Abstract
Hemoglobin A1c (HbA1c) measurement has come to be a cornerstone in modern diabetes therapy. However, the methodological aspects of this type of measurement have been given little attention lately due to its position as an established method of choice. Nevertheless, quite a number of issues face practical application, such as clinically relevant differences between different measurement methods—both lab-based and point-of-care (POCT) systems will show better or worse diabetes management results after switching methods; and there are a number of possible reasons that need to be known and observed in practice. The aim of this review is to draw attention to these problems from a German point of view and provide suggestions for appropriate measures to improve the situation.
Keywords: HbA1c, laboratory determination, National Glycohemoglobin Standardization Program, point-of-care testing instruments
The significance of Hemoglobin A1c (HbA1c) as the central parameter for assessing the quality of glycemic control achieved over time is undisputed. This is documented, for example, by the solid place of HbA1c measurement in the national treatment guidelines of the German Diabetes Association (DDG)1 and the American Diabetes Association (ADA).2,3 HbA1c measurement is now being used for the diagnosis of diabetes.4,5 All therapeutic endeavors to optimize metabolism, as well as new therapeutic agents, must show a certain degree of “success” with this parameter, that is, a reduction in the HbA1c value by a certain percentage. The thinking behind this is based on (older) long-term studies such as the United Kingdom Prospective Diabetes Study (UKPDS) and the Diabetes Control and Complications Trial (DCCT), which have demonstrated a correlation between the glycemic control achieved (measured by the HbA1c value) and the risk of developing diabetes-associated complications.6,7
Just after results from these studies were reported, methodological questions about HbA1c measurement were a hot topic. However, in recent years questions of methodological improvements and standardizations by the National Glycohemoglobin Standardization Program (NGSP) and the International Federation of Clinical Chemists (IFCC) and their adoption by the DDG and the German Society for Clinical Chemistry and Laboratory Medicine (DGKL) have faded into the background. For example, there are now very few contributions on this topic at diabetes conferences or in medical journals—somewhat astonishing, considering the central importance of this parameter to diabetology. As far as we know, there is no academic centre in Germany that focuses mainly on the subject of HbA1c overall (ie, more from a clinical point of view); the Working Group for Diabetes Technology (AGDT) of the German DDG concerns itself to a certain extent with methodological questions relating to HbA1c measurement. A variety of references and literature on this subject can be found on the NGSP website.8
Does this mean that all aspects of this parameter have been clarified, that is, that no further questions about HbA1c measurement remain unanswered? Apparently the aforementioned improvements in standardization and so on that have led to most physicians and patients being currently satisfied with the situation. The aim of this review is to discuss methodological questions that are still unanswered from a German point of view and to mention clinically important aspects that in our view still require clarification.
Confusion About the Units in Which HbA1c Is Stated
First, we need to address a practical problem: In the course of several decades the world of diabetology and everyone else concerned with the question of quality of glycemic control, such as third-party payers, public authorities, the media, and so on have become used to having the HbA1c value stated as a percentage. The standardization introduced a few years ago by the IFCC (see below) presents the practical problem that the HbA1c measurement is stated as an SI unit (in mmol/mol).9,10 Although this unit is supposed to have been used in Germany since January 1, 2009, this has not occurred in practice; neither the patients nor the diabetes teams use the SI unit in their routine communication on this important parameter. So there is now the confusion that the laboratories are obliged to use the SI unit (because legislation requires it), whereas in the real world all information is given as a percentage, as before. The question is whether we really need a “reeducation initiative” (with the problem of how to do this) or whether we leave this duality as it is. The medical association, that is, the Board of the DDG, did take a clear position on this in 2009, but standard practice has changed very little.11 With this position, a consensus devised by the American and European Diabetes Association is being implemented in Germany as a national recommendation. A working group on HbA1c. Standardisation of the IFCC developed a reference measurement system for the measurement of HbA1c. With this method, measured values are about one-third lower and, if stated as a percentage, would easily lead to confusion. Therefore the HbA1c values obtained with this method should be stated in mmol/mol hemoglobin, and at the same time, the value standardized as per NGSP can continue to be stated as a percentage.
