Abstract
Background: The PaQ® insulin delivery system is a simple-to-use patch-on device that provides preset basal rates and bolus insulin on demand. In addition to feasibility of use, safety, and efficacy (reported elsewhere), this study analyzed the impact of PaQ on patient-reported outcomes, including barriers to insulin treatment, diabetes-related distress, and attitudes toward insulin therapy in patients with type 2 diabetes on a stable multiple daily injection (MDI) regimen. Methods: This single-center, open-label, single-arm study comprised three 2-week periods: baseline (MDI), transition from MDI to PaQ, and PaQ treatment. Validated questionnaires were administered during the baseline and PaQ treatment periods: Barriers to Insulin Treatment questionnaire (BIT), Insulin Treatment Appraisal Scale (ITAS), and Problem Areas in Diabetes scale (PAID). Results: Eighteen patients (age 59 ± 5 years, diabetes duration 15 ± 7 years, 21% female, HbA1c 7.7 ± 0.7%) completed the questionnaires. There was a strong, significant effect of PaQ use in mean BIT total scores (difference [D] = −5.4 ± 0.7.7, P = .01, effect size [d] = 0.70). Patients perceived less stigmatization by insulin injection (D = −2.2 ± 6.2, P = .18, d = 0.35), increased positive outcome (D = 1.9 ± 6.6, P = .17, d = 0.29), and less fear of injections (1.3 ± 4.8, P = .55, d = 0.28). Mean change in ITAS scores after PaQ device use showed a nonsignificant improvement of 1.71 ± 5.63 but moderate effect size (d = 0.30, P = .14). No increase in PAID scores was seen. Conclusions: The results and moderate to large effects sizes suggest that PaQ device use has beneficial and clinically relevant effects to overcoming barriers to and negative appraisal of insulin treatment, without increasing other diabetes-related distress.
Keywords: type 2 diabetes, MDI, multiple daily insulin injections, insulin pumps, CSII, PAID, BIT, ITAS
Insulin therapy with basal-bolus regimens is generally considered to be the most physiological approach to achieve optimal glycemic control in individuals with type 2 diabetes requiring insulin therapy.1,2 Studies have also shown that an early intervention with basal-bolus therapy in newly diagnosed type 2 diabetes patients facilitates rapid improvement in glycemic control, reduces glycemic variability, and preserves beta-cell function.3,4 Holman et al5 demonstrated that simple insulin regimens, basal only, failed to achieve satisfactory glycemic control 3 years after initiation of insulin treatment in people with type 2 diabetes. This indicates that over time basal bolus insulin therapy is required to achieve glycemic control.
Administration of multiple daily insulin injections (MDI) by using an insulin syringe or pen device is the most common approach to basal-bolus therapy within the type 2 diabetes population; however, persistent adherence to MDI therapy is often inadequate6 and can result in suboptimal glycemic control.7 Potential barriers to MDI include the need to administer multiple injections, risk of hypoglycemia interference of injections with daily activities, injection pain, and embarrassment.6,8 Although continuous subcutaneous insulin infusion (CSII) has the potential to improve glycemic control and quality of life in individuals with type 2 diabetes who are suboptimally controlled with MDI therapy,9-13 it is not widely used in this population due to the complexity, extensive training requirements, and reimbursement issues associated with current insulin pump devices.14
Many current CSII devices feature advanced functions that allow users to utilize multiple basal insulin rates (in increments as precise as 0.01 units) and a variety of bolus insulin configurations (eg, extended, multiwave). However, evidence suggests that these functions, and the level of complexity they create, may be unnecessary for effective management of type 2 diabetes. Studies have shown that patients with type 2 diabetes are able to achieve good glycemic control with only 1 or 2 daily basal rates.9,15,16 Another recent study showed that type 2 diabetes patients using “simple bolusing” with predetermined dosages could achieve glycemic improvements compared with patients using automated bolus advisors in combination with carbohydrate counting.17 Given the demonstrated benefits of CSII therapy in type 2 diabetes, less complex, lower cost insulin delivery devices may be both clinically practical and economically feasible within this population.
