Abstract
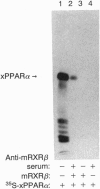
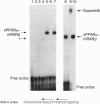
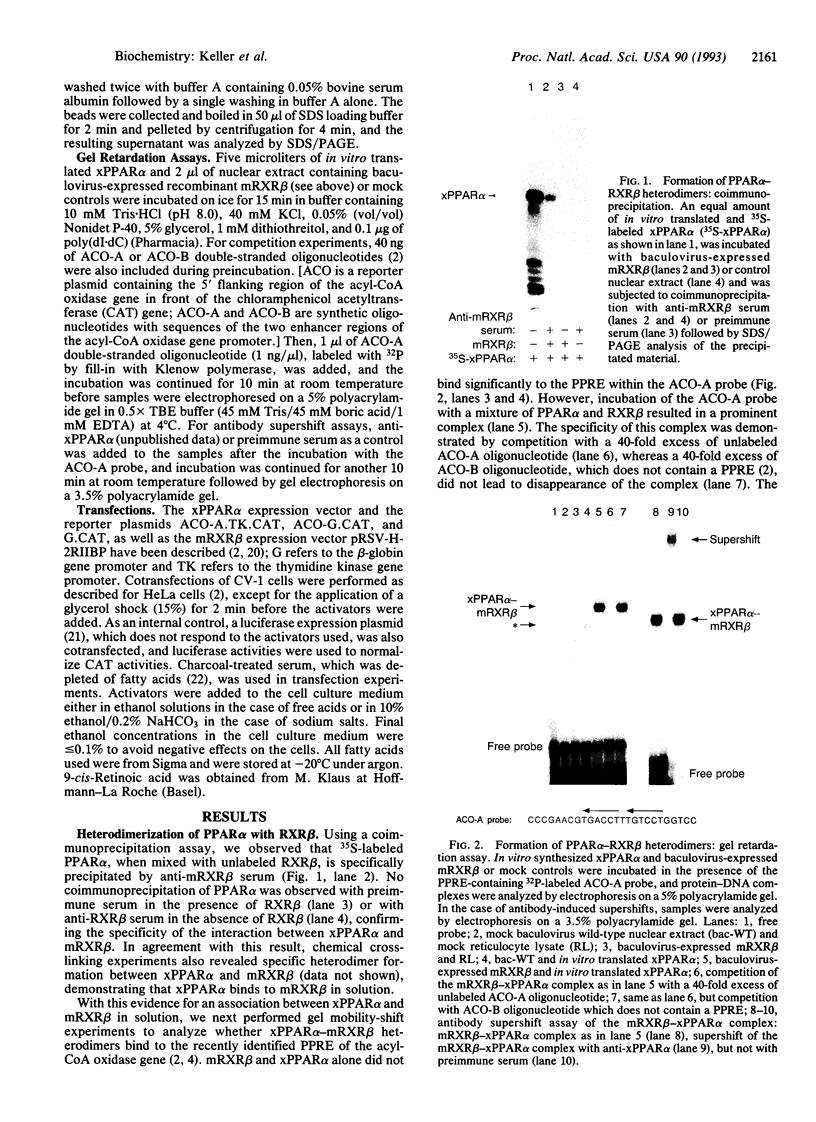
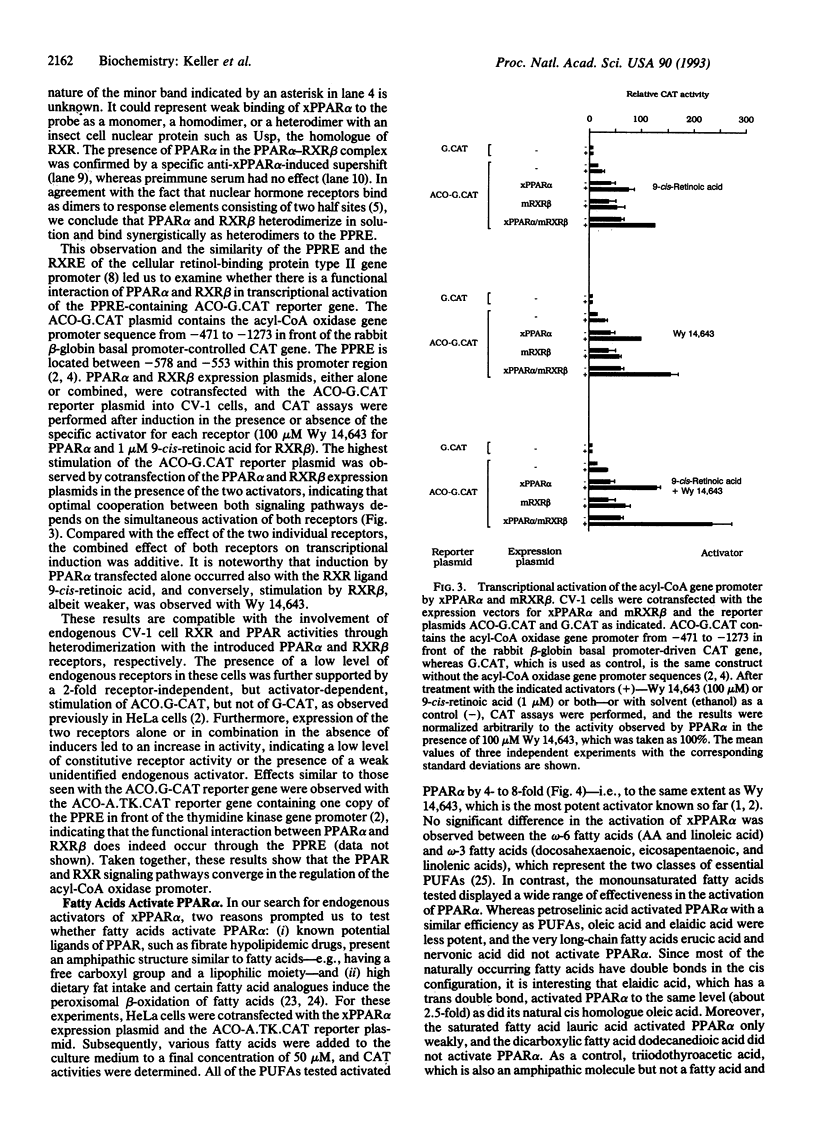
The nuclear hormone receptors called PPARs (peroxisome proliferator-activated receptors alpha, beta, and gamma) regulate the peroxisomal beta-oxidation of fatty acids by induction of the acyl-CoA oxidase gene that encodes the rate-limiting enzyme of the pathway. Gel retardation and cotransfection assays revealed that PPAR alpha heterodimerizes with retinoid X receptor beta (RXR beta; RXR is the receptor for 9-cis-retinoic acid) and that the two receptors cooperate for the activation of the acyl-CoA oxidase gene promoter. The strongest stimulation of this promoter was obtained when both receptors were exposed simultaneously to their cognate activators. Furthermore, we show that natural fatty acids, and especially polyunsaturated fatty acids, activate PPARs as potently as does the hypolipidemic drug Wy 14,643, the most effective activator known so far. Moreover, we discovered that the synthetic arachidonic acid analogue 5,8,11,14-eicosatetraynoic acid is 100 times more effective than Wy 14,643 in the activation of PPAR alpha. In conclusion, our data demonstrate a convergence of the PPAR and RXR signaling pathways in the regulation of the peroxisomal beta-oxidation of fatty acids by fatty acids and retinoids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berge R. K., Aarsland A., Kryvi H., Bremer J., Aarsaether N. Alkylthioacetic acid (3-thia fatty acids)--a new group of non-beta-oxidizable, peroxisome-inducing fatty acid analogues. I. A study on the structural requirements for proliferation of peroxisomes and mitochondria in rat liver. Biochim Biophys Acta. 1989 Aug 22;1004(3):345–356. doi: 10.1016/0005-2760(89)90083-0. [DOI] [PubMed] [Google Scholar]