It is interesting that even diabetes journals do not all follow the same practice in this regard, that is, there are some European journals that require the use of the SI unit, while the American journals do not. The periodical Diabetes Care has recently begun to use both units. If necessary, the value can be converted into mmol/mol with the IFCC-NGSP equation:
-
Conversion from % into mmol/mol
-
Conversion from mmol/mol into %
Point-of-Care Testing Instruments
For the determination of HbA1c a wide range of such instruments for point-of-care testing instruments is available on the German market (see Table 1). Their advantages, including the following, seem convincing in practice.
Table 1.
Overview of a Few POCT Instruments Available on the German Market for HbA1c Measurement.
| Model | Quo-Lab HbA1c Analyser | DCAVantage™ | Afinion™ AS100 Analyzer | HemoCue® HbA1c 501 System |
|---|---|---|---|---|
| Manufacturer | EKF Diagnostics/IME-DC | Siemens | Alere | Infopia/Hemocue |
| Size (H × W × D; mm) | 95 × 205 × 135 | 254 × 287 × 277 | 170 × 170 × 320 | 136 × 198 × 217 |
| Weight (kg) | 0.7 | 3.9 | 5 | 1.6 |
| Blood volume (pl) | 4 | 1 | 1.5 | 4 |
| Measurement time (min) | 4 | 6 | 3 | 5 |
| Capillary whole blood | Yes | Yes | Yes | Yes |
| Venous blood | Yes | Yes | Yes | Yes |
| EDTA, heparin, citrate, NaF | Yes | Yes | Yes | Yes |
| Storage capacity (measured values) | 7000 | 4000 | 1000 | 200 |
| Measurement range according to NGSP | 4.0-15.0% (20.2-140.4 mmol/mol) | 2.5-14.0% (3.8-129.5 mmol/mol) | 4.0-15.0% (20.2-140.4 mmol/mol) | 4.0-14.0% (20.2-129.5 mmol/mol) |
| Measurement method | Boronate affinity | Immunoassay | Boronate affinity | Boronate affinity |
| Imprecision | <3% | <3% | <3% | <3% |
| Data download | Yes | Yes | Yes | Yes |
| Measurement results according to NGSP (%) and IFCC (mmol/mol) | Yes | Yes | Yes | Yes |
Table was compiled with the aid of information accessible on the Internet and therefore does not make any claim to accuracy. As of May 1, 2013.
the use of unit-use reagents, also called ready-to-use/single-use reagents (meaning that the reagents needed do not require any further preparation steps and are intended for single use)
immediate availability of the measurement results in minutes
use of fingerstick vs venous blood
no elaborate blood workup, and so on
Unfortunately there has as yet been only a limited number of head-to-head comparisons of these instruments in respect of their accuracy of measurement.12 Similarly to the differences that can arise in blood glucose measurement systems between different batches of test strips, there are presumably also differences between the test materials that are used in these HbA1c measurement instruments. Associated with this is the question of the relevant quality control (see below) with regard to both the manufacturer and the user. Thus, not only the difference between POCT (point-of-care testing) instruments themselves but also the difference between POCT and laboratory instruments is a topic. For example, systematic differences have been reported between measurement results with a POCT instrument and a central laboratory, in which 98% of the values with the POCT instrument were below the results from the laboratory, and the mean difference was –0.5% (5.5 mmol/mol).13
Quality of the HbA1c Measurement in the Case of Repeated Measurement With 1 Method
An important question is that of the quality of HbA1c measurement in the case of repeated measurement: Does one get approximately the same result if the HbA1c concentration is determined several times with a given measurement method in the same blood sample? Coefficient of variation (CV) (standard deviation [SD]/[mean] × 100) from 0.5% to 2% is stated in the literature.14 In this study, however, measurements were performed with the same instrument and not just with the same measurement method. According to a current publication, most methods show a CV of 4%.11 However, a CV of 4% means that a difference of 0.2% to 0.3% (2.2 to 3.3 mmol/mol) can occur in the HbA1c measurement even when 1 method is used, solely as a result of its variability. If the CV is 7%, HbA1c values between 9.0% and 11.0% (74.9 to 96.7 mmol/mol) cannot be reliably distinguished if the HbA1c value is 10% (85.8 mmol/mol) and the SD 0.7% (7.6 mmol/mol), assuming a “critical difference” of 3 SDs. Therefore, according to the recommendations of the international diabetes association’s such as the European Association for the Study of Diabetes (EASD), the ADA and the International Diabetes Federation (IDF), the CV should be as low as possible, that is, <2%. However, as far as we know, there are no studies on what the quality of the measurement is with the various methods available on the German market under routine conditions (see below). Nevertheless, in the United States the College of American Pathologists (CAP) surveys shows that the between-laboratory within-method CVs are <4% for most methods; some are even <2%.
