Abstract
While being physically active bestows many health benefits on individuals with type 1 diabetes, their overall blood glucose control is not enhanced without an effective balance of insulin dosing and food intake to maintain euglycemia before, during, and after exercise of all types. At present, a number of technological advances are already available to insulin users who desire to be physically active with optimal blood glucose control, although a number of limitations to those devices remain. In addition to continued improvements to existing technologies and introduction of new ones, finding ways to integrate all of the available data to optimize blood glucose control and performance during and following exercise will likely involve development of “smart” calculators, enhanced closed-loop systems that are able to use additional inputs and learn, and social aspects that allow devices to meet the needs of the users.
Keywords: exercise, technology, insulin, artificial pancreas, physical activity, type 1 diabetes
Physical activity (PA) for people of all ages living with type 1 diabetes (T1D) is associated with many well-established health benefits, including improved cardiovascular fitness, better bone-health and enhanced psychological well-being.1,2 Despite these benefits, most adults with T1D participate less frequently in PA than their nondiabetic counterparts3 and may adopt unhealthy lifestyles that contribute to cardiometabolic risk.4 Although the reasons for this are multifactorial, including concerns over loss of control and low fitness levels, the overriding barrier to PA appears to be fear of severe hypoglycemia, coupled with a lack of knowledge of effective strategies for hypoglycemia avoidance.3 Moreover, overall glycemic control (measured with glycated hemoglobin, or A1C, levels) with exercise has shown mixed results in T1D studies, with some demonstrating benefits5-7 and others no improvement in A1C following aerobic or resistance training.8,9 For overall glycemic control to be enhanced, individuals with T1D are required to skillfully balance insulin dosing and food intake to maintain blood glucose levels in a more normal range before, during, and after exercise.10-13 Although many obstacles to safe and effective exercise participation remain, present and future technologies should assist more people with T1D to become and remain physically active to improve their health and reduce the negative impact of diabetes on participation in competitive sports and activities.14,15
What Complicates Diabetes Control During Exercise?
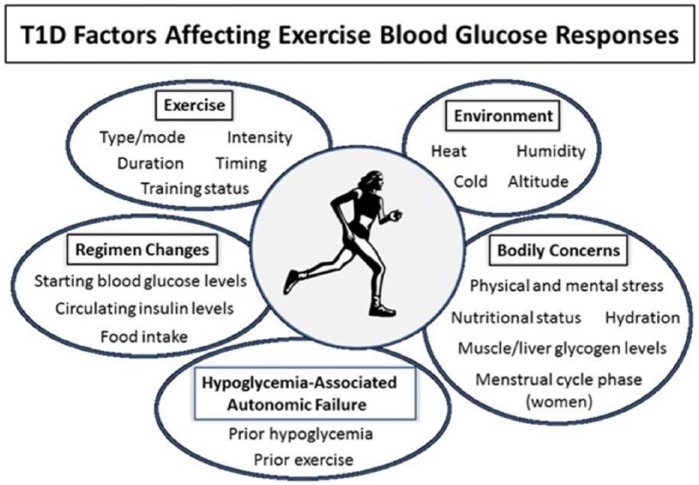
To optimize control of blood glucose levels during exercise, individuals must be aware of factors that can complicate glycemic management. Among these are participation in varying types of PA, differences in insulin regimens and food intake for exercise, and the necessity of maintaining normal or nearly normal blood glucose levels before, during, and after activities (Figure 1). Balancing all of these factors can be overwhelmingly challenging for many, who may choose instead to be less active to avoid hypoglycemia in particular.3
Figure 1.
Type 1 diabetes factors affecting exercise blood glucose responses.
