Abstract
Objective: The purpose of this study was to investigate whether or not adding a fixed preprandial dose of inhaled insulin to a fully automated closed loop artificial pancreas would improve the postprandial glucose control without adding an increased risk of hypoglycemia. Research Design and Methods: Nine subjects with T1DM were recruited for the study. The patients were on closed-loop control for 24 hours starting around 4:30 pm. Mixed meals (~50 g CHO) were given at 6:30 pm and 7:00 am the following day. For the treatment group each meal was preceded by the inhalation of one 10 U dose of Technosphere Insulin (TI). Subcutaneous insulin delivery was controlled by a zone model predictive control algorithm (zone-MPC). At 11:00 am, the patient exercised for 30 ± 5 minutes at 50% of predicted heart rate reserve. Results: The use of TI resulted in increasing the median percentage time in range (70-180 mg/dl, BG) during the 5-hour postprandial period by 21.6% (81.6% and 60% in the with/without TI cases, respectively, P = .06) and reducing the median postprandial glucose peak by 33 mg/dl (172 mg/dl and 205 mg/dl in the with and without TI cases, respectively, P = .004). The median percentage time in range 80-140 mg/dl during the entire study period was 67.5% as compared to percentage time in range without the use of TI of 55.2% (P = .03). Conclusions: Adding preprandial TI (See video supplement) to an automated closed-loop AP system resulted in superior postprandial control as demonstrated by lower postprandial glucose exposure without addition hypoglycemia.
Keywords: artificial pancreas, glucose control, zone-MPC, closed-loop, unannounced meals, Health Monitoring System, hypoglycemia mitigation, telemedicine, type 1 diabetes, inhaled insulin
Intensive glycemic control in type 1 diabetes has been shown to reduce the incidence and progression of microvascular and macrovascular complications.1,2 One validated way to improve glycemic control is the use of insulin pumps and/or continuous glucose monitors (CGMs).3-5 However, many patients do not wear or manage the devices optimally6-9 and clinical penetration is far from complete, even in the top clinical centers.10 The limited and/or suboptimal use of diabetes technology can be traced, in part, to the “hassle factor,” the substantial degree of time, effort, patience, and appropriate decision-making that are required to monitor, operate, and maintain the devices. Thus, systems that automatically modify insulin delivery based on glucose data could facilitate more effective glycemic management, better quality of life, and wider use of diabetes technologies. Such a partially or fully closed-loop system, often referred to as an artificial pancreas (AP), could also lower the risk of acute hypoglycemia by reducing insulin delivery based on the prediction of the control algorithm. Developing an AP has been identified as a priority by both patient advocacy organizations11 and federal health agencies.12 However, for an automated system to improve on the safety and efficacy of current diabetes management, several difficulties must be overcome.
One of the biggest challenges for AP systems is meal time (prandial) glucose control. If the AP system delivers insulin only after the CGM values have started to rise (eg, closed-loop control), then the postprandial glucose excursion will not actually be “covered” by any insulin action for approximately an hour after the start of the meal, due to the slow absorption rate of the subcutaneous insulin injection. Moreover, once the glucose concentration has been lowered back to its target level, some insulin might still be active in the body, due to the slow clearance of currently available insulins when administered subcutaneously. The depot of active insulin can lead to low blood glucose (BG) several hours after the meal (late postprandial hypoglycemia).13 These postprandial risks do not preclude the possibility of safe, effective fully closed-loop control in controlled clinical settings.14 However, the risks can be mitigated with manual, “feedforward” delivery of rapid-acting insulin.15,16 Thus in many AP trials, all or part of patients’ meal time insulin has been calculated and delivered in a manual feedforward manner.17-21
The use of manual subcutaneous insulin boluses in an otherwise closed-loop system has several downsides. For rapid-acting insulin analogs delivered by insulin pump, postprandial control is better with a bolus delivered roughly 15 minutes before a meal rather than right at the start of eating.22 However, preprandial insulin delivery also poses a hypoglycemia risk if a patient eats later or less than expected. Another problem is that both meal announcement and manual bolus require constant diabetes management by the user. This may limit the quality-of-life benefits of an AP system and increase the potential for human error. Also, regardless of when subcutaneous insulin is delivered, its action profile does not closely match that of endogenous insulin release in people without diabetes.23
Among the key differences between subcutaneous insulin delivery and physiological insulin release is the fact that people without diabetes experience first-phase insulin secretion in response to food stimuli (cephalic insulin response). This spike in blood insulin concentration can begin at, or even in anticipation of, the first ingestion of food.24,25 By contrast, subcutaneously injected rapid-acting insulin analogs show a comparatively smooth ascent up to their peak blood concentration (over 40 minutes after injection) and peak glycemic effect (well over an hour after injection).26,27 The action profile of subcutaneously delivered insulin analogs can be enhanced by a variety of methods under investigation, including coadministration with recombinant human hyaluronidase,28 coformulation with disodium EDTA and citrate,29 and heating the site of infusion or injection;30 each of these methods could be useful in an AP. Even more favorable pharmacokinetics and pharmacodynamics might be achievable through a different route of insulin administration, such as intraperitoneal31 or—as we describe in this article—pulmonary.