Quality of HbA1c Measurement Between Laboratories
Another question is that of the quality of the HbA1c measurement if this is carried out in different laboratories (ie, with different methods) and what quality of measurement can be achieved with that. In this regard it is necessary to distinguish between measurements such as those carried out by specialized diabetes practices (DSPs) with POCT unit-use instruments that work with single-use reagents and those carried out by central clinical chemistry laboratories. In the latter laboratories methods that run on automated laboratory machines are usually employed. As far as we know, there is no information as to how many HbA1c measurements are performed in practice in Germany with which measurement method in which facilities, that is, whether more measurements are made nowadays with POCT instruments or in central laboratories. It is worth to consider to establish registers in different countries that “simply” monitor which HbA1c measurements are made at which site.
Reference Method
To determine the HbA1c measurement result, the actual measurement is compared with the value obtained with use of a standard with a known HbA1c value. This means that high importance is attached to this standard and its quality.15,16 The IFCC has established a reference method, that has the great advantage of traceability to reference material. Such metrological aspects are also of considerable importance to blood glucose measurement.17 However, if this IFCC method is used in the HbA1c measurement and the values are stated as a percentage, as before, the results will then be >1.5 percentage points below the results obtained with use of the NGSP method.18,19 To avoid confusion, the measurement result obtained with the IFCC method is expressed as mmol/mol and not as %. In a widely used approach (the designated comparison method), methodological comparisons are made with an (arbitrary) “reference” method. In this way regression equations can be calculated to convert the values into each other.
Requirements for the Use of Appropriate Quality Controls
The quality control that should be used according to the DDG practice guideline is the one defined in the guidelines of the German Federal Medical Association (RiliBÄK):4,20 External quality assurance by means of interlaboratory comparisons is mandatory (1 interlaboratory comparison per quarter) for all clinical chemistry laboratories that use laboratory systems and POCT systems with reagents like those of laboratory systems; that is, in the case of these systems control measurements are carried out every quarter with 2 samples sent by interlaboratory comparison institutions, in which the maximum deviation in the interlaboratory comparison is to be 18%. This obligation does not apply to tests with unit-use reagents in the context of point-of-care on-the-spot diagnostics, that is, with POCT instruments in medical practices; these are subject only to an internal control in accordance with RiliBÄK (see below). It is worth to acknowledge that 18% is a quite wide acceptance range and this may in part be due to the matrix effects of the materials used for the German quality control. For the CAP in the United States, whole blood is used (= no matrix effects), a target value is assigned and the acceptance limits are ±6%.
Looking at the DDG practice recommendations, by way of comparison, one will find the following statement on the diagnosis of diabetes:4 “For the measurement of venous plasma glucose and HbA1c, only standardised and quality-assured laboratory methods may be used.” Apparently there are no specific requirements in the DDG guidelines for the diagnosis of diabetes by HbA1c measurement, whereas they do exist for POCT glucose systems in the gestational diabetes guideline for the diagnosis of gestational diabetes. In a joint DGKL-DDG recommendation on this subject there are relevant instructions. Only when analogous or equivalent requirements are met can POCT HbA1c systems also be used for the primary diagnosis of diabetes mellitus. The minimum requirements are:
These POCT systems must be expressly intended by the manufacturer for medical use in diabetes mellitus screening and diagnosis. We were unable to find references to any such declaration for any POCT HbA1c system.
In the case of use in the sphere of the independent practitioner, external quality assurance in accordance with RiliBÄK rules, if appropriate with use of control samples with method-specific target values, must also (and as a departure from the exception regulated in the RiliBÄK) be carried out. Whether this is currently the voluntary practice, we do not know, but it is not being required so far.
Quality assurance within the institutions (internal QA) is conducted in accordance with the rules laid down by RiliBÄK.