Exercise Variables
The impact of exercise on glucose homeostasis is influenced by the type, intensity, and duration of the activity. Participation in aerobic, sprint, and resistance training can result in widely varying blood glucose responses.16,17 Differences in exercise intensity also impact outcomes, with high intensity activities causing a greater release of counterregulatory hormones like epinephrine and glucagon that can cause immediate and lasting elevations in blood glucose levels.18,19 In fact, an all-out sprint as short as 10 seconds can have a lasting effect on glycemic balance.18,20 Duration also has an impact, with longer periods of exercise generally resulting in greater blood glucose use and the risk of hypoglycemia, although large individual variations in hormonal responses to prolonged exercise of varying types have been demonstrated in athletes with T1D.21,22 Exercising more than once in a day or on more than 1 day in a row can also affect blood glucose outcomes during the exercise itself and afterward.23 While highly trained individuals with T1D can achieve the same cardiopulmonary exercise responses as trained subjects without diabetes, their responses are reduced by poor glycemic control.24 The impact of PA is also complicated by the nature of the sport where periods of intense activity are interspersed with periods of much less activity, for example, American football, soccer, and basketball.
Individuals with T1D are frequently unable to adequately alter endogenous insulin levels and experience normal hormonal glucose counterregulation during and following exercise. Consequently, they are at risk for early and late hypoglycemia and also hyperglycemia. The risk of overnight and next-day hypoglycemia is particularly problematic if moderate to vigorous exercise is undertaken in the afternoon or early evening.25,26 Late-onset hypoglycemia following exercise increases with the duration of exercise and the fitness of the exercisers, and activities lasting 90 to 120 minutes double the risk of hypoglycemia in adolescents and young adults.26
Insulin Regimens
Glycemic control with PA is also complicated by the fact that different insulin regimens and delivery systems are available for T1D. Most insulin regimens are based on a basal-bolus approach.27,28 Insulin pumps using a rapid-acting insulin analog are programmed to deliver small amounts of this insulin frequently in small doses to cover basal needs, with user programming of bolus amounts for meals, snacks, and corrections of glucose levels based on need.29 In a small clinical study, adults with T1D using insulin pump therapy and performing regular moderate-to-heavy intensity aerobic exercise experienced less postexercise hyperglycemia compared with multiple daily injections (MDI) without increasing the risk of postexercise late-onset hypoglycemia.30 However, even when using insulin pump therapies instead of MDI, individuals still can experience wide variability in glucose profiles before, during, and after exercise.31
Carbohydrate and Food Intake
Particularly when insulin adjustments are not made for PA (as is frequently the case for spontaneous activities) or the activity is prolonged in nature, balancing carbohydrate intake with needs based on the intensity and duration of PA and circulating insulin levels becomes critical for prevention of hypoglycemia.32-34 Fuel utilization in T1D adults undertaking aerobic exercise during euglycemia is similar to that in healthy individuals, with a greater reliance on carbohydrate as the primary fuel for moderate to vigorous activity and a shift toward greater lipid oxidation during prolonged exercise. However, during hyperglycemic exercise, carbohydrate and blood glucose use are both increased without sparing muscle glycogen.35 PA undertaken during injected insulin peaks increases reliance on carbohydrate intake to prevent hypoglycemia, but also does not reduce muscle glycogen use.36 After exercise a mix of macronutrients may more efficiently prevent later-onset of hypoglycemia.37,38 Adequate carbohydrate intake during and following exercise is, therefore, critical for effective PA participation and hypoglycemia prevention.
Optimal Glycemic Balance for Athletic Performance
Having a competitive advantage in athletic events requires prevention of both hypoglycemia and hyperglycemia during and following exercise is, therefore, critical. Particularly when individuals experience recurrent low blood glucose levels, they can develop hypoglycemia-associated autonomic failure39,40 that has the potential to affect subsequent counterregulatory responses to PA.23,41 As a corollary, antecedent physiologic increases in cortisol (equivalent to levels during hypoglycemia) have been shown to blunt neuroendocrine and autonomic nervous system responses during subsequent exercise in adults with T1D.42 Inability to prevent hypoglycemia during training and competitive events will assuredly reduce exercise performance.21,43
What Does Current Technology Offer to Diabetes Management With Exercise?
A number of technologies are already available to support PA in people with T1D. Among these are devices that measure and track heart rate, intensity of movement, calories expended, distance traveled as well as diabetes-specific devices including insulin pumps, blood glucose monitoring systems, and continuous glucose monitors.