We have previously proposed that for type 1 diabetes patients using AP systems, both glucose control and quality of life could improve with the meal time use of inhalable Technosphere® Insulin (TI).32 TI (Afrezza™; MannKind Corporation, Valencia, CA) consists of recombinant regular human insulin in a dry powder that can be delivered to the lungs using a breath-powered inhaler (Dreamboat™). For decades scientists have recognized that the lungs’ large surface area and high perfusion could allow for rapid pulmonary uptake of insulin,33 and TI’s profile is particularly favorable. In healthy volunteers without diabetes, TI reached peak insulin concentration in 12-17 minutes and peak glycemic effect at 42-58 minutes, significantly faster than that of regular human insulin; TI’s duration of action was also significantly shorter.34 A similar TI action profile has been reported in patients with type 2 diabetes, among whom TI suppresses endogenous glucose production faster than insulin lispro.35,36 In patients with type 1 diabetes using insulin glargine, a randomized trial showed that prandial TI led to significantly lower fasting and 1- and 2-hour postprandial glucose values compared to prandial insulin lispro, with a lower hypoglycemia event rate.37 Several other published clinical studies have further supported TI’s safety and efficacy as a prandial insulin in type 1 diabetes29,38,39 and type 2 diabetes.29,40,41 At the time of the study TI was an investigational drug that was approved on June 27, 2014, as an ultra rapid-acting insulin for oral inhalation indicated for the treatment of adults with type 1 or type 2 diabetes mellitus in the US by the food and drug administration.42,43
Because of its short action profile, TI can significantly blunt the postprandial glucose excursion even if delivered at the start of a meal rather than in advance. Alternatively, we hypothesize that a similar overall insulin action profile (and thus similar glycemic effects) could be achieved by using a “priming” TI dose followed by subcutaneous infusion of rapid-acting insulin. If this subcutaneous insulin delivery were given by an AP system, then the resulting regimen would be conceptually equivalent to adding unannounced (ie, controller’s lack of awareness of the TI action) meal time TI boluses to closed-loop insulin delivery. As an adjunct to a closed-loop system, TI could be delivered in the same dosage at every meal as a prophylactic dose if meal content is >50 g CHO, with the closed-loop system handling all the meal-specific adjustments. This would circumvent a major limitation of TI in open-loop management of type 1 diabetes: the fact that doses are quantized in increments equivalent to 3-4 units of rapid-acting insulin. Such a protocol would also reduce the burden of meal-size estimation for patients. Indeed, the only necessary calculation would be binary: whether a meal is so small that use of TI would introduce undue risk of hypoglycemia.
A combined TI/closed-loop regimen has been tested in silico with the Food and Drug Administration (FDA)-Accepted University of Virginia/Padova Metabolic Simulator.44 In that experiment, meal time doses of TI equivalent to 4 units of rapid-acting insulin were added to closed-loop subcutaneous insulin delivery as performed in the NIH-funded Control to Zone study.45 Compared to prandial boluses of subcutaneous insulin lispro, use of TI was projected to increase the time that patients spend in glycemic target without causing extra hypoglycemia risk. Higher doses of TI (20 U of TI, equivalent to 6-8 units of rapid-acting insulin) led to further reductions in hyperglycemia, but they also increased the anticipated rate of hypoglycemia.32
Herein we describe the first clinical trial of prandial TI as an adjunct to closed-loop insulin delivery.
Methods
Nine subjects with type 1 diabetes were recruited for the study, which was approved by the FDA and the Santa Barbara Cottage Health System’s Institutional Review Board. This study was a follow-up study to a fully automated AP design that did not use TI. The results summary of that study with the identical subjects IDs are discussed by Harvey et al.45 As discussed in Harvey et al,46 unannounced meals are still a challenge to an automated AP without a priming meal bolus. The use of TI as a simple and user-friendly priming meal bolus that is not announced to the AP system as an alternative to a prandial meal bolus to assist with meal control is the focus of this follow-up study. All subjects signed the Institutional Review Board–approved informed consent form. Inclusion criteria included age between 21 and 65 years, type 1 diabetes duration of at least 1 year, and use of an insulin pump with rapid-acting insulin for at least 6 months. Exclusion criteria included pregnancy, diabetic ketoacidosis within the past 6 months, A1C > 9.0%, severe hypoglycemia within the past year, concomitant disease or medication use affecting metabolic control, diabetic ketoacidosis within 6 months prior to enrollment, severe hypoglycemia resulting in seizure or loss of consciousness within 12 months prior to enrollment, pregnancy, being a nursing mother, active infection, active gastroparesis, abnormal spirometry, having smoked habitually within the 6 months prior to enrollment, and high insulin sensitivity (insulin-to-carbohydrate ratio greater than 1:12). Subjects were screened with a comprehensive metabolic panel, complete blood count, and thyroid tests, height and weight were measured, and the subject’s insulin pump information was downloaded and confirmed (basal rates, average total daily dose, insulin-to-CHO ratios, and correction factors).
The communication platform for closed-loop control was the Artificial Pancreas System (APS).47 The CGM used was the Dexcom G4™ Platinum (Dexcom Inc, San Diego, CA), which was unmodified from its FDA-approved version. Continuous subcutaneous insulin infusion (CSII) was performed with the OmniPod® Insulin Management System (Insulet Corp, Bedford, MA). Each volunteer received the same rapid-acting insulin analog used in their typical diabetes management: either insulin aspart (NovoLog®; Novo Nordisk A/S, Bagsvaerd, Denmark), insulin lispro (HumaLog®; Eli Lilly and Co, Indianapolis, IN), or insulin glulisine (Apidra®; Sanofi SA, Paris, France).