For all HbA1c POCT unit-use and laboratory systems, laboratory or practice controls of the systems should be performed with a control solution supplied by the manufacturer on all testing days. In these, a deviation of up to 10% from the target value of the control solution is permissible. For POCT unit-use systems this control measurement need be performed only once a week if the manufacturer supplies electronic/physical standards for its instrument, which can then be used as a substitute for the daily control. With these instruments it is thus sufficient to obtain a satisfactory deviation once a week; it is not clearly explained what is to be done if this is not obtained.
Results From Interlaboratory Comparisons
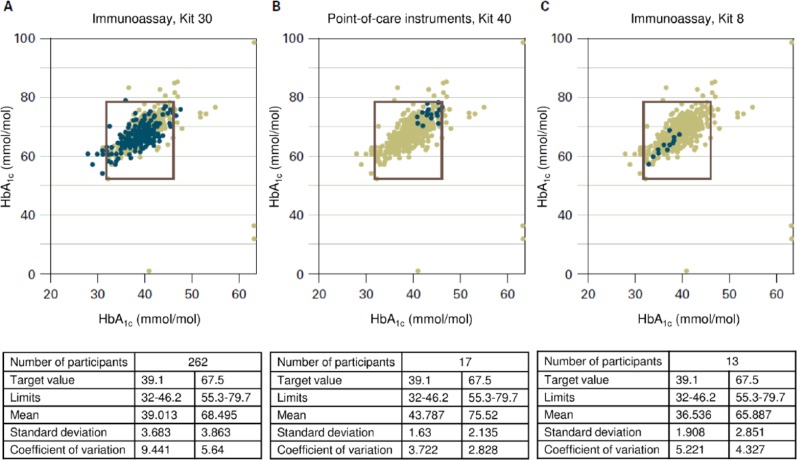
Looking at the results from previous interlaboratory comparisons in Germany (Figure 1),21 it is clear not only that some measurement methods are used considerably more frequently than others and that there are apparently several manufacturers and methods (133 and about 70), but also that there are clinically relevant discrepancies in the results, depending on the method: At a target value of 39.1 mmol/mol (5.7%) for sample A, the median of all measurements is 39.6 mmol/mol (5.8%). This appears quite good, but the extremes were 28 mmol/mol (4.7%) and 89 mmol/mol (10.3%)! The acceptable limits in the interlaboratory comparison (18% according to RiliBÄK) are also quite far apart, at 32 mmol/mol to 46.2 mmol/mol (5.08% to 6.38%).
Figure 1.
Results in mmol/mol of HbA1c measurements obtained in interlaboratory comparisons with 3 different methods.20 The results plotted were obtained with the measurement method in question on the HbA1c samples A (x axis) and B (y axis). The square drawn in defines the maximum permitted deviation of ±18% from the target value. As the differing number of blue points shows, these are used with varying frequency. The results from all measurements with the different methods are entered in grey. In the tables below the figures, the results with samples A (left column) and B (right column) are shown next to the number of participants using the method in question. The target value of these samples is stated with the limits within which the results should lie. The mean of all results, with the standard deviation and the variation coefficient gives an idea of how good the method is overall, but also what the observed deviations are. Figure 1A presents the results from a frequently used immunological assay method. The results in Figure 1B are all in the upper right corner and those in Figure 1C in the lower left corner; that is, with 1 method there is a trend toward higher measurements and with the other toward lower values. Mean values obtained in the interlaboratory comparison with the 2 HbA1c samples (B: 6.16% and 9.06% [43.8 and 75.5 mmol/mol]; C: 5.49% and 8.18% [36.5 and 65.9 mmol/mol]) differ accordingly.
Looking at 2 types of instrument, by way of example (see Figures 1A and 1B; immunoassay kit versus POCT instrument), with the immunoassay kit there is not only a whole series of results that are outside the acceptable limits, but the CV stated in the analyses of the interlaboratory comparisons, that is, the relative scatter between the results is also remarkably high at 9.4% (see above). With the POCT instrument the measured values are all within the limits and the CV is lower, at 3.7%, but all results are clearly higher than the mean (43.8 mmol/mol versus 39.1 mmol/mol [6.16% vs 5.7%]).
