccipital craniotomy for the purpose of obtaining another biopsy sample. During this operation, sampling was reported from an ill-defined, firm, granulomatous lesion with caseous material at the left cerebellar peduncle; however, Gram staining of this biopsy sample revealed no organisms, and was negative for microbial growth on culture medium as well as negative on AFB and fungal stains, and for M. tuberculosis on PCR. Serum tests for melioidosis antibodies were negative for IgM and positive for IgG antibodies (at a ratio of 1:200).
Initial Posttreatment Course
After 1 month of hospitalization without a specific diagnosis and after an empirical course of parenteral meropenem of less than 8 weeks, the patient’s mental status had improved and he was able to converse intelligently. He was afebrile with a normal serum WBC count, and his left hemiparesis had improved to antigravity strength in the left lower extremity and to 1/5 strength in the left upper extremity. He was discharged from the hospital without antibiotics and returned to the US.
Second Admission, Workup Results, and Treatment Decision
Within days of his return to the US, the patient presented to another institution reporting worsening headaches and worsening left-sided weakness, and was transferred to the Brigham and Women’s Hospital for further workup. He was admitted to the hospital and a broad infectious workup failed to reach a conclusive diagnosis, with the following findings reported: an LP with mild lymphocytic pleocytosis; an MRI study demonstrating diffuse mild decrease in size of previous lesions; unremarkable results on echocardiogram; negative serological findings for HIV-1 and syphilis; Toxoplasma IgM–negative; indeterminate values for galactomannan and β-1,3-glucan; CSF flow cytometry consistent with generalized inflammation; and review of pathology slides from the Thai hospital indicative of global inflammation but no granulomata, with all stains for infectious organisms negative. In addition, results of stool ova and parasite testing were negative; CSF studies demonstrated a glucose level of 62 mg/dl, a protein level of 111 mg/dl, and 23 WBCs/μl with 95% lymphocytes; angiotensin-converting enzyme was low, and CSF bacterial/fungal/AFB culture and ova and parasite tests were negative. After the patient demonstrated stability of symptoms with no fever during his stay and MRI lesions that were mildly decreasing in size, the decision was made not to place him on antibiotics, and he was discharged to a rehabilitation facility after a short hospital admission.
Third Admission and Examination
Three weeks after discharge the patient again presented to the hospital, although this time with a 3-day history of low-grade fever in the setting of nausea, vomiting, increased headaches, diplopia, new left-sided ptosis, and recent transient episodes of marked somnolence. During this admission, results of an LP were equivocal, and the patient became increasingly somnolent; repeat MRI demonstrated an increase in the size of his cerebellar lesion, including a new area of decreased diffusivity, with surrounding enhancement in the lateral cerebellum—probably representing a new infectious nidus and causing an obstructive hydrocephalus (Fig. 1).
Fig. 1.
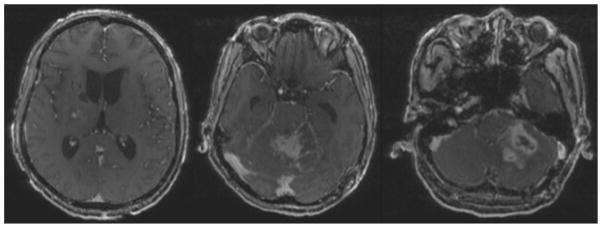
Axial T1-weighted postcontrast MRI studies illustrating obstructive hydrocephalus, previous right basal ganglia biopsy site from the procedure performed at the Thai hospital, and diffuse left cerebellar ring-enhancing cavitary lesion extending into the left cerebellar peduncle with fourth ventricular and aqueductal compression.
Operation
To establish a definitive diagnosis and relieve mass effect, the patient was taken to the operating room for a left suboccipital craniectomy through the site of the previous suboccipital biopsy, where a caseous cerebellar lesion was removed. During the operation, a right frontal ventriculostomy was also placed to relieve his hydrocephalus. Several days later, a third ventriculostomy was performed (instead of mechanical shunt placement given the patient’s infection) for prophylactic diversion of flow and prevention of further hydrocephalus if the lesion were to continue to grow.