Subcutaneous insulin delivery was controlled by a zone model predictive control algorithm (zone-MPC), which modified insulin delivery only if current or predicted CGM values were outside of the prespecified zone: 80-140 mg/dl.48 The controller was initialized using only each patient’s total daily insulin dose. The system also had knowledge of the individual’s nominal basal profile. Operating simultaneously to the zone-MPC algorithm was a safety algorithm; the Health Monitoring System (HMS), which independently analyzed CGM data.49 If the HMS predicted that sensor glucose would fall below 70 mg/dl within the next 15 minutes, it would alert the main APS interface and send a text message to the attending physician. The HMS suggested giving the subject roughly 16 g of carbohydrate (CHO).
Two to 3 days before each patient visited the clinical research center (CRC), she or he made an outpatient visit to have 2 CGM sensors inserted by the study staff and to receive training in CGM use. For 2 to 3 days, the CGM was used in blinded mode and calibrated according to manufacturer’s instructions with the OneTouch® Ultra® Blood Glucose Monitoring System (LifeScan, Inc, Milpitas, CA). Two to 3 days after sensor insertion, the patient arrived at the CRC at approximately 4:00 pm, and the CGM was unblinded. If the patient’s BG was greater than 250 mg/dl on arrival, the visit was rescheduled; otherwise, closed-loop control was initiated at roughly 4:30 pm (all times are ± 30 minutes). Mixed meals (~50 g CHO) were given at 6:30 pm and 7:00 am the next day. Each meal was preceded by the inhalation of one 10 U dose of TI via the Dreamboat inhaler (MannKind Corp, Valencia, CA), roughly equivalent to 4 U of subcutaneous insulin. See the online video supplement for demonstration.
Patients were also studied for a similar time period using the exact same protocol except for the fact that no priming bolus was given. At 11:00 am, the patient was given a snack (~16 g CHO) if her or his BG was < 120 mg/dl per protocol and then exercised on a stationary bike for 30 ± 5 minutes at 50% of her or his predicted heart rate reserve based on the formula of Karvonen et al.50 At 2:00 pm, the patient was given another snack (~16 g CHO). Closed-loop control ended at 4:30 pm, roughly 24 hours after it had begun. Food intake, TI inhalation, and exercise were not announced to the closed-loop control system. However, during the closed-loop period, the CGM was calibrated roughly 30 minutes before each meal and before bedtime in addition to the calibrations that followed the manufacturer’s instructions.
Throughout the CRC visit, reference BG values were measured with the YSI 2300 STAT Plus (YSI Life Sciences, Yellow Springs, OH): every 15 ± 5 minutes during hypoglycemia (YSI BG < 70 mg/dl or CGM-predicted glucose level of 70 mg/dl in the next 15 minutes, until YSI BG > 80 mg/dl), every 15 ± 5 minutes during extreme hyperglycemia (YSI BG > 400 mg/dl, until YSI BG < 300 mg/dl), every 15 ± 5 minutes during and 1 hour after exercise, and every 30 ± 10 minutes otherwise.
Results
The study’s primary endpoint was the comparison of the glucose time in range (70-180 mg/dl) during the 5-hour postprandial period with and without the use of TI. Secondary endpoints included analyses of hypo- and hyperglycemic excursions (minimum and maximum values), time spent outside target range, whether outside intervention was provided, as well as the time in range for the following periods: 70-150 mg/dl during and for 3 hours after exercise and 80-140 mg/dl during the entire study period, including the overnight period (12:00 am to 7:00 am).
The 9 subjects with type 1 diabetes participated in both 24-h closed-loop sessions. Demographics and closed-loop details are shown in Table 1. A summary of results is shown in Figure 1, with data comparing cumulative percentage time in range including both YSI BG and CGM. Figure 2 presents percentage time in different glycemic ranges based on YSI BG for both arms (with and without TI) for the full duration of the study (panel A), 5 hours postdinner and postbreakfast (panel B and C, respectively), 3 hours postexercise and overnight (panels D and E, respectively). The median percentage time in range (70-180 mg/dl, YSI BG) during the 5-hour postprandial period with the use of TI was 81.6% (IQ range 72.5-85%, Breakfast 75%, Dinner 93.3%) as compared to percentage time in range without the use of TI 60.0% (IQ range 50.8-75.8%, breakfast 61.6%, dinner 56.6%, P = .059). The median percentage time in range 80-140 mg/dl during the entire study period was 67.5% (IQ range 48.5-73.1%) as compared to percentage time in range without the use of TI of 55.2% (IQ range 52.0-60.1%, P = .032).
Table 1.
Results Summary as Provided by YSI BG and CGM With Demographic and Clinical Parameters of the Individual Subjects.