Wearable Tracking Devices and Performance Indicators
There is increasing interest in the use of wearable sensors that are able to measure physiological parameters including heart rate, distance traveled, and calorie expenditure.44,45 Many utilize smartphone technologies (apps) that can communicate with a variety of fitness and health tracking platforms. For example, continuous data from ankle triaxial accelerometers can be transmitted from the home and community via Wi-Fi or a smartphone to a remote data analysis server and compile data and reports on walking speed, duration of exercise, and even the type, duration, and energy used during exercise.46,47 Other devices can track food intake (entered by the user); calculate calories in and out; estimate carbohydrate intake (in grams); measure body weight, percentage body fat, and body mass index; estimate power (wattage) and transmit results wirelessly to online accounts; and display graphs, trends, daily, and weekly goals.48
For people with T1D accurate estimation of carbohydrate (higher glycemic index carbohydrates in particular) and calorie intake, balanced with energy expenditure during exercise, is critical for maintaining euglycemia.49 The glycemic effects of protein and fat may also be relevant for PA.50-52 With current devices, although it may be possible to estimate carbohydrate and other fuel use based on heart rate, characteristics of the activity (such as intensity and duration), calorie expenditure, and blood glucose responses, a considerable amount of variability is still likely to exist due to the potential impact of other factors like variations in subcutaneous insulin absorption and fluctuations in insulin sensitivity.53-55
Insulin Delivery and Pumps
Despite many advances in insulin pumps, syringes, and pens over the past several decades, the delivery of pumped or injected insulin remains hindered by its subcutaneous delivery and rates of absorption, which generally lags behind carbohydrate digestion and absorption and creates a mismatch between the postprandial rise in blood glucose and insulin availability. For metabolism to be normal, exogenous insulin would need to be delivered through the portal vein as occurs in nondiabetic individuals. The liver maintains glycemic balance at rest and during exercise by either releasing or storing glucose in response to changes in blood glucose levels; in people without diabetes, insulin released directly from the pancreas following food ingestion results in a dual trigger of elevated glucose and insulin postprandially that promotes glycogen storage. In individuals with T1D, the liver is less effective at both storing glucose as glycogen for later release and releasing adequate amounts of glucose in response to decreases in blood levels during exercise with elevated peripheral insulin levels, even though increased rates of hepatic glucose production apparently occur in T1D at both times through increased gluconeogenesis.56,57
One advance that insulin pumps offer over MDI regimens is that pumps allow users to change basal insulin delivery around the time of exercise to more closely replicate a normal physiological response, whereas users of basal insulin that is injected once or twice daily are less able to respond quickly enough to changes in insulin needs.29,30 In 1 study, for adults with T1D performing 45 minutes of regular moderate-to-heavy intensity aerobic exercise, use of insulin pump therapy limited postexercise hyperglycemia compared with MDI and was not associated with increased risk for postexercise late-onset hypoglycemia. 30 Most pumps also now incorporate bolus calculators to take into account any remaining insulin from the last bolus, but the duration of insulin action used in such calculators has yet to be standardized to assist in making a more effective reduction of circulating insulin levels in the hour or 2 prior to exercise.58 Using pumps leads to additional practical considerations based on the type of PA, though, such as what to do with the infusion set (if the pump is not disconnected59), whether the pump is waterproof, and how environmental factors may affect the insulin in the pump (if still connected or tubeless).
Home Glucose Monitors
The ability to test blood glucose levels before, during, and after PA is clearly important for managing glycemia and improving exercise performance, regardless of the athletic abilities of the exerciser.60 Although the time required and size of the blood drop needed for an accurate measurement have decreased considerably over recent years, a lancing device is still required to obtain a glucose sample. The smallest self-monitoring blood glucose device is now smaller than the usual flash drive (and can fit into a port of an iPhone to display results), and most of these monitors are still stand-alone devices. Some of them are integrated with insulin pumps, but remain open-loop systems leaving decision making up to users and/or their health care teams.