To get to the point, switching from one HbA1c measurement method to another can lead to an apparently substantial change in the glycemic control of all patients in a specialized diabetes practice, without any concrete change in the treatment. In an extreme case, which is very unrealistic, it would be possible for a patient with a doctor who used HbA1c measurement method A, to have an HbA1c value of 4.7% (27.9 mmol/mol), that is, optimal glycemic control, and for the same patient with a different doctor, who used measurement method B, to have an HbA1c value of 10.3% (89.1 mmol/mol), and for the doctor to initiate extensive adjustments to his or her treatment.
The question is why there are such differences between the HbA1c measurements between laboratories, despite efforts at standardization. The size of the differences in HbA1c results obtained with POCT systems not participating in interlaboratory comparison is still not known. The differences observed in the interlaboratory comparisons can be explained not only by different measurement methods, there are also considerable differences when the same method is used. As far as we know, there are very few publications on such questions.
Correlation Between the Blood Glucose Measurement and the HbA1c Value
When a diabetologist discusses his or her patient’s documented blood glucose values with him or her, he or she usually refers to his or her HbA1c values. It is usually assumed that there is a quite close correlation between the documented blood glucose values and/or the data downloaded from the measurement instruments (ie, the prevailing blood glucose level) and the HbA1c value: If the estimated mean glucose (eAG) is 140 mg/dl, the HbA1c value should be 6.5% (47.5 mmol/mol) and at a value of 200 mg/dl it should be 8.6% (70.5 mmol/mol) (professional.diabetes.org). It is assumed with this view that the blood glucose is measured with a high degree of reliability; thus, the mean absolute relative deviation (MARD) should be < 10%. However, if the blood glucose measurement is systematically measured too high or too low, because of deviations of 10% or 20% in one or the other direction (eg, in the case of a bad batch of test strips), this naturally has an influence on the quality of the correlation. It is worth to keep in mind that the HbA1c reflects a weighted average blood glucose which is, in itself, difficult to measure directly. Any single blood glucose measurement or small daily profile is going to give only an approximation of the mean glucose. The health care professional can only use the eAG as an education tool—this was the intention of the original international group for when it would be used clinically.
The measuring quality of blood glucose measurement systems was not really a topic until a few years ago, or at least there were very few studies of it. Looking at up-to-date studies, these prove to be quite high-quality (measured by International Organization for Standardization [ISO] standard 15197 in the still current version of 2003 and the version of 2013, which will be valid in the future) with a whole series of measurement instruments, especially those from well-known manufacturers.22,23
Causes of Discrepancies Between HbA1c and Blood Glucose Values
A recurring problem in practice is that there are individual patients in whom, after all factors known to have an influence on the HbA1c value have been ruled out; there are considerable discrepancies between the documented blood glucose values and repeatedly measured HbA1c values.24,25 In the case of such discrepancies the spontaneous reaction is first to suspect that the blood glucose measurement system is giving inaccurate readings—the quality of the HbA1c measurement, on the other hand, is almost never doubted. As mentioned above, however, the exact opposite may be the case. Many diabetologists can report several such patients and his or her frustration in the attempt to solve such mysteries. As in the central laboratories the HbA1c value is only 1 parameter among many; in many cases, there is no time or know-how for focused handling of such questionable measurements. Ideally there would be a centre that could deal primarily with such cases and support diabetologists in the treatment of these patients.
The HbA1c literature provides a whole series of possible causes for the discrepancies mentioned (see Table 2). Practically all of these are based on a deviation of the red blood cell survival time from the norm, different forms of red blood cells/ hemoglobin variants, and so on. But first it is important to make sure that the patient really is correctly recording his or her blood glucose values. Psychological causes of false documentation of blood glucose values are (this list was compiled by Christoph von Boxberg, Leverkusen, Germany):
Table 2.