Pathological samples obtained from the biopsy procedure were examined histologically, demonstrating a few scattered gram-negative organisms with classic bipolar appearance. Microbiological investigation of the CSF again showed no organisms on Gram staining and failed to grow any organisms in any culture medium. However, when plated in culture media, the tissue biopsy grew out B. pseudomallei that was sensitive to meropenem, confirming the patient’s diagnosis of cerebral melioidosis.
Treatment and Follow-Up
After review of the limited literature and collaboration with experts in the Infectious Disease Division at the Brigham and Women’s Hospital and in Australia’s Royal Darwin Hospital (B. Currie and A. Cheng, personal communications, 2010–2011), it was decided that the patient should be placed on high-dose meropenem (2 g delivered intravenously every 8 hours) and oral SMX-TMP (40 mg SMX and 8 mg TMP/kg twice daily at induction doses) for at least 6–8 weeks, with an ultimate duration of antibiotic course to depend on the patient’s clinical and radiographic progression. It was determined that, after the induction phase, the patient should then undergo an eradication phase including continued SMX-TMP for at least 6 months after conclusion of the intravenous meropenem.
Posttreatment Course
To date, the patient has consistently attended follow-up and has recovered well, with progressive radiographic resolution of melioid abscesses, and with improvement in strength in his left upper and lower extremities, despite persistent diplopia. He has completed an 8-week course of intravenous meropenem and is currently continuing to take SMX-TMP.
Discussion
Features of Melioidosis
Epidemiological Data
In the largest and most recent study, a 20-year prospective examination of melioidosis in northern Australia, only 14 of 540 cases of melioidosis presented with neurological sequelae, with pneumonia being the most common secondary focus. In that study, neurological melioidosis carried a 21% mortality, as opposed to the more general 14% mortality associated with other presentations of the disease.10 Worldwide, there have been fewer than 50 reported cases of CNS melioidosis in the last 50 years; none of which have presented in the western hemisphere.19 On review of the literature, cerebral melioidosis does not affect any specific age or sex with preference, with case reports in endemic areas recounting patients affected by the disease who range in age from 12 to 69 years, with males and females equally affected, although these statistics frequently do not adjust for age or sex as treatment or reporting biases. Peak incidence of the disease in endemic regions is in the 4th and 5th decades of life.33
Risk Factors
The risk factors for general melioidosis are diabetes, alcohol abuse, and chronic lung and renal failure.10,24 Melioidosis as well as neurological melioidosis can recur years after initial infection. Melioidosis has been called the “Vietnamese time bomb” in military reporting because of acquisition by US soldiers during the Vietnam War and recurrence after long periods of latency in that population.6 Although cerebral melioidosis is quite rare, the first examples of Australian melioidosis in both animals (1949) and humans (1951) demonstrated neurological sequelae—and it has been suggested that cerebral melioidosis (vs general melioidosis) is more common in Australia than eastern Asia, the 2 areas of the globe where the disease is endemic.7,23
Presenting Symptoms
In endemic areas, most cases of cerebral melioidosis present during the rainy season with prominent headache and fever—both symptoms that are typical of general melioidosis as well. Other predictive neurological features of the presentation of neurological melioidosis include unilateral limb weakness, predominant cerebellar signs, mixed cerebellar and brainstem features with peripheral weakness, and flaccid paraparesis.9 Additionally, patients presenting with neurological melioidosis also demonstrate fluctuating levels of consciousness as well as cranial nerve palsies (in particular, unilateral seventh nerve palsies).38 A hallmark of general melioidosis is abscess formation, most typically in the lungs, and it therefore follows that cases of neurological melioidosis are also associated with intracranial or intraspinal abscesses. 21,30,31,33,37 Both general and neurological melioidosis are most frequently acquired through inoculation or inhalation but not by ingestion. Vertical (mother to child) and sexual transmission have been described but are much less common.1,12,13,17,18 After exposure, antibodies to the disease are developed but have not been shown to be protective in prevention after additional disease exposure.33
Characteristics of CSF
In the workup of many cases of neurological melioidosis, LPs are frequently performed. On review of the published literature, we found that CSF from patients with neurological melioidosis often displays a leukocytosis with a mononuclear cell predominance, high protein levels, and normal glucose.9,20 The CSF frequently demonstrates no organisms on Gram staining as well as in both aerobic and anaerobic media.
