Abstract
Background: Although physical exercise (PE) is recommended for individuals with type 1 diabetes (DM1), participation in exercise is challenging because it increases the risk of severe hypoglycemia and the available therapeutic options to prevent it frequently result in hyperglycemia. There is no clear recommendation about the best timing for exercise. The aim of this study was to compare the risk of hypoglycemia after morning or afternoon exercise sessions up to 36 hours postworkout. Methods: This randomized crossover study enrolled subjects with DM1, older than 18 years of age, on sensor-augmented insulin pump (SAP) therapy. Participants underwent 2 moderate-intensity exercise sessions; 1 in the morning and 1 in the afternoon, separated by a 7 to 14 day wash-out period. Continuous glucose monitoring (CGM) data were collected 24 hours before, during and 36 hours after each session. Results: Thirty-five subjects (mean age 30.31 ± 12.66 years) participated in the study. The rate of hypoglycemia was significantly lower following morning versus afternoon exercise sessions (5.6 vs 10.7 events per patient, incidence rate ratio, 0.52; 95% CI, 0.43-0.63; P < .0001). Most hypoglycemic events occurred 15-24 hours after the session. On days following morning exercise sessions, there were 20% more CGM readings in near-euglycemic range (70-200 mg/dL) than on days prior to morning exercise (P = .003). Conclusions: Morning exercise confers a lower risk of late-onset hypoglycemia than afternoon exercise and improves metabolic control on the subsequent day.
Keywords: diabetes mellitus type 1, exercise, hypoglycemia, insulin infusion system
Physical exercise (PE) is a strong stimulant of glucose absorption by the skeletal muscles, a phenomenon that results from an increase in the rates of glucose release, transmembranal transport of glucose, and substrate flow at the intracellular level through glycolysis.1
Although PE is an important tool for maintaining or improving cardiovascular fitness, most studies on the impact of PE on DM1 have not shown objective improvements on glycemic control.2 It has been described that type 1 diabetic athletes show alterations in their metabolic control compared to sedentary type 1 diabetics.2 The fear of a hypoglycemic event underlies this finding because overcompensation generally occurs in terms of additional carbohydrate intake prior to exercise and excessive reductions to insulin dosages.2 In fact, in a pediatric population, hypoglycemia during or after exercise is the most frequent specific cause of severe hypoglycemia, with most of the severe events occurring at night.3
It has been established that hypoglycemia associated with exercise is determined by an increase in glucose absorption, the inability of PE per se to decrease insulin levels, and the presence of autonomous diabetic neuropathy.4 A history of hypoglycemia can deteriorate even further the adrenergic activity in response to hypoglycemia caused by exercise.4
Most DM1 patients identify exercise as a risk factor for hypoglycemia even several hours afterward.2 Furthermore, PE masks some of the symptoms of hypoglycemia, like sweating, vertigo, and tiredness.
Even though there are documented, descriptive articles that report the presence of hypoglycemia up to 31 hours after a workout session,4 there are no accurate data available in terms of the benefits on metabolic control, either in adult patients or on the differential impact of morning or afternoon exercise on the characteristics of a subsequent hypoglycemic event.
The primary outcome of this randomized crossover study on adult individuals with DM1 using insulin pump therapy and CGM systems was the incidence of hypoglycemia and the glycemic patterns after morning versus afternoon sessions up to 36 hours postworkout. We also evaluated the impact of exercise sessions on glycemic control up to 36 hours postworkout determined by CGM.
Methods
This was a randomized, crossover study, with all subjects serving as their own controls. A total of 35 patients with DM1 were studied. They were recruited from the Diabetes Clinic of the San Ignacio Hospital in Bogota, Colombia. Inclusion criteria were patients older than 18 years old, on SAP therapy (Paradigm REAL-Time System, Medtronic Diabetes, Northridge, CA, USA), who have been on this therapy for at least 3 months, using continuous glucose monitoring (CGM) and the Bolus Wizard more than 80% of the time. The exclusion criteria were pregnancy, physical or mental incapacity to perform PE, history of acute myocardial infarction or positive exercise-ECG test, diabetic ketoacidosis or active infection at the moment of the trial, use of beta blockers or glucocorticoids, peripheral vascular disease, severe proliferative diabetic retinopathy or nonproliferative diabetic retinopathy, severe peripheral neuropathy, and chronic stage 5 kidney failure on dialysis therapy. All patients gave written informed consent.