Reasons Why the HbA1c Measurement Gives a False Low or False High.
| Physiological causes | ||
|---|---|---|
| False low | False high | |
| Red blood cell production | Increased | Slowed by the lack of available iron |
| High altitude | Anemia induced by iron deficiency | |
| Pregnancy | Anemia induced by infection | |
| Hemorrhages, blood loss | Anemia induced by tumors | |
| Blood transfusion | ||
| Administration of erythropoietin | ||
| Iron supplementation | ||
| Red blood cell destruction | Premature | Late |
| Hemolytic anemia | Splenectomy | |
| Chronic kidney failure | Aplastic anemia | |
| Cirrhosis of the liver | ||
| Folic acid deficiency | ||
| Hemoglobinopathies: HbS, HbC, HbD | Hemoglobinopathies: HbH, HbF (thalassemia) | |
| Spherocytosis | ||
| Options for objective determination | ||
| • Determination of an “HbF-purified” HbA1c | ||
| • Reticulocytes plus ferritin | ||
| • Urea | ||
| • Hb-electrophoresis | ||
| • In the case of Hb variants, determine HbA1c with an immunological method | ||
| • Fructosamin | ||
| Laboratory causes and options for avoidance | ||
| False high—only in HPLC HbA1c measurements by carbamylation | ||
| Terminal kidney failure, uraemia, creatinine > 5 mg/dl | ||
| Alcoholism (acetaldehyde) | ||
| Aspirin (upward of 500 mg/day over weeks) | ||
| False high—only in immunological HbA1c measurements | ||
| Beta-lactam antibiotics | ||
| Contraceptive pill | ||
| HydroxyethyI starch | ||
| Options for objective determination | ||
| • Newer HPLC columns are no longer influenced by carbamylation, ask the laboratory | ||
| • Request a laboratory method other than HPLC (written note on the laboratory request form): immunological or enzymatic method | ||
| Other causes | ||
| False low | False high | |
| Nutritional (alcohol, fat) | Drugs: immunosuppressants, protease inhibitors | |
| Genetic hyperglycation in certain ethnic groups | ||
| Elderly patient | ||
| Organ transplant | ||
| Hypertriglyceridemia | ||
| Hereditary causes | Hereditary causes | |
Source: Compiled by C. von Boxberg, Leverkusen, Germany.
Head in sand mechanism: The patient is “afraid” of high blood glucose values and diabetes in general. To avoid any triggers of these fearful feelings, he or she ignores the diabetes by repression/displacement: not measuring his or her blood glucose generally, not measuring if elevated blood glucose values can be expected, not injecting insulin.
Pseudo-rationalization and isolation: In the case of various high blood glucose measurements the patient knows the cause (eg, biscuits beforehand) (rationalization). He or she then consciously does not write down these blood glucose values or records a normal blood glucose value instead, as if the dietary “sin” had not been committed (isolation).
Shame before the judgment of others: The patient is ashamed of his or her supposed or actual unhealthy lifestyle and is unwilling to face his or her family and friends and his or her doctor/diabetes team with it. By not writing down the blood glucose values or writing down fictive (“good”) blood glucose values, he or she protects himself or herself from embarrassing exposure.
Shame before his or her own sense of self-worth: The patient is disappointed and remorseful that he or she is not managing to achieve better blood glucose values. To avoid these unpleasant feelings, he or she resorts to various repression/displacement strategies to the extent of not documenting any blood glucose values or not measuring the blood glucose value at all in the first place.
Options for objective detection of these problems are spot checks of the data stored in the blood glucose measuring instrument manually or with suitable software.
Use of the HbA1c Value in the Diagnosis of Diabetes
HbA1c measurement has been used for a few years also to diagnose diabetes.26 This approach is based on a proposal by the ADA and has in consequence led to a large number of publications and responses. According to the DDG practice recommendations for Germany, HbA1c is suitable “as a primary diagnostic test to rule out diabetes with great certainty and to make the diagnosis in some patients.”4 We do not know how many of our colleagues in Germany actually use HbA1c as a diagnostic measure. The methodological aspects mentioned here are of great relevance to the reliability with which a diabetes diagnosis can be made on the basis of 1 single value. This might be 1 reason why the ADA recommendation is to have 2 HbA1c measurements for diagnosis.
Significance of the HbA1c Measurement to the Long-Term Prognosis
Similar to the situation with blood glucose measurement, there is no endpoint study that proves that an HbA1c measurement with a narrow margin of error (CV <2%) leads to an improvement in hard endpoints compared to an HbA1c measurement with a wide margin of error (eg, with a CV >4%). Whereas in the case of blood glucose measurements there are model calculations which provide an idea of the MARD (as a possible parameter for characterizing the measuring quality of blood glucose systems) from which the size of insulin dosage errors become so great that a serious impact can be expected on the safety and efficacy of the insulin treatment in patients with diabetes; we do not know of corresponding models in respect of the significance of the quality of the HbA1c measurement.