PaQ® (CeQur SA, Horw, Switzerland) is a small and simple-to-use insulin delivery device that has been specifically designed for individuals with type 2 diabetes. Applied directly to the skin, the device infuses insulin at a constant basal rate for up to 3 days, utilizing 1 of 5 preset basal rates: 20 U/day (0.83 U/hour), 24 U/day (1.0 U/hour), 32 U/day (1.33 U/hour), 40 U/day (1.67 U/hour), and 50 U/day (2.08 U/hour). Users administer bolus insulin manually by pushing the bolus delivery button, which delivers 2 U of insulin with each button push.
We have recently reported findings from a 6-week, prospective, single-arm, single-center pilot study that evaluated the feasibility of use, safety and efficacy of PaQ in 20 individuals with type 2 diabetes currently treated with MDI.18 The 18 study participants who completed the study demonstrated competency in assembling, placing and using the device. Data from self-monitored blood glucose (SMBG) and masked continuous glucose monitoring (CGM) showed glycemic control similar to MDI with no increase in hypoglycemia. A secondary objective of this study was to analyze the impact of the use of PaQ on patient-reported outcomes (PROs), including barriers against insulin treatment, diabetes-related distress, and negative attitudes toward insulin therapy. This publication reports on these results as well as greater detail on patient satisfaction and device acceptance.
Research Design and Methods
The pilot study comprised three 2-week study periods: baseline (MDI therapy), transition from MDI to PaQ, and PaQ treatment. Details of the study design and interventions have been presented elsewhere.18 The objective of this evaluation was to determine the study participants’ diabetes-related distress and attitudes toward insulin treatment and insulin pump therapy. The study was approved by the local ethics committee and performed in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice at Medical University of Graz, Austria. All participants provided written informed consent prior to study participation.
Participants
Participants were recruited from a single clinical site in Austria. Main inclusion criteria were type 2 diabetes, age 30-65 years, and glycosylated hemoglobin (HbA1c) ≤9.0% (75mmol/mol) with stable MDI regimens. Main exclusion criteria were insulin requirements >100 U/day, basal insulin alone or premixed insulin, and concomitant sulfonylurea therapy.
Interventions
Three validated PRO tools were used to assess patient health-related quality of life (QoL) factors at the beginning of the baseline period. In the transition period, participants received up to 1 hour of training and were started on 1 of the 5 available preset basal doses using insulin aspart (NovoNordisk AS, Baegsvard, Denmark). The first selected basal rate was equal to or less than the basal dose used during baseline. Meal boluses were adapted to current blood glucose and carbohydrate intake. Participants spent the first 24 hours of device use at the clinical research site and were then seen every 3 days, thereafter. There was no optimization of the insulin dose to achieve glycemic targets. During the treatment period, participants managed their blood glucose independently. At study end investigators administered the patient-outcome tools.
Measures
Patient health-related QoL was assessed by using 3 validated PRO tools, which have been independently validated for use in diabetes: the Barriers to Insulin Treatment (BIT) questionnaire,19 Insulin Treatment Appraisal Scale (ITAS),20 and Problem Areas in Diabetes (PAID) scale.21,22 Patient satisfaction with and acceptance of the PaQ device was assessed using a sponsor-generated questionnaire.
The BIT questionnaire assesses aspects of psychological obstacles to insulin treatment in individuals with type 2 diabetes. The questionnaire includes 3 positively and 11 negatively worded items. Answers range from 0 (completely disagree) to 10 (completely agree). As a 5-dimensional scale, BIT deals with the following 4 negative and 1 positive dimensions: fear of injections and self-testing, expectations regarding positive insulin-related outcomes, expected hardship from insulin therapy, stigmatization by insulin injections, and fear of hypoglycemia.
The ITAS is used with individuals with type 2 diabetes to obtain their current appraisal of insulin therapy and track changes in their perception regarding insulin therapy over time. ITAS comprises 16 negative and 4 positive statements, which also form the 2 dimensions of the scale. Possible answers rank from 1 (strongly disagree) to 5 (strongly agree) on a 5-point Likert-type scale. For analysis, the coding of the positive items was inversed to acquire a homogenous interpretation. Positive mean differences should be interpreted as increased satisfaction with insulin treatment, while negative mean differences should be interpreted as a decline in satisfaction.