Participants were randomized to 2 consecutive moderate-intensity exercise sessions, 1 in the morning (at 7 am) and 1 in the afternoon (at 4 pm), separated by a 7 to 14 day wash-out period. A computer-generated random number list was used to allocate the order of exercise sessions. All patients changed their CGM sensor 36 to 48 hours prior to each of their workout sessions. The first 6 hours were used to calibrate the equipment. The data obtained during the 24 hours prior to the workout session were used as information for day of rest.
Subjects’ preexercise meals (dinner on the night before morning sessions and lunch prior to afternoon sessions) were standardized to provide 25% of the total daily caloric intake in the following way: 50% carbohydrates, 20% protein, and 30% fat. Forty-five minutes after finishing morning exercise, patients were provided with a standardized breakfast. Prior to afternoon exercise, the patients were asked to have lunch at 12 pm. During the following 36 hours after each exercise session the patients were allowed to continue eating as they would on a regular basis, recording a meal diary, and using the Bolus Wizard to register the amount of carbohydrates consumed during the study.
Exercise sessions started only if subjects’ blood glucose concentrations were in the 120-200 mg/dL range. If the result was less than 120 mg/dl, we offered 15 to 30 grams of carbohydrates to the patient, and if the result was more than 200 mg/dl, we administrated a fast-acting insulin analog, according to the bolus wizard calculation. In both of the later cases, a new blood glucose measurement was performed 15 minutes later.
Before the start of each session, the pump was stopped and started again 45 minutes after the exercise was over. This action was based on a prior study in children and adolescents that found that discontinuing basal insulin during and for 45 minutes following postabsorptive afternoon exercise significantly reduced the frequency of hypoglycemic episodes.5 The exercise session involved a 60-minute moderate aerobic workout on a treadmill machine, divided into 4 cycles 15 minutes each, separated by three 5-minute breaks. Capillary blood glucose readings were taken at the start of the session, after the second 15-minute bout of exercise, and 45 minutes after the end of the session. Capillary blood glucose readings were also obtained before and 2 hours after meals, at 10 pm and 3 am on the day of sensor insertion, on the day of the workout session, and 36 hours after the session.
The primary outcome was the rate of hypoglycemic episodes during exercise and in the 36 hours following morning and afternoon sessions. Secondary outcomes were the number of CGM readings in the 70-200 mg/dL range on the day after exercise and the accuracy of CGM data compared to self-monitored blood glucose (SMBG) measurements.
Statistical Analysis
Standard statistical methods were used for the calculation of means and standard deviation (SD). Hypoglycemia was defined as a CGM value of <70 mg/dL. If values of <70 mg/dL were sustained for >20 min, an additional event was counted. Severe hypoglycemic events were defined as hypoglycemia plus altered consciousness, seizures, or the requirements of assistance from a third person. We evaluated the differences using incidence rate ratios. Time spent in euglycemia (70-200 mg/dL) was measured on days before and after the workout session and compared via paired t tests. The accuracy of the data obtained through CGM was estimated with the mean absolute relative difference (MARD) between the CGM and contemporaneous SMBG values. The CGM information was downloaded using Personal Care Link software. It was a priori established that the quality of the CGM readings would be acceptable if each of the MARD reports were below 20%. Data were analyzed using Stata statistical software version 10 (StataCorp LP, College Station, TX).
Results
Among 35 patients initially randomized, 3 subjects were excluded from the final analysis due to noncompliance with the protocol and unexpected interruption in the CGM. The basal characteristics of included patients are presented in Table 1.
Table 1.
Basal Characteristics of Included Patients.
| Age, years (mean ± SD) | 30.31 ± 12.66 |
|---|---|
| Male (n, %) | 17 (51.4) |
| Time since diagnosis of DM1 in years (mean ± SD) | 13.67 ± 9.15 |
| Weight, kg (mean ± SD) | 62.47 ± 12.21 |
| Height, cm (mean ± SD) | 164 ± 9 |
| BMI, kg/m2 (mean ± SD) | 23.3 ± 3.4 |
| A1c level (mean ± SD) | 7.28 ± 1.03 |
| Time on SAP therapy (months) | 9.58 ± 9.77 |
| Sensor use (80-100% of time) (n, %) | 35 (100) |
| Use of bolus wizard (100% of boluses) | 35 (100) |
| Easy bolus | 0 (0) |
| Insulin total daily dose (U/kg/day) | 0.75 ± 0.34 |
A1c, glycated hemoglobin A1c; BMI, body mass index; SAP, sensor-augmented pump therapy; SD, standard deviation.