| Subject ID | Total/mean ± SD | 1 | 2 | 3 | 6 | 7 | 8 | 9 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 6 F/3 M | F | M | M | F | F | F | M | F | F |
| Height (cm) | 170 ± 10 | 157 | 167 | 193 | 165 | 165 | 175 | 174 | 165 | 163 |
| Weight (kg) | 71 ± 17 | 61.4 | 94.1 | 95.5 | 66 | 59.5 | 65.5 | 90 | 61.4 | 48.7 |
| Age (years) | 49 ± 10 | 40 | 43 | 57 | 43 | 53 | 29 | 62 | 56 | 54 |
| TDD (U) | 35 ± 15 | 20 | 69.1 | 40.2 | 37.5 | 23.1 | 36.6 | 38.4 | 26.1 | 21.5 |
| CF (mg/dl/U) | 55 ± 24 | 70 | 27 | 33 | 35 | 100 | 50 | 40 | 60 | 80 |
| C:I (g CHO/U) | 11 ± 2.3 | 15 | 8 | 10 | 15 | 10 | 9 | 12 | 10 | 11 |
| Duration of diabetes (years) | 29 ± 14 | 24 | 22 | 44 | 15 | 44 | 8 | 39 | 18 | 46 |
| Duration of CL (hours) | 24 ± 1.2 | 21.5 | 24 | 24 | 23.9 | 23.9 | 24 | 25 | 24 | 21.4 |
| Number of HMS treatments | 3.4 ± 2.2 | 2 | 5 | 5 | 1 | 6 | 0 | 4 | 6 | 2 |
| Sensor glucose (CGM) | ||||||||||
| BG at start of CL (mg/dl) | 120 ± 27 | 85 | 87 | 140 | 130 | 129 | 161 | 108 | 116 | 87 |
| BG at end of CL (mg/dl) | 130 ± 39 | 81 | 141 | 154 | 159 | 195 | 112 | 122 | 79 | 100 |
| BG at start of dinner (mg/dl) | 108 ± 36 | 71 | 95 | 91 | 85 | 120 | 189 | 88 | 137 | 93 |
| BG at 5 hours after dinner (mg/dl) | 128 ± 30 | 142 | 102 | 91 | 179 | 102 | 141 | 101 | 141 | 152 |
| BG at start of breakfast (mg/dl) | 118 ± 22 | 109 | 100 | 94 | 140 | 103 | 133 | 120 | 103 | 158 |
| BG at 5 hours after breakfast (mg/dl) | 109 ± 34 | 168 | 98 | 74 | 96 | 131 | 135 | 81 | 136 | 65 |
| BG at start of exercise (mg/dl) | 150 ± 25 | 193 | 162 | 144 | 138 | 133 | 189 | 156 | 135 | 123 |
| BG 3 hours after exercise (mg/dl) | 100 ± 11 | 95 | 81 | 106 | 109 | 121 | 101 | 96 | 93 | 100 |
| LBGI | 0.66 ± 0.54 | 0.37 | 0.31 | 1.41 | 0.10 | 0.51 | 0.03 | 1.20 | 0.60 | 1.45 |
| HBGI* | 2.7 ± 1.8 | 2.90 | 1.61 | 0.59 | 4.18 | 1.66 | 6.38 | 2.01 | 3.32 | 1.38 |
| Maximum BG (mg/dl)* | 230 ± 42 | 230 | 220 | 186 | 241 | 214 | 239 | 231 | 329 | 185 |
| Minimum BG (mg/dl) | 68 ± 12 | 68 | 72 | 71 | 76 | 61 | 92 | 52 | 67 | 53 |
| Time at glucose level (%) | ||||||||||
| <60 | 0.43 ± 0.87 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| <70 | 1.6 ± 2.4 | 1 | 0 | 0 | 0 | 1 | 0 | 5 | 1 | 6 |
| <80 | 6.8 ± 6.8 | 5 | 3 | 6 | 1 | 4 | 0 | 11 | 9 | 23 |
| 80-140* | 60 ± 16 | 57 | 77 | 79 | 42 | 62 | 39 | 74 | 65 | 41 |
| 140-180 | 20 ± 10 | 23 | 10 | 14 | 32 | 22 | 26 | 4 | 14 | 35 |
| 180-250* | 13 ± 11 | 16 | 10 | 1 | 25 | 12 | 35 | 12 | 4 | 2 |
| >250 | 0.82 ± 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 |
| Plasma glucose (YSI BG) | ||||||||||
| BG at start of CL (mg/dl) | 110 ± 37 | 76.2 | 74.5 | 95.2 | 130 | 109 | 192 | 105 | 84.2 | 85.8 |
| BG at end of CL (mg/dl) | 120 ± 39 | 48.9 | 118 | 164 | 158 | 161 | 101 | 122 | 98.4 | 82.8 |
| BG at start of dinner (mg/dl) | 101 ± 33 | 67 | 89 | 83 | 94 | 100 | 183 | 89 | 109 | 94 |
| BG at 5 hours after dinner (mg/dl) | 121 ± 31 | 122 | 121 | 82 | 168 | 82 | 139 | 91 | 138 | 152 |
| BG at start of breakfast (mg/dl) | 116 ± 24 | 106 | 94 | 104 | 153 | 89 | 123 | 136 | 95 | 146 |
| BG at 5 hours after breakfast (mg/dl) | 106 ± 31 | 101 | 81 | 102 | 156 | 121 | 131 | 78 | 132 | 58 |