Continuous Glucose Monitors
A second advance in home-based glucose monitoring is the development of continuous glucose monitors (CGM). In 1 study on runners with T1D, CGM use was found to be helpful in identifying asymptomatic hypoglycemia or hyperglycemia during and after a marathon.61 In adolescents with T1D using CGM, participating in afternoon moderate-to-vigorous PA was found to increase the risk of overnight and next-day hypoglycemia.26 At a summer sports camp, adolescents with T1D were able to use CGM coupled with a novel carbohydrate intake algorithm to prevent hypoglycemia and maintain euglycemia during exercise, particularly if they ingested carbohydrates in adequate amounts when trend arrows on the device alerted them of a drop in glycemia.32
Current CGM systems have their limitations, including changes in accuracy with duration of sensor wear, precision issues, and skin irritation and problems with sensor adhesiveness.62-64 However, the marked inaccuracy and lag time of CGM readings when blood glucose levels change rapidly within the physiological range (such as during exercise) should be minimized for optimal CGM use in glycemic management.64 Future CGM systems will benefit from further increasing their accuracy and reliability and ideally utilize algorithms that can adapt over time to individual differences in user physiology.65,66 The most critical improvement in CGM systems that would increase their use (without breaks) by a larger proportion of individuals with T1D during exercise is the ability to measure blood glucose (not interstitial glucose) to give the user (or any integrated system) “real-time” values that are actually reflective of blood glucose at that exact point in time and not lagging behind when glucose levels are changing rapidly during exercise,64 and such technologies are currently not available.
Other advances combining some of these devices are in the early stages. One promising approach includes the measurement of heart rate variability (HRV). Adolescents who engage in spontaneous moderate aerobic activity have improved autonomic regulation.67 Moreover, in a very recent study, hypoglycemia detection accuracy and lead time were significantly improved by a novel algorithm involving both HRV and CGM data together.68 This approach, as compared to the CGM device alone, has the potential to make the combination of such tools of immense potential value to individuals with T1D to detect hypoglycemia during and following PA, particularly if they experience hypoglycemia unawareness or autonomic failure. Hypoglycemia-associated autonomic failure following prior exercise or hypoglycemia is only experienced with peripheral insulin delivery in T1D.23,41,57,69-75
What Should Future Technology Offer to Best Manage Diabetes With Exercise?
An even bigger challenge than perfecting the devices themselves (and many of them have advanced substantially over several decades) is finding ways to integrate all of the available data to optimize blood glucose control and performance during and following exercise undertaken by individuals of all ages with T1D. Integration will likely involve “smart” calculators, better closed-loop systems that are able to do adapt and learn, and social aspects that allow devices to meet the needs of the users.
Calculators for Exercise
Currently, insulin bolus calculators are available on most insulin pumps that take into account the timing of prior insulin dosing, usual absorption rates, insulin sensitivity, and correction factors. These calculators are also being integrated into glucose meters and portable device applets for use with multiple daily injections.76 However, none of these calculators currently account for the effects of PA beyond offering a percentage correction for exercise in the general sense (Roche device), and they may use inappropriately short estimates of duration of insulin action that can cause unrecognized “stacking” of insulin, leading to unexplained hypoglycemic events, particularly when exercise is an added variable.58
The primary goal of an ideal exercise calculator for T1D is prevention of both hypoglycemia and hyperglycemia during the activity and during the 24- to 48-hour period following exercise. Hypoglycemia most commonly develops during, in the immediate postexercise period, and then again around 7 to 11 hours later in a biphasic manner,25 meaning that many individuals experience a secondary decline in their postactivity glucose levels during nighttime hours. Glycemic management with intensive insulin therapy should ideally be accomplished without inducing weight gain or increasing the number of hypoglycemic events, which frequently requires fine-tuning of both carbohydrate intake and insulin dosing.49
Prediction of glycemic responses for the purposes of a calculator may be further complicated by use of other medications with insulin like pramlintide.77 Likewise, the presence of certain diabetes-related complications like gastroparesis, which is estimated to affect about 40% of those with T1D, can make the absorption of food erratic and delayed and glycemic responses less predictable.78,79