- Bugge T. H., Pohl J., Lonnoy O., Stunnenberg H. G. RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors. EMBO J. 1992 Apr;11(4):1409–1418. doi: 10.1002/j.1460-2075.1992.tb05186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Dreyer C., Krey G., Keller H., Givel F., Helftenbein G., Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992 Mar 6;68(5):879–887. doi: 10.1016/0092-8674(92)90031-7. [DOI] [PubMed] [Google Scholar]
- Flatmark T., Christiansen E. N., Kryvi H. Evidence for a negative modulating effect of erucic acid on the peroxisomal beta-oxidation enzyme system and biogenesis in rat liver. Biochim Biophys Acta. 1983 Oct 11;753(3):460–466. doi: 10.1016/0005-2760(83)90071-1. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Reddy J. K. Peroxisomes (microbodies) in cell pathology. Int Rev Exp Pathol. 1984;26:45–84. [PubMed] [Google Scholar]
- Göttlicher M., Widmark E., Li Q., Gustafsson J. A. Fatty acids activate a chimera of the clofibric acid-activated receptor and the glucocorticoid receptor. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K. C., Khosla P. Dietary fatty acid thresholds and cholesterolemia. FASEB J. 1992 May;6(8):2600–2607. doi: 10.1096/fasebj.6.8.1592210. [DOI] [PubMed] [Google Scholar]
- Hertz R., Bar-Tana J. Induction of peroxisomal beta-oxidation genes by retinoic acid in cultured rat hepatocytes. Biochem J. 1992 Jan 1;281(Pt 1):41–43. doi: 10.1042/bj2810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990 Oct 18;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Lock E. A., Mitchell A. M., Elcombe C. R. Biochemical mechanisms of induction of hepatic peroxisome proliferation. Annu Rev Pharmacol Toxicol. 1989;29:145–163. doi: 10.1146/annurev.pa.29.