Median age was 30.31 ± 12.66 years, HbA1c of 7.28 ± 1.03 %, and BMI of 23.3 ± 3.4 kg/m2, with 13.67 ± 9.15 years since their diagnosis with DM1.
There were no significant differences in the pump and CGM settings and patients’ behaviors during days before and after the exercise sessions. The SAP patterns are presented in Table 2.
Table 2.
Patient Behaviors and Pump Settings During Days Before and After the Exercise Sessions.
| Day before exercise | First day after exercise | Second day after exercise | P | |
|---|---|---|---|---|
| Morning exercise | ||||
| Number of bolus (mean ± SD) | 5.67 ± 2.55 | 5.70 ± 2.19 | 6.06 ± 2.18 | .95 |
| Basal insulin units (mean ± SD) | 22.4 ± 16.99 | 21.91 ± 16.58 | 22.12 ± 16.73 | .9 |
| Basal percentage (% of total doses) | 46.12 ± 11.69 | 45.18 ± 15.26 | 46.09 ± 12.49 | .77 |
| SMBG number/day | 6.88 ± 4.03 | 6.76 ± 2.08 | 6.79 ± 2.32 | .87 |
| Number of programmed insulin basals | 3.58 ± 1.68 | 3.64 ± 1.73 | 3.73 ± 1.72 | .88 |
| Average carbohydrate intake (g) | 218.58 ± 105.69 | 227.03 ± 111.44 | 218.48 ± 114.03 | .74 |
| Afternoon exercise | ||||
| Number of bolus (mean ± SD) | 6.28 ± 2.91 | 6.97 ± 2.03 | 5.55 ± 2.43 | .61 |
| Basal insulin units (mean ± SD) | 22.92 ± 17.55 | 23.68 ± 21.1 | 23.36 ± 20.82 | .87 |
| Basal percentage (% of total doses) | 46.07 ± 12.55 | 47.14 ± 10.17 | 46.86 ± 14.39 | .69 |
| SMBG number/day | 6.07 ± 2.52 | 7.14 ± 2.77 | 6.48 ± 2.38 | .09 |
| Number of programmed insulin basals | 3.66 ± 1.32 | 3.66 ± 1.32 | 3.62 ± 1.18 | .99 |
| Average carbohydrate intake (g) | 222.07 ± 99.87 | 203.66 ± 81.91 | 219.72 ± 108.52 | .40 |
SD, standard deviation; SMBG, self-monitored blood glucose.
Hypoglycemic Events
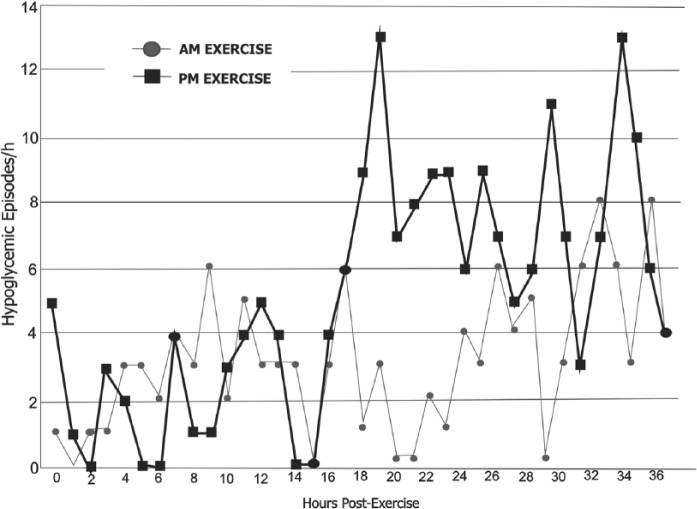
At least 1 hypoglycemic event was noted in 53% of the patients in the 36 hours after the exercise session. Figure 1 shows the distributions of hypoglycemic events. There were 180 events after the morning sessions (5.6 per patient during 36 hours), with most occurring 15-24 hours after the sessions (10 pm to 7 am). There were no hypoglycemic events during or immediately after the morning sessions. There were 322 hypoglycemic events after the afternoon sessions (10.7 per patient during 36 hours), with most occurring 15-21 hours after the sessions (7 am to noon). Six hypoglycemic events took place during the afternoon exercise session. The comparison of postexercise hypoglycemia rates significantly favored morning exercise (incidence rate ratio, 0.52; 95% CI, 0.43-0.63; P < .0001).