The PAID scale is a widely used 20-item self-report scale that assesses the current level of diabetes-related emotional distress both in type 1 and type 2 diabetes. PAID items are rated on a Likert-type scale ranging from 0 (not a problem) to 4 (a serious problem); scores are summed and standardized to a 0 to 100 scale, with higher scores indicating higher emotional distress. In our analysis, a positive mean difference in PAID means that diabetes-related distress is declining, while negative mean differences indicate that distress is rising.
A 4th tool, the Device Satisfaction and Acceptance (DSA) questionnaire, was developed by investigators to assess patient satisfaction with and acceptance of PaQ device. The DSA questionnaire consists of 2 subscales in which each subscale has its own answering mode. Items from the Satisfaction subscale mention neutral aspects of the PaQ device; possible answers range from 1 (very dissatisfied) to 5 (very satisfied). Items from the Acceptance subscale describe worries or uncomfortable situations and include the possible answers from 0 (never) to 4 (always). In addition, items 11 and 12 ask for open answers concerning Advantages/Benefits and Disadvantages/Weaknesses.
Statistical Analysis
The paired t test was used to analyze changes in questionnaire scores before and after applying the PaQ to detect mean differences when 1 sample and different points in time were analyzed. Cohen’s d was used to measure the strength of the effect of PaQ device use. It represents a standardized (by standard deviation) mean difference, with, for example, d = 1 representing a reduction of 1 standard deviation. An effects size d ≥ 0.50 is considered as a large effect, effect sizes between 0.2 and 0.5 are regarded as a medium effect, whereas effects smaller than 0.2 are small effects.23 Analysis was extended to nonparametric data by use of the Wilcoxon test to compare the rank values of the variables before and after PaQ use, pair by pair, and displays the count of positive and negative differences as well as the level of significance for these differences. For our analyses only the Wilcoxon P value for significance is reported.
Results
Twenty individuals were enrolled in the study. Baseline characteristics included HbA1c 7.7 ± 0.7%, age of 59 ± 5 years, weight of 96.1 ± 13.7 kg, BMI 32.1 ± 5.6 kg/m2, and diabetes duration of 15 ± 7 years. One participant was withdrawn from the study during the baseline period due to a protocol violation, that is, he stopped taking his basal insulin. Nineteen participants successfully transitioned from MDI to PaQ; 1 participant withdrew informed consent after the transition period because of no improvement in glycemic control. As a result, 18 participants completed the questionnaires at the 2 measurement points.
Barriers to Insulin Treatment
There was a strong and significant reduction in overall barriers to insulin therapy after 2 weeks of PaQ device use. The total score showed a large effect size (Cohen’s d > 0.5), whereas 4 of the 5 subscales had moderate effects (between d > 0.2 and d < 0.5). The changes in the total and 5 subscales before and after the PaQ device use are shown in Figure 1.
Figure 1.
Change of BIT total and BIT subscales scores.
Insulin Treatment Appraisal Scale
The mean change in negative appraisals toward insulin therapy after PaQ device use showed a nonsignificant improvement of 1.71 ± 5.63, P = .14. Single items that most contributed to the positive changes in negative appraisal toward insulin treatment after PaQ device use were the perception that managing insulin injections took less time and energy and that taking insulin does not make life less flexible. After using the PaQ device, participants also perceived it less difficult to inject the correct amount of insulin at the correct time every day.
The Problem Areas in Diabetes
The assessment of diabetes-related distress showed a nonsignificant decrease of diabetes-related distress from 21.67 at baseline to 20.97 after PaQ device use (Δ = 0.69 ± 6.7, P = .79). The greatest improvements were seen in “less deprivation of food and meals” (Δ = 0.28 ± 0.90, d = 0.31, P = .19) and the perception that “diabetes is overwhelming” (Δ = 0.22 ± 0.73, d = 0.30, P = .21). A large improvement was seen in patients “worrying about the future and the possibility for serious complications” (Δ = 0.39 ± 0.98, d = 0.40, P = .12); however, this was likely not associated with PaQ device use.