Consequences and Conclusion
We hope that the points discussed will lead to an enlivening of interest in HbA1c measurement and help to improve the reliability of this measurement that is so important to the monitoring of treatment and to primary diagnosis. As in many other cases, the question is ultimately who is concerning themselves with it specifically. Usually one refers to the “industry,” as it earns its money with this measurement. In the United States the group of respective manufacturers have in fact worked quite well with the IFCC and NGSP to improve methods and standardize accordingly. However, in Germany this was not the case until now; there is no spokesperson who feels responsible. One option could be for the German Association of the Diagnostics Industry (VDGH) to feel called on to become active. Whether it would take the very welcome step of becoming active without relevant regulations from the European authorities remains to be seen. The ideal would be a constructive cooperation between the manufacturers and the academic world in Europe or Germany on this subject; establishing a quality control system like CAP with its lower limits appears to be a good model. There are proposals for an independent institute that would be concerned with subjects connected with the quality of blood glucose measurement; the subject of HbA1c measurement could be one that such an institute would concern itself with.26
To answer the question of how accurately the various methods/instruments used measure HbA1c, there are—as discussed—rather few studies, and above all not many up-to-date studies.
Acknowledgments
Heartfelt thanks to Cornelia Haug and Theodor Koschinsky for the critical reading of the manuscript. We are indebted to Christoph von Boxberg for permitting us to use Table 2 and other information.
Footnotes
Abbreviations: ADA, American Diabetes Association; AGDT, Working Group for Diabetes Technology; CAP, College of American Pathologists; CV, coefficient of variation; DCCT, Diabetes Control and Complications Trial; DDG, German Diabetes Association; DGKL, German Society for Clinical Chemistry and Laboratory Medicine; DSPs, specialized diabetes practices; eAG, estimated mean glucose; EASD, European Association for the Study of Diabetes; HbA1c, hemoglobin A1c; IDF, International Diabetes Federation; IFCC, International Federation of Clinical Chemists; ISO, International Organization of Standardization; MARD, mean absolute relative deviation; NGSP, National Glycohemoglobin Standardization Program; POCT, point-of-care testing; QA, quality assurance; RiliBÄK, guidelines of the German Federal Medical Association; SD, standard deviation; UKPDS, United Kingdom Prospective Diabetes Study; VDGH, German Association of the Diagnostics Industry.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The manuscript was prepared as part of the honorary membership of Lutz Heinemann and Guido Freckmann in an Advisory Board of Sanofi Germany. GF is general manager of the IDT (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out studies evaluating BG meters and medical devices for diabetes therapy on behalf of various companies. GF/IDT received speakers’ honoraria or consulting fees from Abbott, Bayer, Berlin-Chemie, Becton-Dickinson, Dexcom, Menarini Diagnostics, Roche Diagnostics, Sanofi, and Ypsomed.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This review article was supported by being member of a German advisory member of Sanofi Germany for blood glucose monitoring.
References
- 1. Bundesärztekammer, Kassenärztliche Vereinigung, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Nationale VersorgungsLeitlinie Therapie des Typ-2-Diabetes—Langfassung, 1. Auflage. Version 3. Available at: http://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Leitlinien/Evidenzbasierte_Leitlinien/NVL_Typ-2_Therapie-lang_Apr_2014.pdf.
- 2. Böhm B, Dreyer M, Fritsche A, Füchtenbusch M, Gölz S, Martin S. Therapie des Typ-1-Diabetes. Diabetologie. 2011;6(suppl 2):S120-S130. [Google Scholar]
- 3. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerner W, Brückel J. Definition, Klassifikation und Diagnostik des Diabetes mellitus. Diabetologie und Stoffwechsel. 2012;7(suppl 2):S84-S87. [Google Scholar]
- 5. Schindhelm R, Lenters-Westra E, Fokkert M, Slingerland R. Glucose and glycated haemoglobin point-of-care testing and early diagnosis of diabetes and pre-diabetes. Eur Endocrinol. 2010;6:24-28. [Google Scholar]
- 6. Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676-1685. [DOI] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 8. National Glycohemoglobin Standardization Program. 2013. Available at: http://www.ngsp.org/. Accessed July 14, 2014.