| BG at start of exercise (mg/dl) | 150 ± 19 | 172 | 134 | 154 | 124 | 140 | 174 | 134 | 130 | 164 |
| BG 3 hours after exercise (mg/dl) | 94 ± 22 | 60.1 | 69.1 | 100 | 106 | 108 | 83.3 | 125 | 113 | 82.8 |
| LBGI | 1.1 ± 0.88 | 1.21 | 0.88 | 2.31 | 0.10 | 1.24 | 0.11 | 0.82 | 0.99 | 2.72 |
| HBGI* | 1.9 ± 1.3 | 0.82 | 0.96 | 0.85 | 3.71 | 0.95 | 4.38 | 1.95 | 2.28 | 1.24 |
| Maximum BG (mg/dl) | 210 ± 25 | 194 | 191 | 196 | 229 | 204 | 211 | 227 | 269 | 199 |
| Minimum BG (mg/dl) | 65 ± 13 | 48 | 66.6 | 64.3 | 81.3 | 67.3 | 83.3 | 64.3 | 60.4 | 45.9 |
| Time at glucose level (%) | ||||||||||
| <60 | 0.81 ± 1.7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| <70 | 2.5 ± 2.7 | 5 | 2 | 3 | 0 | 1 | 0 | 1 | 1 | 8 |
| <80 | 11 ± 8.8 | 8 | 8 | 20 | 0 | 13 | 0 | 10 | 12 | 27 |
| 80-140* | 63 ± 15 | 77 | 79 | 64 | 40 | 73 | 46 | 73 | 68 | 48 |
| 140-180 | 16 ± 13 | 12 | 6 | 11 | 45 | 7 | 27 | 4 | 11 | 19 |
| 180-250 | 9.6 ± 7.6 | 2 | 7 | 5 | 15 | 7 | 27 | 12 | 6 | 5 |
| >250 | 0.49 ± 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
BG, blood glucose; CF, correction factor ; CGM, continuous glucose monitor; C:I, carbohydrate to insulin ratio; CL, closed-loop; HBGI, high blood glucose index; HMS, health monitoring system; LBGI, low blood gluucose index. *Indicates the P value from the paired t test < .05 comparing the results of the TI and the no TI. Subjects 4, 5, and 10 did not participate in the TI study. This study was a follow-up study to a fully automated artificial pancreas design that did not use TI. The results of that study with the identical subjects IDs are discussed by Harvey et al.46
Figure 1.
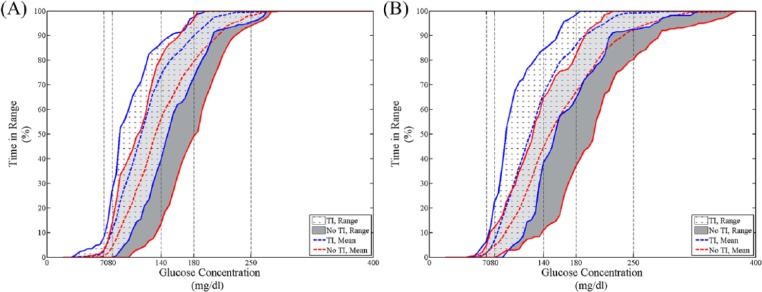
Cumulative percentage time in different ranges for all subjects in the TI case (white with cross marks) and the no TI case (gray) based on YSI BG (A) and CGM (B). The mean values for the TI case and the no TI case are represented by the blue dashed line and the red dashed line, respectively.
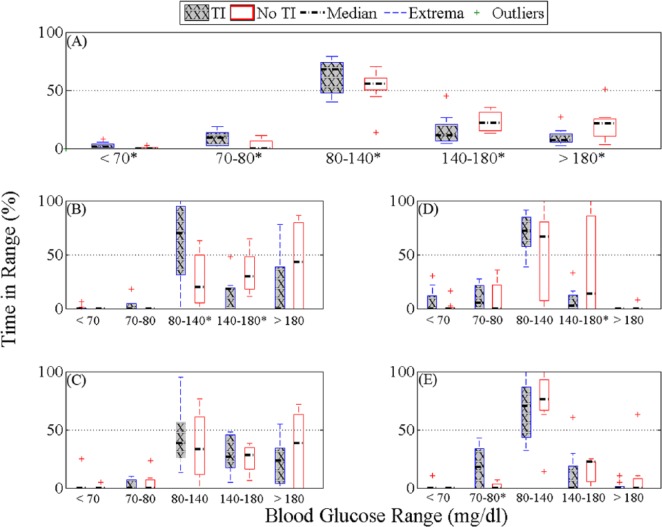
Figure 2.
Percentage time in different glycemic ranges based on YSI BG in the TI case (gray with diagonal line) and the no TI case (white) for the whole study (A), start of dinner to 5 hours after (B), start of breakfast to 5 hours after (C), start of exercise to 3 hours after (D), and the overnight period (12:00 am to 7:00 am) (E). *P value from the paired t test < .05 comparing the results of the TI and the no TI cases.
The median percentage time in range (70-150 mg/dl, YSI BG) during and for 3 hours after exercise was 94.4% (IQ range 91.6-94.4%) as compared to percentage time in range without the use of TI 83.3% (IQ range 30.5-94.4%, P = .19). Three hours after exercise the median glucose was 100 mg/dl (IQ range 82.8-107 mg/dl) with TI and 120 mg/dl (IQ range 112-127 mg/dl) without TI (P = .02). There was no significant difference during the overnight period (12:00 am to 7:00 am) in range (70-180 mg/dl, YSI BG), the median BG during the period in the range were 100% (IQ range 95.2-100%) with the use of TI as compared to percentage time in range without the use of TI 100% (IQ range 92.9-100%, P = .18).