Therefore, to be truly effective, a calculator will benefit from being able to do most, if not all, of the following:
Make general suggestions about insulin and food intake to allow for adjustments prior to the start of PA (such as lowering basal insulin prior to the activity and not just during and following it);80
Make more specific recommendations for regimen changes based on user input or measured physiological data in real time related to the type, intensity, and duration of PA that is being undertaken by the individual;17,81,82
Create updated recommendations when the plan for PA is altered during the activity, such as a change in duration, intensity, or type;17,82
Factor in the impact of the individual’s starting blood glucose level on subsequent responses to PA;83
Predict hypoglycemia in advance or in real time based on specific user input about PA, prior insulin timing and dosage, and prior or planned food intake during activity and give recommendations for its prevention during and following activity;84
Predict hyperglycemia and give recommendations for regimen changes to prevent or correct PA-induced increases in blood glucose;83
Account for use of medications or the presence of diabetes complications that slow absorption of ingested carbohydrates taken for PA;85
Factor in the potential confounding effects of prior hypoglycemia and exercise resulting in hypoglycemia-associated autonomic failure during subsequent exercise;71,72 and
Adapt to prior input to make even more “intelligent” recommendations for maintenance of glycemia during subsequent PA of a similar nature.
Artificial Pancreas Systems
This involves integration of CGM devices, insulin delivery via a pump, and an algorithm control system to manage blood glucose levels with minimal or without user input, thereby creating an external device-driven artificial pancreas (AP) system. Some investigators have suggested that dual hormone delivery (ie, both insulin and glucagon) may work more effectively in preventing hypoglycemia than insulin alone,86-91 while others continue to work on developing a system using insulin delivery only.92-98
Researchers have begun to collect a variety of PA data from existing devices to assist them in creating functioning algorithms.99-103 Early work by Turksoy et al,103 Breton et al,100 and Stenerson et al104 suggest that early detection of PA is feasible using heart rate and/ or a multisensory device like the Bodymedia armband and that this information will enhanced closed-loop control and improve both the immediate hypoglycemia risk and the latent effect. Use of adaptive control and/or predictive design that will use additional PA inputs may be beneficial especially in the prevention of latent hypoglycemia. However, any closed-loop system that is developed using current CGM technologies and insulin delivery methods (subcutaneous) will be limited by slow insulin action and slow insulin clearance and lagging of glucose response in case of fast negative rate of change. This may affect AP systems that are based solely on glucose measurements.64,105 PA variables that are introduced and/or exacerbated by activity include the rates of blood glucose and glycogen use during activity, effects of any residual insulin being absorbed into the bloodstream from a subcutaneous depot, and inability of the system to respond to rapid changes in blood glucose. For more information on the progress in the development of automated glucose control, readers are directed to other comprehensive review articles.106-109
Ideally any AP closed-loop system will be able to cope with PA with minimal or no user intervention. In a recent study, adolescents and adults with T1D involved in a closed-loop insulin delivery system participated in midafternoon exercise (brisk walking for 60 minutes, a moderate activity) and still exhibited a lower risk of nocturnal hypoglycemia following exercise and an increased percentage of time spent in their target blood glucose range.110 Moreover, adding a heart rate signal to another closed-loop system further limited the risk for hypoglycemia during and immediately after exercise.100 The challenge of the current AP systems that are based on subcutaneous delivery of insulin and glucose sensing is that the system is limited by the fact that even if PA is detected at the onset of the event, it may be too late to prevent the acute onset of hypoglycemia without user intervention or the use of glucagon. These results suggest that, while waiting for technology to advance to the point that a full closed-loop AP system would work optimally, simply perfecting a system that could provide closed-loop insulin delivery at night or help make better exercise adjustments may be of benefit to maintaining glycemic balance during and following PA participation.