Effect Sizes
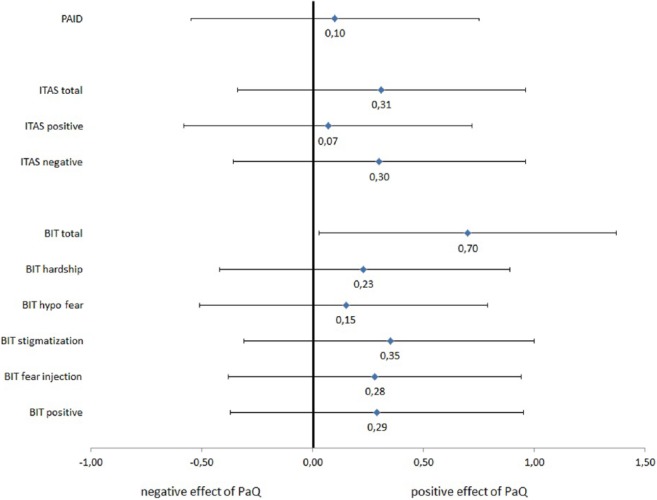
To compare the impact of the PaQ use on the different self report measures the changes in the different questionnaires and its subscales were converted into a standardized effect size measure (Cohen’s d). The observed effects sizes in the BIT, ITAS, and PAID are shown in Figure 2. The total score of the BIT showed a large effect size (Cohen’s d > 0.5), whereas 4 of the 5 subscales had moderate effects. The effect size of PAQ use on the ITAS was medium for the total score and the negative appraisal subscale, whereas effect size for the positive appraisal scale was small. Diabetes-related distress is reduced instead of increased by the use of the PaQ device; the effect size of the PaQ use on the PAID questionnaire is small.
Figure 2.
Standardized effects sizes of change from baseline to follow up after PaQ use in the questionnaires PAID, ITAS and BIT expressed as Cohen’s d.
Items Sensitive for Change After PaQ Use
PROs obtained in the BIT, ITAS, and PAID instruments showed that several items were specifically sensitive to the effects of the PaQ, while other items showed effects regarding insulin treatment in general. Table 1 presents a breakout of the items that showed a moderate size effect (Cohen’s d > 0.3).
Table 1.
Items Most Sensitive to PaQ Use From BIT, ITAS, and PAID (Selection Criterion Cohen’s d ≥ 0.3.
| Item | Cohen’s d |
|---|---|
| Specifically related to PaQ | |
| Managing insulin injections takes a lot of time and energy. (ITAS) | 0.48 |
| I can’t organize my day as carefully as insulin treatment requires. (BIT) | 0.46 |
| I am afraid of the pain when injecting insulin. (BIT) | 0.34 |
| Feelings of deprivation regarding food and meals? | 0.31 |
| General items about insulin treatment | |
| Worrying about the future and the possibility of serious complications? (PAID) | 0.40 |
| Insulin can reliably prevent long-term complications due to diabetes. (BIT) | 0.36 |
| Taking insulin helps to prevent complications of diabetes. (ITAS) | 0.36 |
| An insulin overdose can lead to extremely low blood glucose levels (hypoglycemia). I have concerns about possible permanent damage to my health. (BIT) | 0.33 |
| Feeling overwhelmed by your diabetes? (PAID) | 0.30 |
| Feeling that diabetes is taking up too much of your mental and physical energy every day? (PAID) | −0.30 |
Device Satisfaction and Acceptance
The mean for all Satisfaction items but 1 was > 4.00, ranking between high and very high device satisfaction. The mean for all Acceptance items was < 1.00, ranking between high and very high acceptance. Descriptive analyses are presented in Table 2.
Table 2.
Descriptive Results for Device Satisfaction and Acceptance.