Figure 1.
Hourly hypoglycemic episodes following morning (circles) or afternoon (squares) exercise.
The probability of detecting hypoglycemia by SMBG was 20.1% lower than the probability of detecting hypoglycemia by CGM (P = .0004). Four events were documented by capillary blood glucose testing and were not detected by CGM during the morning exercise. There were no severe hypoglycemic events during the study period.
Hyperglycemic Events
When comparing morning versus afternoon sessions, there was no difference in the incidence of hyperglycemic events; (Incidence rate ratio 1.0; 95%CI: 0.93-1.14)
Time Spent in Euglycemia
On the day prior to morning exercise sessions, 63 ± 30.7% (mean ± SD) of the CGM readings were in the 70-200 mg/dl range, compared to 83 ± 17.6% of readings on the day after the session. This improvement on the day after morning exercise was statistically significant (P = .003). On the days following afternoon exercise, there were more CGM readings reflecting hypoglycemia (<70 mg/dl) than on the day prior to afternoon exercise (7.5 vs 1.1%, P = .011) and there was no difference in the number of CGM readings in the 70-200 mg/dl range.
Accuracy of CGM Data
The mean absolute relative difference (MARD) for the morning exercise sessions compared to the previous day of rest was within the prespecified acceptable quality ranges according to the ISO criteria available at the moment in which the study was conducted.6 Rest MARD 13.8% ± 9.6% (mean ± SD); post–morning exercise MARD 18.3% ± 7.2%. (No significant difference between groups.) The MARD for the afternoon exercise sessions compared to the previous day of rest did not show difference between groups (rest MARD: 13.8% ± 9.5%; post–afternoon exercise MARD: 17.4% ± 9.1%).
Discussion
People with diabetes must balance the benefits of exercise against the risk of hypoglycemia during or after the PE. This is the first study conducted in adult people with DM1 to determine the safety of PE, comparing the risk of late-onset hypoglycemia following morning versus afternoon exercise.
We found significantly fewer hypoglycemic events if the exercise was conducted in the morning. There are several possible explanations for these findings. Cortisol, the main counterregulatory hormone, has a circadian rhythm and is not highly affected by hypoglycemia and PE. Its lower concentrations in the afternoon and particularly at midnight may favor hypoglycemia by reducing gluconeogenesis and responses to glucagon.7,8 Further research is needed to better quantify this phenomenon. Other possible explanation could be the absence of insulin boluses before the morning exercise since these was performed in a fasting state, however, we aimed to reduce this difference as the afternoon exercise session was conducted 4 hours after lunch, which is considered enough time to minimize the effect of the fast-acting insulin used for that meal.
In our study we observed similar rates of postexercise nocturnal hypoglycemia to those reported by the DirectNet Workgroup.9,10 They evaluated 50 patients with DM1 (11-17 years old) after moderate PE in the afternoon versus a day of sedentarism. In this study, mean glucose between 10 pm and 6 am on the day after exercise was significantly lower compared to the resting day (131 vs 154 mg/dL, P = .003).
We found a low rate of hypoglycemia during and immediately after exercise, which differs from other published studies10 which report that 30% of adolescents and children with DM1 had either biochemical (glucose < 60 mg/dL) or symptomatic hypoglycemia during or immediately after the exercise session. This discrepancy may be explained because we required our subjects to start exercise only if glucose levels were in the range between 120-200 mg/dL, based on previous findings and on expert recommendations.11-13 In addition, we suspended basal insulin infusion during exercise, restarting it only 45 minutes after finishing it. The real-time availability of CGM data may have also contributed to the relatively low rates of early- and late-onset hypoglycemia.14,15
Comparing days before versus after morning exercise sessions, we observed an increase in the CGM readings in the euglycemic range, showing the benefits of exercise on glycemic control. When we compared CGM data in days pre– and post–afternoon exercise, this was not evident, but we observed a clear tendency toward more hypoglycemic events.