Pattern Recognition and Learning
For an exercise calculator or a closed-loop AP system to work effectively, access to real-time data about physiological variables like heart rate, movement (with an accelerometer), and possibly other biometric data like blood lactate levels during more intense training, is likely a necessity.111 Beyond just receiving these data, it would benefit from having the capacity to analyze and integrate these inputs into algorithms that would identify regimen changes in real time and be able to predict such a change prior to the start of the PA, enabling the system to take preventive actions to help to maintain glycemia and optimize performance outcomes.
For real-time learning to take place with a calculator or for any integrated (eg, closed-loop) system, it should be able to collect and analyze data in real time, use algorithms developed using mathematical modeling that can adapt to new input from users and associated devices, and decrease variability in outcomes over time with more relevant and individualized recommendations for diabetes regimen management.65,66 Ideally, such systems or machines would also be able to quantify or integrate users’ more subjective responses to them, thereby enhancing their perceived value.112 For that to be the case, technologies will eventually have to advance to be even more accurate and adaptable than they currently are to integrate analyses from multiple sources, participants and types of PA, along with having adaptive machine learning to allow for personalization of advice.
Regulated and Unregulated Devices
To be effective in both preventing glycemic swings above and beyond target values and supporting the achievement of personal PA goals, technologies will benefit from being able to
Be interoperative;
Avoid barriers related to health literacy;
Understand the potential value of physiological data affected by PA; and
Provide specific decision support.
Providing specific decisions for an insulin-regulated person with T1D will require evidence of effectiveness and safety of any devices or integrated system. The journey along a regulatory pathway can be problematic and dissuade innovators from participating or focusing on the consumer electronic market in general. One option would be to consider approval of hybrid systems that incorporate regulated devices measuring specific variables like glucose levels and insulin pumps or other technologies that can predict the potential for hypoglycemia, but with a less burdensome approval pathway for PA devices that indirectly contribute to the development of decision support exercise. Of course, with the complexity of blood glucose responses to PA, any hybrid system would still have to be able to lower the risk of hypoglycemia compared to using those same devices with greater user input.
Social Integration
Systematic reviews of internet-delivered interventions for PA have shown modest effectiveness when self-monitoring, goal-setting, or feedback was included.113,114 Recently, the use of social networking has demonstrated a modest benefit for health and weight management,115-117 although at present there is a paucity of studies focusing on diabetes. One potential area of benefit involves the use of video games, which are especially popular with younger people.118-120 A recent approach has been to create augmented reality games whereby participants combine real-world with virtual activities with PA embedded within the game (such as Wii Fit).119,121,122 This type of approach may be especially beneficial for individuals of all ages who are geographically isolated or have lower levels of peer support.
Currently, many web sites and apps allow users to share their exercise goals and results with friends, family, and health care team, but these programs currently lack the ability to integrate all of the biometric and other collected data to be shared with others in real time to enhance the experience. In a recent review of mobile apps, the available ones for diabetes management support self-management tasks like physical exercise, insulin dosage or medication, blood glucose testing, and diet, but also include other support tasks decision support, notification and alerts, tagging of input data, and integration of social media.123 Overall, app usage appears to improve attitudes toward diabetes self-management, but app use may be limited by lack of personalized feedback, issues with ease of data entry, and ineffective integration with patients and electronic health records.
In adults with T1D, the use of a diabetes-related smartphone application combined with weekly text-message support from a health care professional and usual care was able to significantly improve glycemic control.124 Imagine the diabetic exerciser of the future who receives feedback from a friend or diabetes educator in real time who has access to his or her data and can share successes, failures, and learning that can improve the next exercise experience. In a world severely lacking in exercise motivation, such a system may be the answer to keeping everyone more active and healthy, not just individuals with T1D.
Conclusions
While technological advances have allowed exercisers with diabetes to progress toward more effectively managing their blood glucose levels during various types of physical activities, technology is still far from fully removing the fear of hypoglycemia that is the strongest impediment to undertaking regular exercise with T1D. The use of present and future technologies will likely assist more people dealing with T1D to become and remain physically, as well as perform optimally in competitive sports and activities. The future integration of various technologies will likely make exercise a much safer and more effective undertaking and result in a greater perceived value related to both health and glycemic management.