Microbiological and Pathological Characteristics
Microbiologically, B. pseudomallei has an extensive history. First described in humans in 1912, it was characterized as a “glanders”-like illness (a disease affecting predominantly horses).35,36 By 1932, Stanton and Fletcher had documented sufficient cases in animals (mostly horses and donkeys) and humans to coin the disease name melioidosis, from the Greek for “a distemper of Asses.”27 The pathogen causing the disease was officially incorporated into its own genus (B. pseudomallei) in 1992. Found in more than 50% of all rice paddies in Thailand, the organism is a motile, aerobic, non–spore-forming bacterium that is inherently resistant to penicillins, first- and second-generation cephalosporins, and most aminoglycosides.33,39 It grows aerobically on most simple agar–containing media and produces colonies with a stereotypical cornflower or rugose appearance.2,32 Although Gram stains of the organism show gram-negative rods with bipolar staining resembling safety pins, the morphology in clinical specimens is extremely variable.11 Because of the extremely high risk of acquisition of infection when handling human samples with B. pseudomallei, suspected cases should be reported to the microbiology laboratory so that appropriate precautions can be maintained, including prophylactic treatment of any exposed laboratory staff.
Radiological Characteristics
Brain CT scans often show normal results on initial presentation, whereas MRI studies often demonstrate dramatic changes.9 In endemic regions, MRI abnormalities described in several case reports demonstrate frequently increased diffuse parenchymal signal intensity on T2-weighted imaging, although when discreet findings were evident they tended to correlate with clinical symptoms. Gadolinium enhancement of affected cranial regions is variable, and reports have described enhancement predominantly in the internal capsule, brainstem, and cerebellum as well as the frontal and occipital lobes, with reported extension across the midline. 9,15 If cases are identified later in the course of the disease, they are frequently mistaken for malignant glioma, although in the majority of late-presenting cases, the cerebral lesions often resemble typical intracranial abscesses with characteristic ring-enhancing lesions and frequent satellite microabscesses.
Treatment
A definitive treatment regimen for isolated cerebral melioidosis has yet to be established given the rarity of the disease. In addition, even nonneurological melioidosis is quite difficult to treat, requiring a minimum of 2 weeks of intravenous antibiotics followed by a minimum of 3 months of oral antibiotics to prevent relapse.33 Over the last 20 years there have been several randomized trials of parenteral and oral antibiotics in the treatment of general melioidosis. From these trials it has emerged that melioid septicemia is best treated with long-term intravenous ceftazidime or carbapenem therapy.3,5,22,25,26,29,34 Intravenous therapy should be continued for at least 10 days for systemic treatments, and the switch to oral antibiotics should not be made until there is a clear clinical improvement. The median time to resolution of fever is 9 days in melioid septicemia; however, fevers have been reported to fluctuate for periods of more than 1 month when the septicemia is complicated by abscess.