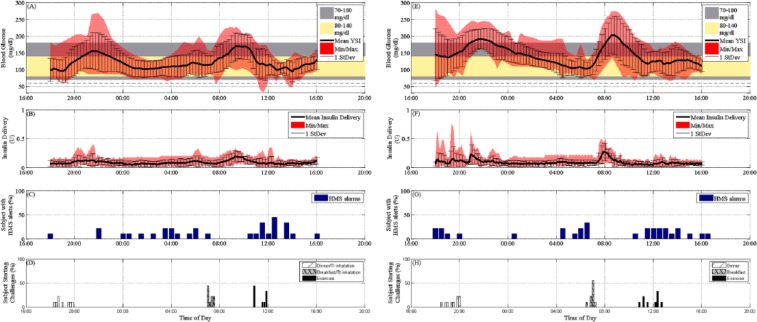
The total median insulin/24 hours was 29.6 U for the TI group (21.6 U of SC + 8 U of TI) and 20.9 U for the group without TI (Figures 3B and 3F, respectively). The percentage of subjects experiencing HMS alerts per 30 minutes is shown in Figure 3C and Figure 3G for the TI arm and the non-TI arm, respectively. There was no statistical difference in 30-minute alarms. Information regarding the starting time of each of the 3 unannounced challenges for both studies (dinner, breakfast, exercise), if shown as the percentage of subjects who have commenced the challenge (Figure 3D and Figure 3H for the TI and non-TI groups, respectively). All the results of the individual subjects are available in Online Supplementary Figures 1 to 9.
Figure 3.
Glucose summary results of all TI trials are summarized in (A) as mean YSI BG, 1 standard deviation (SD), and minimum and maximum YSI BG values. The control objective (80-140 mg/dl) is shown as the light yellow band and the clinically accepted region (70-180 mg/dl) as the gray band. The mean insulin delivery via CSII for all trials is shown in (B) along with 1 SD and the minimum and maximum of the insulin delivery values. The percentage of subjects received HMS alarms in a 30-minute interval is shown in (C). The time of starting the challenges and TI inhalation is shown in (D). Glucose summary results of all no TI trials are summarized in (E) as mean YSI BG, 1 SD, and minimum and maximum YSI BG values. The control objective (80-140 mg/dl) is shown as the light yellow band and the clinically accepted region (70-180 mg/dl) as the gray band. The mean insulin delivery via CSII for all trials is shown in (F) along with 1 SD and the minimum and maximum of the insulin delivery values. The percentage of subjects received HMS alarms in a 30-minute interval is shown in (G). The time of starting the challenges and TI inhalation is shown in (H).
As can be seen in the glucose tracing in Figure 3A, there was an overall a mild postprandial rise in glucose concentrations as compared to the non-TI case (Figure 3E). Some subjects had a higher postprandial peak, generally when they had started at a higher value at meal time. Nevertheless, a significant reduction in postprandial excursion was observed with the TI group compared to the control group (median delta YSI BG of 57 mg/dl and 95.5 mg/dl from the baseline at meal time by YSI BG in the with and without TI cases, respectively, P = .006). After dinner, the median delta glucose peaks by YSI BG were 64 mg/dl (IQ range, 30-120 mg/dl) and 85 mg/dl (IQ range, 50-112 mg/dl) in the with and without TI cases, respectively, with P values of .15, and the median peak times by YSI BG were at 150 (IQ range, 120-180 minutes) and 121 minutes (IQ range, 105-159 minutes) in the with and without TI cases, respectively, with P values of .18. Median baseline predinner glucose values by YSI BG were 94 mg/dl (IQ range, 89-100 mg/dl) and 124 mg/dl (IQ range, 91-134 mg/dl) in the with and without TI cases, respectively.
After breakfast, the median peak postprandial glucose excursion was 61 mg/dl (IQ range, 56-85 mg/dl) and 99 mg/dl (IQ range, 75-118 mg/dl) by YSI with and without TI, respectively, with a P value of .008, and the median time to peak occurred at 120 minutes (IQ range, 90-150 minutes) and 107 minutes (IQ range, 95-131 minutes) in the with and without TI cases, respectively, with a P value of .22. Median baseline prebreakfast glucose values by YSI BG were 106 mg/dl (IQ range, 95-136 mg/dl) and 110 mg/dl (IQ range, 89-131 mg/dl) in the with and without TI cases, respectively.
Mild activity was undertaken for all subjects for 30 minutes beginning between 11:10 am and 12:40 pm for the TI case and between 11:15 am to 12:20 pm for the control group. The median YSI values were relatively steady during exercise and for the rest of the study, with median values at the start of exercise by YSI BG of 133 mg/dl (IQ range, 131-159 mg/dl) and 156 mg/dl (IQ range, 127-163 mg/dl) in the with and without TI cases, respectively, and median peak YSI BG values of 142 mg/dl (IQ range, 133-159 mg/dl) and 172 mg/dl (IQ range, 127-177 mg/dl, P = .2) in the with and without TI cases, respectively.
Conclusions
A major rate-limiting factor in the development of an AP is the delay in insulin action associated with currently available rapid acting insulin analogs. Previous investigations have demonstrated that meal control is one of the major challenges of an automated insulin management and even a premeal bolus has its limitation in preventing postprandial hyperglycemia. We have previously demonstrated that fully automated AP closed-loop systems can safely control glucose concentrations in patients with type 1 diabetes under limited CHO consumption. The postprandial performance of this type of system as currently designed is less than ideal, since if one wants optimal control, meal sizes would need to be restricted or one would need to have a system with the addition of glucagon. The addition of an ultra-rapid insulin at meal time allows for superior postprandial control. Using this hybrid semiautomated approach has several advantages. First the patient would have to do only a rough estimation of the CHO content. The controller will take care of any remaining insulin requirement. Second, the use of TI mimics first phase secretion of insulin with a fast-peak and rapid clearance, which results in lower postprandial peaks and less late postprandial hypoglycemia. Third, its use also results in both lower insulin delivery and glucose concentration variability.