Sensor accuracy during PE is a concern because rapidly changing blood glucose concentrations may lead to a large gradient between blood and interstitial glucose values. One study suggests that CGM data overestimate glucose levels during aerobic exercise compared with measures made simultaneously in capillary blood, due to the 10- to 20-minute time delay in equilibration between interstitial fluid and capillary glucose.16 Our data showed a good accuracy of CGM data during PE, with MARD in acceptable ranges and the 6 hypoglycemic events presented during exercise were all corroborated by capillary glucose measurements.
The crossover design of this study, with all participants serving as their own controls has advantages as it reduces the variability between subjects, letting us evaluate the response of each patient to both interventions. Our study results may not be generalizable to pediatric or geriatric populations or to those with recently diagnosed DM1.
Conclusion
Among adult people with DM1 using SAP therapy, PE in the morning confers a lower risk of postexercise hypoglycemia than does afternoon exercise. Most episodes of postexercise hypoglycemia occur between 15 and 24 hours after the cessation of exercise. Exercise in the morning improves metabolic control on the subsequent day and maintains patients in the euglycemic range for a longer time. In addition, our data showed a good accuracy of CGM data during PE.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; DM1, type 1 diabetes mellitus; MARD, mean absolute relative difference; PE, physical exercise; SAP, sensor-augmented pump; SMBG, self-monitored blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AMG reports speaker fees from NovoNordisk, Elli Lilly, MSD, Novartis, and Medtronic and research grants from Medtronic, Novartis, and Abbott. PA has participated in advisory boards and has received speaker fees from AstraZeneca, BMS, Boehringer, GSK, Jansen, J&J, Lilly, MSD, Novartis, and Sanofi and has participated in original research involving Sitagliptin and insulin Glargine. AV reports receiving speaker fees for insulin pump patient training and research grant from Medtronic. CR receives fees for a patient education program with NovoNordisk and insulin pump patient training with Medtronic. No other potential conflicts of interest relevant to this article were reported.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Suh SH, Paik IY, Jacobs K. Regulation of blood glucose homeostasis during prolonged exercise. Mol Cells. 2007;3(23):272-279. [PubMed] [Google Scholar]
- 2. Riddell M, Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol. 2009;3(4):914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo-Scharer R. Preventing post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr. 2010;157:784-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in non-trained patients with type 1 diabetes. Diabetes Technol Ther. 2010;12(10):763-768. [DOI] [PubMed] [Google Scholar]
- 5. Ertl AC, Davis SN. Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab Res Rev. 2004;20(2):124-130. [DOI] [PubMed] [Google Scholar]
- 6. In vitro diagnostic test systems— requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197:2003. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 7. Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Acute, sane-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglysemia in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab. 2006;290(6):E1331-E1338. [DOI] [PubMed] [Google Scholar]
- 8. Galassetti P, Mann S, Tate D, et al. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab. 2001;280(6):E908-E917. [DOI] [PubMed] [Google Scholar]
- 9. Tsalikian E, Mauras N, Beck RW, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tansey MJ, Tsalikian E, Beck RW, et al. The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care. 2006;29(1):20-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillip M, Battelino T, Rodriguez H, et al. Use of insulin pump therapy in the pediatric age-group: Consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2007;30(6):1653-1662. [DOI] [PubMed] [Google Scholar]
- 12. Riddell MC, Bar-Or O, Ayub BV, Calvert RE, Heigenhauser GJ. Glucose ingestion matched with total carbohydrate utilization attenuates hypoglycemia during exercise in adolescents with IDDM. Int J Sport Nutr. 1999;9(1):24-34. [DOI] [PubMed] [Google Scholar]
- 13. Tsalikian E, Kollman C, Tamborlane WB, et al. Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care. 2006;29(10):2200-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Admon G, Weinstein Y, Falk B, et al. Exercise with and without an insulin pump among children and adolescents with type 1 diabetes mellitus. Pediatrics. 2005;116(3):e348-e355. [DOI] [PubMed] [Google Scholar]
- 15. Iscoe KE, Campbell JE, Jamnik V, Perkins BA, Riddell MC. Efficacy of continuous real-time blood glucose monitoring during and after prolongs high-intensity cycling exercise: spinning with a continuous glucose monitoring system. Diabetes Technol Ther. 2006;8(6):627-635. [DOI] [PubMed] [Google Scholar]
- 16. Iscoe KE, Davey RJ, Fournier PA. Increasing the low glucose alarms of a continuous monitoring system prevents exercise induced hypoglycaemia without triggering any false alarm. Diabetes Care. 2011;34:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]