If abscesses (normally located in the lung or abdomen in general melioidosis) are large and accessible, it is recommended that these should be drained, although enlargement of the original abscess or the appearance of new ones is not uncommon in melioid septicemia, nor is this necessarily associated with treatment failure.33 Despite long-term antibiotic therapy, the relapse rate of melioid sepsis is almost 30% if antibiotic treatment lasts for less than 8 weeks.3,4,8,23,28 A majority of these relapses occur with the same strain of B. pseudomallei and, in approximately 75% of cases, those strains do not demonstrate resistance to the drug used for initial therapy.14 Given this patient’s short course in the Thai hospital and less than 8 weeks of initial antibiotic treatment, it is likely that he was inadequately treated with meropenem on his initial presentation and that he therefore was experiencing a relapse of the disease when he presented again in the US. It is thought that treatments similar to the abovementioned are effective in cases of cerebral melioidosis, but no definitive treatment for isolated melioid infection of the nervous system has been established.16
Conclusions
Cerebral melioidosis is an extremely rare disease, and this is the first report of it presenting in the western hemisphere. Given the increasing globalization of the modern economy, combined with the ease and availability of international travel, there exists the potential for a marked increase in the incidence of previously unseen diseases with possible neurosurgical sequelae. Therefore, it is becoming increasingly important to remain vigilant in the care of individuals who present with a persistently confounding diagnostic picture, because the offering of targeted and timely neurosurgical intervention can often not only assist with the formulation of diagnoses but in many cases confirm those diagnoses.
As diseases previously limited to the tropics become more and more common in the developed world, it is important to keep an open mind to rare diagnoses and encourage collaboration across departments and fields of expertise because, in many cases, this is exactly what is needed to properly diagnose and treat disease in the modern world.
Acknowledgments
The authors acknowledge Dr. Bart Currie of Australia’s Royal Darwin Hospital and Dr. Allen Cheng of Australia’s Alfred Hospital for their correspondence and treatment recommendations during this patient’s care. The authors also acknowledge Rochelle Walensky, M.D., M.P.H., and Paul Sax, M.D., of the Brigham and Women’s Hospital Department of Infectious Disease for their participation in the care of this patient.
Abbreviations used in this paper
- AFB
acid-fast bacilli
- LP
lumbar puncture
- PCR
polymerase chain reaction
- SMX-TMP
sulfamethoxazole-trimethoprim
- WBC
white blood cell
Footnotes
Author contributions to the study and manuscript preparation include the following. Conception and design: Vestal, Gormley, Dunn. Acquisition of data: Vestal, Dunn. Analysis and interpretation of data: Vestal, Dunn. Drafting the article: Vestal. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Vestal. Administrative/technical/material support: Milner, Dunn. Study supervision: Gormley, Dunn.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Abbink FC, Orendi JM, de Beaufort AJ. Mother-to-child transmission of Burkholderia pseudomallei. N Engl J Med. 2001;344:1171–1172. doi: 10.1056/NEJM200104123441516. [DOI] [PubMed] [Google Scholar]
- 2.Ashdown LR. An improved screening technique for isolation of Pseudomonas pseudomallei from clinical specimens. Pathology. 1979;11:293–297. doi: 10.3109/00313027909061954. [DOI] [PubMed] [Google Scholar]