The convenience/ease of use was commented on by all of the study participants. Adding TI to an automated closed-loop AP system results in superior postprandial control as demonstrated by lower postprandial glucose exposure by 81% and 60% for dinner and breakfast, respectively, without additional hypoglycemia. There is less variability in both insulin delivery and glucose concentrations in the TI arm. As seen in most AP studies to date there was no difference in glucose control during the overnight period.
We acknowledge that we are giving a partial premeal bolus for the TI arm, but we wanted to demonstrate that the addition of an ultra-rapid insulin to an automated AP system improves postprandial control without the need to estimate the meal content. We acknowledge that glucose control in the postbreakfast period is still difficult to control. We anticipate the in future studies there would be a proportional dose escalation around periods of increased insulin resistance and meals with increased CHO intake. We also acknowledge that using a full meal announcement in combination with closed-loop control will most likely result in more time in range. This study was designed to demonstrate a proof of concept that giving a small, consistent bolus of ultra-rapid insulin that provides a first phase meal correction could improve the postprandial glucose profile as compared to fully automated closed-loop control with very minimal user intervention or need to count CHOs and enter estimation to the closed-loop system.
Acknowledgments
MannKind Corporation provided Technosphere pharmacokinetics and pharmacodynamics data. Joseph P. Shivers (Sansum Diabetes Research Institute) assisted with the writing of the manuscript. We also acknowledge product support from Insulet Corp, Dexcom Inc, and Lifescan Inc. Clinicaltrials.gov NCT01874392. Video supplement: http://thedoylegroup.org/ultra-rapid-acting-inhaled-insulin-trial-funded-by-jdrf/.
Footnotes
Abbreviations: AP, artificial pancreas; APS, Artificial Pancreas System; BG, blood glucose; CGM, continuous glucose monitor; CHO, carbohydrate; CRC, clinical research center; CSII, continuous subcutaneous insulin infusion; FDA, Food and Drug Administration; HMS, Health Monitoring System; T1DM, type 1 diabetes mellitus; TI, Technosphere® Insulin; Zone-MPC, Zone Model Predictive Controller.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HZ was a consultant to Mankind during employment at Sansum Diabetes Research Institute.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the JDRF (17-2011-765) and the National Institutes of Health (DP3 DK094331 and R01 DK085628).
References
- 1. Abbes IB, Richard PY, Lefebvre MA, Guilhem I, Poirier JY. A closed-loop artificial pancreas using a proportional integral derivative with double phase lead controller based on a new nonlinear model of glucose metabolism. J Diabetes Sci Technol. 2013;7(3):699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15(5):386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yaturu S. Insulin therapies: current and future trends at dawn. World J Diabetes. 2013;4(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsukamoto Y, Kinoshita Y, Kitagawa H, et al. Evaluation of a novel artificial pancreas: closed loop glycemic control system with continuous blood glucose monitoring. Artif Organs. 2013;37(4):E67-E73. [DOI] [PubMed] [Google Scholar]
- 5. Nimri R, Danne T, Kordonouri O, et al. The “Glucositter” overnight automated closed loop system for type 1 diabetes: a randomized crossover trial. Pediatr Diabetes. 2013;14(3):159-167. [DOI] [PubMed] [Google Scholar]
- 6. Thabit H, Hovorka R. Closed-loop insulin delivery in type 1 diabetes. Endocrinol Metab Clin North Am. 2012;41(1):105-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013;185(4):297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayrak ES, Turksoy K, Cinar A, Quinn L, Littlejohn E, Rollins D. Hypoglycemia early alarm systems based on recursive autoregressive partial least squares models. J Diabetes Sci Technol. 2013;7(1):206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Heusden K, Dassau E, Zisser HC, Seborg DE, Doyle FJ., III Control-relevant models for glucose control using a priori patient characteristics. IEEE Trans Biomed Eng. 2012;59(7):1839-1849. [DOI] [PubMed] [Google Scholar]
- 10. Valla V. Continuous subcutaneous insulin infusion (CSII) pumps. Adv Exp Med Biol. 2012;771:414-419. [DOI] [PubMed] [Google Scholar]
- 11. Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther. 2009;11:S113-S119. [DOI] [PubMed] [Google Scholar]
- 12. Stenvers DJ, Devries JH, la Fleur SE. What’s the time? Does the artificial pancreas need to know? Diabetes. 2013;62(7):2173-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344-3350. [DOI] [PubMed] [Google Scholar]
- 14. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a fully automated personalized artificial pancreas with an unannounced meal using mpMPC and insulin-on-board. Diabetes Care. 2013;36:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934-939. [DOI] [PubMed] [Google Scholar]
- 16. Ricotti L, Assaf T, Dario P, Menciassi A. Wearable and implantable pancreas substitutes. J Artif Organs. 2013;16(1):9-22. [DOI] [PubMed] [Google Scholar]
- 17. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35(11):2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743-751. [DOI] [PubMed] [Google Scholar]