| Item | Mean | Confidence interval |
|
|---|---|---|---|
| Lower bound | Upper bound | ||
| Device satisfaction (1 = minimum to 5 = maximum satisfaction) | |||
| 1. Current insulin dosing method. | 4.61 ± 0.78 | 4.22 | 5.00 |
| 2. Amount of time it takes to maintain your current insulin device. | 4.28 ± 1.02 | 3.77 | 4.78 |
| 5. Time it takes to administer an insulin dose | 4.83 ± 0.38 | 4.64 | 5.02 |
| 6. Amount of training time you had with a health care professional learning your insulin device for the first time | 4.88 ± 0.33 | 4.71 | 5.05 |
| 8. Number of items you must carry to manage your diabetes | 3.72 ± 1.36 | 3.04 | 4.40 |
| 9. Amount of time it took to learn how to use your insulin device | 4.78 ± 0.43 | 4.57 | 4.99 |
| 10. Knowledge of your insulin device | 4.61 ± 0.61 | 4.31 | 4.91 |
| Acceptance (4 = minimum to 0 = maximum acceptance) | |||
| 3. Skip an insulin dose rather than have someone see your insulin device | 0.0 ± 0.00 | 0 | 0 |
| 4. Worry about whether your insulin device will malfunction | 0.89 ± 0.76 | 0.51 | 1.27 |
| 7. Pain administering your insulin to yourself | 0.39 ± 0.61 | 0.09 | 0.69 |
Discussion
Despite the already demonstrated benefits of intensive insulin therapy in individuals with type 2 diabetes,1-4 it is widely recognized that many individuals within this population are not persistent in adhering to their therapy regimens.6 Among those individuals treated with MDI therapy, the common barriers to treatment adherence are interference of injections with daily activities, injection pain, and embarrassment.6 Although use of insulin pumps can potentially alleviate many of these barriers, the extensive training requirements, device complexity, and associated costs often limits use of this therapy in type 2 diabetes.14
The PaQ insulin delivery device was designed specifically for individuals with insulin-treated diabetes. In this study of 20 adults with MDI-treated type 2 diabetes, we assessed the impact of PaQ device use on participants’ reported outcomes, regarding barriers to insulin treatment, attitudes toward insulin therapy, and diabetes-related distress.
Results from our study suggest that barriers to insulin treatment can be greatly reduced with the use of the PaQ device. Although PaQ device use resulted in a significant reduction in the overall BIT score, the effect sizes of the BIT subscales seem to indicate that reduction of “hardship of insulin therapy,” “less feelings of stigmatization,” and “less fear about hypoglycemia” were the most important factors in overall reduction of barriers to insulin treatment. Reduction of “hardship of insulin treatment” and “feeling of stigmatization” may be explained by the fact that the use of PaQ device replaced an average of 5.2 daily insulin injections.
Negative attitudes toward insulin treatment were also reduced after using the PaQ device. Key contributors to this were participants’ expectations that managing insulin injections takes less time and energy, injecting the right amount of insulin correctly at the right time every day is easier and taking insulin does not have negative effects on flexibility in life. Although the changes in PAID scores were not significant, it is noteworthy that initiation and utilization of the PaQ device resulted in no increase in diabetes-related distress; rather, results showed a trend toward reduced distress. Results from the DSA questionnaire showed a very high level of satisfaction and acceptance toward the PaQ among study participants, which may support greater adherence to therapy among device users.
From a research perspective, it is interesting that the BIT instrument, as a complete questionnaire, appeared to be the most promising in detecting relevant changes in PROs. It may be that the BIT instruments is more capable of covering practical aspects and barriers of insulin treatment, whereas the ITAS may be more focused on cognitive appraisal of insulin treatment and emotional reactions toward insulin treatment. This suggests that researchers may want to consider combining the complete BIT questionnaire with those items from the ITAS and PAID instruments that showed the greatest sensitivity to PaQ device use (Table 1).
The study does have important limitations that should be considered. It is difficult to disentangle specific effects from PaQ device use on PROs from more general effects of study participation because the study was uncontrolled. The small sample size is another key limitation; the moderate to large changes seen in many of the patient-reported measures were not significant due to a lack of statistical power. In addition, because participants were not blinded to the intervention, there may be some bias in answering the DSA questionnaire. Participants may have been aware that the purpose of the study was to assess the device and, therefore, gave more positive responses.
Conclusions
Despite the aforementioned limitations, the moderate to large effects sizes seen in our study strongly suggest that the use of the PaQ device has beneficial and clinically relevant effects to overcome barriers to insulin therapy without increasing diabetes-related distress. Large, controlled, clinical trials are needed to further elucidate the impact of PaQ device use on the barriers to insulin treatment in participants with suboptimal glycemic control and whether reduction of these barriers will result in improved glycemic control.
Acknowledgments
The authors wish to thank Christopher G. Parkin (CGParkin Communications, Inc, Boulder City, NV, USA) for editorial assistance in developing this article.