- 3.Chaowagul W, Simpson AJ, Suputtamongkol Y, Smith MD, Angus BJ, White NJ. A comparison of chloramphenicol, trimethoprim-sulfamethoxazole, and doxycycline with doxycycline alone as maintenance therapy for melioidosis. Clin Infect Dis. 1999;29:375–380. doi: 10.1086/520218. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul W, Suputtamongkol Y, Dance DA, Rajchanuvong A, Pattara-arechachai J, White NJ. Relapse in melioidosis: incidence and risk factors. J Infect Dis. 1993;168:1181–1185. [PubMed] [Google Scholar]
- 5.Chetchotisakd P, Porramatikul S, Mootsikapun P, Anunnatsiri S, Thinkhamrop B. Randomized, double-blind, controlled study of cefoperazone-sulbactam plus cotrimoxazole versus ceftazidime plus cotrimoxazole for the treatment of severe melioidosis. Clin Infect Dis. 2001;33:29–34. doi: 10.1086/320878. [DOI] [PubMed] [Google Scholar]
- 6.Clayton AJ, Lisella RS, Martin DG. Melioidosis: a serological survey in military personnel. Mil Med. 1973;138:24–26. [PubMed] [Google Scholar]
- 7.Cottew GS. Melioidosis in sheep in Queensland; a description of the causal organism. Aust J Exp Biol Med Sci. 1950;28:677–683. [PubMed] [Google Scholar]
- 8.Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94:301–304. doi: 10.1016/s0035-9203(00)90333-x. [DOI] [PubMed] [Google Scholar]
- 9.Currie BJ, Fisher DA, Howard DM, Burrow JN. Neurological melioidosis. Acta Trop. 2000;74:145–151. doi: 10.1016/s0001-706x(99)00064-9. [DOI] [PubMed] [Google Scholar]
- 10.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dance DA, Wuthiekanun V, Naigowit P, White NJ. Identification of Pseudomonas pseudomallei in clinical practice: use of simple screening tests and API 20NE. J Clin Pathol. 1989;42:645–648. doi: 10.1136/jcp.42.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green RN, Tuffnell PG. Laboratory acquired melioidosis. Am J Med. 1968;44:599–605. doi: 10.1016/0002-9343(68)90060-0. [DOI] [PubMed] [Google Scholar]
- 13.Halder D, Abdullah WA, Johari MR, Choo KE. Neonatal melioidosis. Singapore Med J. 1993;34:85–86. [PubMed] [Google Scholar]
- 14.Jenney AW, Lum G, Fisher DA, Currie BJ. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int J Antimicrob Agents. 2001;17:109–113. doi: 10.1016/s0924-8579(00)00334-4. [DOI] [PubMed] [Google Scholar]
- 15.Kasantikul V, Lerdlum S, Suwanwela N. Cerebral abscesses due to Pseudomonas pseudomallei. J Med Assoc Thai. 1992;75:536–541. [PubMed] [Google Scholar]
- 16.Kumar GS, Raj PM, Chacko G, Lalitha MK, Chacko AG, Rajshekhar V. Cranial melioidosis presenting as a mass lesion or osteomyelitis. J Neurosurg. 2008;108:243–247. doi: 10.3171/JNS/2008/108/2/0243. [DOI] [PubMed] [Google Scholar]
- 17.Lumbiganon P, Pengsaa K, Puapermpoonsiri S, Puapairoj A. Neonatal melioidosis: a report of 5 cases. Pediatr Infect Dis J. 1988;7:634–636. doi: 10.1097/00006454-198809000-00007. [DOI] [PubMed] [Google Scholar]
- 18.McCormick JB, Sexton DJ, McMurray JG, Carey E, Hayes P, Feldman RA. Human-to-human transmission of Pseudomonas pseudomallei. Ann Intern Med. 1975;83:512–513. doi: 10.7326/0003-4819-83-4-512. [DOI] [PubMed] [Google Scholar]
- 19.Muthusamy KA, Waran V, Puthucheary SD. Spectra of central nervous system melioidosis. J Clin Neurosci. 2007;14:1213–1215. doi: 10.1016/j.jocn.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Padiglione A, Ferris N, Fuller A, Spelman D. Brain abscesses caused by Burkholderia pseudomallei. J Infect. 1998;36:335–337. doi: 10.1016/s0163-4453(98)94639-4. [DOI] [PubMed] [Google Scholar]
- 21.Puthucheary SD, Vadivelu J. Human Melioidosis. Singapore: Singapore University Press; 2002. [Google Scholar]