- 19. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96(5):1402-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castle JR, Engle JM, Youssef JE, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33(6):1282-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JJ, Dassau E, Zisser H, Harvey RA, Jovanovic L, Doyle FJ., III In silico evaluation of an artificial pancreas combining exogenous ultrafast-acting technosphere insulin with zone model predictive control. J Diabetes Sci Technol. 2013;7(1):215-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polonsky KS, Given BD, Hirsch L, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988;81(2):435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brange J. Chemical stability of insulin. 4. Mechanisms and kinetics of chemical transformations in pharmaceutical formulation. Acta Pharm Nord. 1992;4(4):209-222. [PubMed] [Google Scholar]
- 25. Harvey RA, Dassau E, Zisser HC, et al. Clinically relevant hypoglycemia prediction metrics for event mitigation. Diabetes Technol Ther. 2012;14(8):719-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teutsch SM, Herman WH, Dwyer DM, Lane JM. Mortality among diabetic patients using continuous subcutaneous insulin-infusion pumps. New Engl J Med. 1984;310(6):361-368. [DOI] [PubMed] [Google Scholar]
- 27. Siperstein MD. Diabetic ketoacidosis and hyperosmolar coma. Endocrinol Metab Clin North Am. 1992;21(2):415-432. [PubMed] [Google Scholar]
- 28. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824-833. [DOI] [PubMed] [Google Scholar]
- 29. Dassau E, Hennings T, Fazio J, Atlas E, Phillip M. Closing the loop. Diabetes Technol Ther. 2013;15(suppl 1):S29-S39. [DOI] [PubMed] [Google Scholar]
- 30. Raz I, Weiss R, Yegorchikov Y, Bitton G, Nagar R, Pesach B. Effect of a local heating device on insulin and glucose pharmacokinetic profiles in an open-label, randomized, two-period, one-way crossover study in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Clin Ther. 2009;31(5):980-987. [DOI] [PubMed] [Google Scholar]
- 31. Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery. Diabetes Care. 2010;33(1):121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeFelippis MR, Chance RE, Frank BH. Insulin self-association and the relationship to pharmacokinetics and pharmacodynamics. Crit Rev Ther Drug Carrier Syst. 2001;18(2):201-264. [PubMed] [Google Scholar]
- 33. Gaensslen M. Uber inhalation von Insulin. Klin Wochenschr. 1925;2:71-72. [Google Scholar]
- 34. Rave K, Potocka E, Boss AH, Marino M, Costello D, Chen R. Pharmacokinetics and linear exposure of AFRESA compared with the subcutaneous injection of regular human insulin. Diabetes Obes Metab. 2009;11(7):715-720. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Rajaraman S, DeHennis A. Real-time lag compensation for a subcutaneously implanted fluorescent glucose sensor. Paper presented at: 6th Conference on Advanced Technologies & Treatments for Diabetes, 2013; Paris, France. [Google Scholar]
- 36. Saudek CD, Selam JL, Pitt HA, et al. A preliminary trial of the programmable implantable medication system for insulin delivery. N Engl J Med. 1989;321(9):574-579. [DOI] [PubMed] [Google Scholar]
- 37. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457-2471. [DOI] [PubMed] [Google Scholar]
- 38. Renard E. Implantable insulin delivery pumps. Minim Invasive Ther Allied Technol. 2004;13:328-335. [DOI] [PubMed] [Google Scholar]
- 39. Colvin AE, Jiang H. Increased in vivo stability and functional lifetime of an implantable glucose sensor through platinum catalysis. J Biomed Mater Res A. 2013;101:1274-1282. [DOI] [PubMed] [Google Scholar]
- 40. Broussolle C, Jeandidier N, Hanaire-Broutin H. French multicentre experience of implantable insulin pumps. The EVADIAC Study Group. Evaluation of Active Implants in Diabetes Society. Lancet. 1994;343(8896):514-515. [DOI] [PubMed] [Google Scholar]
- 41. Benefit/risk evaluation of insuman implantable versus insuplant using medtronic minimed implantable pump system in patients with type 1 diabetes. ClinicalTrials.gov NCT01194882; 2010.
- 42. Food and Drug Administration. FDA approves Afrezza to treat diabetes. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm403122.htm. Accessed August 28, 2014.
- 43. Klonoff DC. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties. J Diabetes Sci Technol. 2014;8(6):1071-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. In Silico model and computer simulation approximating the human glucose/insulin utilization: Food and Drug Administration Master File MAF-1521 [computer program]. 2008.
- 45. Zisser CH, Dassau E, Bevier W, Harvey RA, Jovanovic L, Doyle FJ., III Clinical evaluation of a fully-automated artificial pancreas using zone-model predictive control with health monitoring system. In: 72nd American Diabetes Association Meeting. Vol. 61 (suppl. 1). Philadelphia, PA: Diabetes 2012:LB8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harvey RA, Dassau E, Bevier WC, et al. Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol Ther. 2014;16(6):348-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dassau E, Jimenez ND, Zisser H, Jovanovič L, Doyle FJ., III The artificial pancreas system: a comprehensive system for the clinical evaluation of control algorithms. Paper presented at: 4th International Conference on Advanced Technologies & Treatments for Diabetes, 2011; London, UK. [Google Scholar]
- 48. Grosman B, Dassau E, Zisser HC, Jovanovič L, Doyle FJ., III Zone model predictive control: a strategy to minimize hyper- and hypoglycemic events. J Diabetes Sci Technol. 2010;4(4):961-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harvey RA, Dassau E, Zisser CH, Seborg DE, Jovanovič L, Doyle FJ., III Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol. 2012;6(2):1345-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307-315. [PubMed] [Google Scholar]