- 9. Sacks DB, Bergenstal RM, McLaughlin S. Point: the reporting of estimated glucose with hemoglobin A1c. Clin Chem. 2010;56(4):545-546. [DOI] [PubMed] [Google Scholar]
- 10. Sacks DB. Measurement of hemoglobin A(1c): a new twist on the path to harmony. Diabetes Care. 2012;35(12):2674-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deutsche Diabetes Gesellschaft, diabetesDE. Stellungnahme der Deutschen Diabetes Gesellschaft, diabetesDE und des Kompetenznetzes Diabetes mellitus zur Verwendung des HbA1c-Wertes als Biomarker zur Diabetesdiagnose. 2013. Available at: http://www.diabetesde.org/ueber_diabetes/diabetesde_bezieht_stellung/wissenschaftliche_stellungnahmen/verwendung_des_hba1c_wertes_als_biomarker_zur_diabetesdiagnose_102010/. Accessed July 14, 2014.
- 12. Lenters-Westra E, Slingerland RJ: Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem 60:1062-1072, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Leca V, Ibrahim Z, Lombard-Pontou E, et al. Point-of-care measurements of HbA(1c): simplicity does not mean laxity with controls. Diabetes Care. 2012;35(12):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapelle JP, Teixeira J, Maisin D, et al. Multicentre evaluation of the Tosoh HbA1c G8 analyser. Clin Chem Lab Med. 2010;48(3):365-371. [DOI] [PubMed] [Google Scholar]
- 15. Little RR, Rohlfing CL. The long and winding road to optimal HbA1c measurement. Clin Chim Acta. 2013;418:63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40(1):78-89. [DOI] [PubMed] [Google Scholar]
- 17. Andreis E, Appel M., Küllmer K. Kalibrierung von Messsystemen zur Blutzuckerselbstkontrolle. Diabetes Stoffw Herz. 2013;22:149-155. [Google Scholar]
- 18. Miedema K. Standardization and reference method for HbA1c. In: Baba S, Kaneko T, eds. Diabetes 1994. Amsterdam, Netherlands: Elsevier; 1995:1021-1025. [Google Scholar]
- 19. Hoelzel W, Miedema K. Development of a reference system for the international standardization of HbA1c/glycohemoglobin determinations. J Int Fed Clin Chem. 1996;8(2):62-67. [PubMed] [Google Scholar]
- 20. Bundesärztekammer. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen. Deutsches Ärzteblatt. 2008;105(7):341-355. [Google Scholar]
- 21. Referenzinstitut für Bioanalytik. Ringversuche. 2013. Available at: http://www.dgkl-rfb.de/cgi/displayAnaStats?rv_type=GH8crvTypeForDetails=GH8cyear=20138crv_num=18canaIyte=all&searchType=rv_type&anaV=l.
- 22. Pfützner A, Mitri M, Musholt PB, et al. Clinical assessment of the accuracy of blood glucose measurement devices. Curr Med Res Opin. 2012;28(4):525-531. [DOI] [PubMed] [Google Scholar]
- 23. Freckmann G, Baumstark A, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther 2010; 12(3); 221-31 [DOI] [PubMed] [Google Scholar]
- 24. Cohen RM, Lindsell CJ. When the blood glucose and the HbA(1c) don’t match: turning uncertainty into opportunity. Diabetes Care. 2012;35(12):2421-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sacks DB. Hemoglobin A1c in diabetes: panacea or pointless? Diabetes. 2013;62(1):41-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheta D, Rusu E, Stirban A, Constantin C. HbA1c—Importance in diagnosis and treatment. Diabetes Stoffw Herz. 2012;21:371-382. [Google Scholar]
- 27. Heinemann L, Freckmann G, Koschinsky T. Considerations for an institution for evaluation of diabetes technology devices to improve their quality in the European Union. J Diabetes Sci Technol. 2013;7(2):542-547. [DOI] [PMC free article] [PubMed] [Google Scholar]