- 22.Rajchanuvong A, Chaowagul W, Suputtamongkol Y, Smith MD, Dance DA, White NJ. A prospective comparison of coamoxiclav and the combination of chloramphenicol, doxycycline, and co-trimoxazole for the oral maintenance treatment of melioidosis. Trans R Soc Trop Med Hyg. 1995;89:546–549. doi: 10.1016/0035-9203(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 23.Rimington RA. Melioidosis in north Queensland. Med J Aust. 1962;49:50–53. doi: 10.5694/j.1326-5377.1962.tb76106.x. [DOI] [PubMed] [Google Scholar]
- 24.Scott IA, Bell AM, Staines DR. Fatal human melioidosis in south-eastern Queensland. Med J Aust. 1997;166:197–199. doi: 10.5694/j.1326-5377.1997.tb140075.x. [DOI] [PubMed] [Google Scholar]
- 25.Simpson AJ, Suputtamongkol Y, Smith MD, Angus BJ, Rajanuwong A, Wuthiekanun V, et al. Comparison of imipenem and ceftazidime as therapy for severe melioidosis. Clin Infect Dis. 1999;29:381–387. doi: 10.1086/520219. [DOI] [PubMed] [Google Scholar]
- 26.Sookpranee M, Boonma P, Susaengrat W, Bhuripanyo K, Punyagupta S. Multicenter prospective randomized trial comparing ceftazidime plus co-trimoxazole with chloramphenicol plus doxycycline and co-trimoxazole for treatment of severe melioidosis. Antimicrob Agents Chemother. 1992;36:158–162. doi: 10.1128/aac.36.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanton AT, Fletcher W. Melioidosis: Studies From the Institute for Medical Research, Federated Malay States. Vol. 21 London: John Bale and Sons and Danielson; 1932. [Google Scholar]
- 28.Suputtamongkol Y, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, White NJ. Amoxycillin-clavulanic acid treatment of melioidosis. Trans R Soc Trop Med Hyg. 1991;85:672–675. doi: 10.1016/0035-9203(91)90391-b. [DOI] [PubMed] [Google Scholar]
- 29.Suputtamongkol Y, Rajchanuwong A, Chaowagul W, Dance DA, Smith MD, Wuthiekanun V, et al. Ceftazidime vs. amoxicillin/clavulanate in the treatment of severe melioidosis. Clin Infect Dis. 1994;19:846–853. doi: 10.1093/clinids/19.5.846. [DOI] [PubMed] [Google Scholar]
- 30.Thin RN, Brown M, Stewart JB, Garrett CJ. Melioidosis: a report of ten cases. Q J Med. 1970;39:115–127. [PubMed] [Google Scholar]
- 31.Vatcharapreechasakul T, Suputtamongkol Y, Dance DA, Chaowagul W, White NJ. Pseudomonas pseudomallei liver abscesses: a clinical, laboratory, and ultrasonographic study. Clin Infect Dis. 1992;14:412–417. doi: 10.1093/clinids/14.2.412. [DOI] [PubMed] [Google Scholar]
- 32.Walsh AL, Wuthiekanun V. The laboratory diagnosis of melioidosis. Br J Biomed Sci. 1996;53:249–253. [PubMed] [Google Scholar]
- 33.White NJ. Melioidosis. Lancet. 2003;361:1715–1722. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- 34.White NJ, Dance DA, Chaowagul W, Wattanagoon Y, Wuthiekanun V, Pitakwatchara N. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;334:697–701. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- 35.Whitmore A. An account of a glanders-like disease occurring in Rangoon. J Hyg. 1913;13:1–34. doi: 10.1017/s0022172400005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitmore A, Krishnaswami CS. An account of the discovery of a hitherto undescribed infective disease occurring among the population of Rangoon. Indian Med Gaz. 1912;47:262–267. [PMC free article] [PubMed] [Google Scholar]
- 37.Wong KT, Puthucheary SD, Vadivelu J. The histopathology of human melioidosis. Histopathology. 1995;26:51–55. doi: 10.1111/j.1365-2559.1995.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 38.Woods ML, II, Currie BJ, Howard DM, Tierney A, Watson A, Anstey NM, et al. Neurological melioidosis: seven cases from the Northern Territory of Australia. Clin Infect Dis. 1992;15:163–169. doi: 10.1093/clinids/15.1.163. [DOI] [PubMed] [Google Scholar]
- 39.Wuthiekanun V, Smith MD, Dance DA, White NJ. Isolation of Pseudomonas pseudomallei from soil in north-eastern Thailand. Trans R Soc Trop Med Hyg. 1995;89:41–43. doi: 10.1016/0035-9203(95)90651-7. [DOI] [PubMed] [Google Scholar]