Footnotes
Abbreviations: A1C, glycated hemoglobin; AP, artificial pancreas; CGM, continuous glucose monitors; HRV, heart rate variability; MDI, multiple daily injections; PA, physical activity; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chimen M, Kennedy A, Nirantharakumar K, Pang TT, Andrews R, Narendran P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia. 2012;55:542-551. [DOI] [PubMed] [Google Scholar]
- 2. Brazeau AS, Leroux C, Mircescu H, Rabasa-Lhoret R. Physical activity level and body composition among adults with type 1 diabetes. Diabet Med. 2012;29:e402-e408. doi: 10.1111/j.464-5491.2012.03757.x. [DOI] [PubMed] [Google Scholar]
- 3. Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31:2108-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leroux C, Brazeau AS, Gingras V, Desjardins K, Strychar I, Rabasa-Lhoret R. Lifestyle and cardiometabolic risk in adults with type 1 diabetes: a review. Can J Diabetes. 2014;38:62-69. [DOI] [PubMed] [Google Scholar]
- 5. Cuenca-Garcia M, Jago R, Shield JP, Burren CP. How does physical activity and fitness influence glycaemic control in young people with type 1 diabetes? Diabet Med. 2012. [DOI] [PubMed] [Google Scholar]
- 6. Aouadi R, Khalifa R, Aouidet A, et al. Aerobic training programs and glycemic control in diabetic children in relation to exercise frequency. J Sports Med Phys Fitness. 2011;51:393-400. [PubMed] [Google Scholar]
- 7. Schweiger B, Klingensmith G, Snell-Bergeon JK. Physical activity in adolescent females with type 1 diabetes. Int J Pediatr. 2010;2010:328318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramalho AC, de Lourdes Lima M, Nunes F, et al. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res Clin Pract. 2006;72:271-276. [DOI] [PubMed] [Google Scholar]
- 9. Aman J, Skinner TC, de Beaufort CE, Swift PG, Aanstoot HJ, Cameron F. Associations between physical activity, sedentary behavior, and glycemic control in a large cohort of adolescents with type 1 diabetes: the Hvidoere Study Group on Childhood Diabetes. Pediatr Diabetes. 2009;10:234-239. doi: 10.1111/j.399-5448.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 10. Younk LM, Mikeladze M, Tate D, Davis SN. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev Endocrinol Metab. 2011;6:93-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallen IW, Hume C, Lumb A. Fueling the athlete with type 1 diabetes. Diabetes Obes Metab. 2011;13:130-136. [DOI] [PubMed] [Google Scholar]
- 12. Chu L, Hamilton J, Riddell MC. Clinical management of the physically active patient with type 1 diabetes. Phys Sportsmed. 2011;39:64-77. [DOI] [PubMed] [Google Scholar]
- 13. Riddell M, Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol. 2009;3:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovatchev BP. Diabetes technology: markers, monitoring, assessment, and control of blood glucose fluctuations in diabetes. Scientifica (Cairo). 2012;2012:283821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heinemann L, Faber-Heinemann G, Roberts R, Walsh J. Future of diabetes-technology: certificate of competency for insulin pumps and continuous glucose monitors. J Diabetes Sci Technol. 2012;6:725-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yardley JE, Kenny GP, Perkins BA, et al. Resistance versus aerobic exercise: acute effects on glycemia in type 1 diabetes. Diabetes Care. 2013;36:537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yardley J, Mollard R, Macintosh A, et al. Vigorous intensity exercise for glycemic control in patients with type 1 diabetes. Can J Diabetes. 2013;37:427-432. [DOI] [PubMed] [Google Scholar]
- 18. Fahey AJ, Paramalingam N, Davey RJ, Davis EA, Jones TW, Fournier PA. The effect of a short sprint on postexercise whole-body glucose production and utilization rates in individuals with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:4193-4200. [DOI] [PubMed] [Google Scholar]