Footnotes
Abbreviations: BIT, Barriers to Insulin Treatment; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; DSA, Device Satisfaction and Acceptance; HbA1c, glycated hemoglobin; ITAS, Insulin Treatment Appraisal Scale; MDI, multiple daily insulin injections; PAID, Problem Areas in Diabetes; PRO, patient-reported outcome; QoL, quality of life; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LL and JW are employees of CeQur Corp USA. The remaining authors declare no duality of interest associated with this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the study was provided by CeQur SA, Horw, Switzerland.
References
- 1. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289(17):2254-2264. [DOI] [PubMed] [Google Scholar]
- 2. Edelman S, Dailey G, Flood T, Kuritzky L, Renda S. A practical approach for implementation of a basal-prandial insulin therapy regimen in patients with type 2 diabetes. Osteopath Med Prim Care. 2007;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou J, Jia W, Bao Y, et al. Glycemic variability and its responses to intensive insulin treatment in newly diagnosed type 2 diabetes. Med Sci Monit. 2008;14(11):CR552-558. [PubMed] [Google Scholar]
- 4. Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753-1760. [DOI] [PubMed] [Google Scholar]
- 5. Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736-1747. [DOI] [PubMed] [Google Scholar]
- 6. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of Insulin Injection Omission. Diabetes Care. 2010;33(2):240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung BM, Ong KL, Cherny SS, Sham PC, Tso AW, Lam KS. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122(5):443-453. [DOI] [PubMed] [Google Scholar]
- 8. Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008;371(9618):1073-1084. [DOI] [PubMed] [Google Scholar]
- 9. Edelman SV, Bode BW, Bailey TS, et al. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther. 2010;12(8):627-633. [DOI] [PubMed] [Google Scholar]
- 10. Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598-2603. [DOI] [PubMed] [Google Scholar]
- 11. Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568-1573. [DOI] [PubMed] [Google Scholar]
- 12. Wainstein J, Metzger M, Boaz M, et al. Insulin pump therapy vs. multiple daily injections in obese type 2 diabetic patients. Diabet Med. 2005;22(8):1037-1046. [DOI] [PubMed] [Google Scholar]
- 13. Berthe E, Lireux B, Coffin C, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224-229. [DOI] [PubMed] [Google Scholar]
- 14. Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clin Diabetes. 2007;25(2):50-56. [Google Scholar]
- 15. King AB, Clark D, Wolfe GS. The number of basal rates required to achieve near-normal basal glucose control in pump-treated type 2 diabetes. Diabetes Technol Ther. 2012;14(10):900-903. [DOI] [PubMed] [Google Scholar]
- 16. Parkner T, Moller MK, Chen JW, et al. Overnight CSII as supplement to oral antidiabetic drugs in type 2 diabetes. Diabetes Obes Metab. 2008;10(7):556-563. [DOI] [PubMed] [Google Scholar]
- 17. Leinung MC, Thompson S, Luo M, Leykina L, Nardacci E. Use of insulin pump therapy in patients with type 2 diabetes after failure of multiple daily injections. Endocr Pract. 2013;19(1):9-13. [DOI] [PubMed] [Google Scholar]
- 18. Pieber T, Mader J, Lilly L, et al. A feasibility study of a 3-day basal-bolus insulin delivery device in individuals with type 2 diabetes. Diabetes Care. 2014;37:1476-1479. [DOI] [PubMed] [Google Scholar]
- 19. Petrak F, Stridde E, Leverkus F, Crispin AA, Forst T, Pfutzner A. Development and validation of a new measure to evaluate psychological resistance to insulin treatment. Diabetes Care. 2007;30(9):2199-2204. [DOI] [PubMed] [Google Scholar]
- 20. Snoek FJ, Skovlund SE, Pouwer F. Development and validation of the insulin treatment appraisal scale (ITAS) in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, Schwartz CE. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754-760. [DOI] [PubMed] [Google Scholar]
- 22. Snoek FJ, Pouwer F, Welch GW, Polonsky WH. Diabetes-related emotional distress in Dutch and U.S. diabetic patients: cross-cultural validity of the Problem Areas in Diabetes scale. Diabetes Care. 2000;23(9):1305-1309. [DOI] [PubMed] [Google Scholar]
- 23. Bland M. An Introduction to Medical Statistics. Oxford, UK: Oxford University Press; 2000. [Google Scholar]