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Umesono K., Kliewer S. A., Borgmeyer U., Ong E. S., Evans R. M. A direct repeat in the cellular retinol-binding protein type II gene confers differential regulation by RXR and RAR. Cell. 1991 Aug 9;66(3):555–561. doi: 10.1016/0092-8674(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. S., Levi B. Z., Segars J. H., Driggers P. H., Hirschfeld S., Nagata T., Appella E., Ozato K. H-2RIIBP expressed from a baculovirus vector binds to multiple hormone response elements. Mol Endocrinol. 1992 Feb;6(2):219–230. doi: 10.1210/mend.6.2.1569965. [DOI] [PubMed] [Google Scholar]
- Nagata T., Segars J. H., Levi B. Z., Ozato K. Retinoic acid-dependent transactivation of major histocompatibility complex class I promoters by the nuclear hormone receptor H-2RIIBP in undifferentiated embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):937–941. doi: 10.1073/pnas.89.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J. Effects of N-3 fatty acids on lipid metabolism. Annu Rev Nutr. 1990;10:149–167. doi: 10.1146/annurev.nu.10.070190.001053. [DOI] [PubMed] [Google Scholar]
- Osmundsen H., Bremer J., Pedersen J. I. Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys Acta. 1991 Sep 11;1085(2):141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989 Apr 15;259(2):315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias L. D., Hamilton J. G. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids. 1979 Feb;14(2):181–193. doi: 10.1007/BF02533870. [DOI] [PubMed] [Google Scholar]
- Tugwood J. D., Issemann I., Anderson R. G., Bundell K. R., McPheat W. L., Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5' flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992 Feb;11(2):433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Martinez E. Superfamily of steroid nuclear receptors: positive and negative regulators of gene expression. FASEB J. 1991 Jun;5(9):2243–2249. doi: 10.1096/fasebj.5.9.1860615. [DOI] [PubMed] [Google Scholar]
- Widom R. L., Rhee M., Karathanasis S. K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992 Aug;12(8):3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]