- 19. Adolfsson P, Nilsson S, Albertsson-Wikland K, Lindblad B. Hormonal response during physical exercise of different intensities in adolescents with type 1 diabetes and healthy controls. Pediatr Diabetes. 2012;13:587-596. doi: 10.1111/j.399-5448.2012.00889.x. [DOI] [PubMed] [Google Scholar]
- 20. Iscoe KE, Riddell MC. Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with type 1 diabetes mellitus. Diabet Med. 2011;28:824-832. [DOI] [PubMed] [Google Scholar]
- 21. Koivisto VA, Sane T, Fyhrquist F, Pelkonen R. Fuel and fluid homeostasis during long-term exercise in healthy subjects and type I diabetic patients. Diabetes Care. 1992;15:1736-1741. [DOI] [PubMed] [Google Scholar]
- 22. Turner D, Luzio S, Gray BJ, et al. Impact of single and multiple sets of resistance exercise in type 1 diabetes [published online ahead of print March 20, 2014]. Scand J Med Sci Sports. [DOI] [PubMed] [Google Scholar]
- 23. Galassetti P, Mann S, Tate D, Neill RA, Wasserman DH, Davis SN. Effect of morning exercise on counterregulatory responses to subsequent, afternoon exercise. J Appl Physiol. 2001;91:91-99. [DOI] [PubMed] [Google Scholar]
- 24. Baldi JC, Cassuto NA, Foxx-Lupo WT, Wheatley CM, Snyder EM. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med Sci Sports Exerc. 2010;42:1454-1459. [DOI] [PubMed] [Google Scholar]
- 25. McMahon SK, Ferreira LD, Ratnam N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab. 2007;92:963-968. [DOI] [PubMed] [Google Scholar]
- 26. Metcalf KM, Singhvi A, Tsalikian E, et al. Effects of moderate-to-vigorous intensity physical activity on overnight and next-day hypoglycemia in active adolescents with type 1 diabetes. Diabetes Care. 2014;37:1272-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zachariah S, Sheldon B, Shojaee-Moradie F, et al. Insulin detemir reduces weight gain as a result of reduced food intake in patients with type 1 diabetes. Diabetes Care. 2011;34:1487-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Leeuw I, Vague P, Selam JL, et al. Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab. 2005;7:73-82. [DOI] [PubMed] [Google Scholar]
- 29. Hanaire-Broutin H, Melki V, Bessieres-Lacombe S, Tauber JP. Comparison of continuous subcutaneous insulin infusion and multiple daily injection regimens using insulin lispro in type 1 diabetic patients on intensified treatment: a randomized study. The Study Group for the Development of Pump Therapy in Diabetes. Diabetes Care. 2000;23:1232-1235. [DOI] [PubMed] [Google Scholar]
- 30. Yardley JE, Iscoe KE, Sigal RJ, Kenny GP, Perkins BA, Riddell MC. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients. Diabetes Technol Ther. 2013;15:84-88. [DOI] [PubMed] [Google Scholar]
- 31. Kapitza C, Hovelmann U, Nosek L, Kurth HJ, Essenpreis M, Heinemann L. Continuous glucose monitoring during exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2010;4:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011;13:819-825. [DOI] [PubMed] [Google Scholar]
- 33. Kilbride L, Charlton J, Aitken G, Hill GW, Davison RC, McKnight JA. Managing blood glucose during and after exercise in type 1 diabetes: reproducibility of glucose response and a trial of a structured algorithm adjusting insulin and carbohydrate intake. J Clin Nurs. 2011;20:3423-3429. [DOI] [PubMed] [Google Scholar]
- 34. Perrone C, Laitano O, Meyer F. Effect of carbohydrate ingestion on the glycemic response of type 1 diabetic adolescents during exercise. Diabetes Care. 2005;28:2537-2538. [DOI] [PubMed] [Google Scholar]
- 35. Jenni S, Oetliker C, Allemann S, et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus—a prospective single-blinded randomised crossover trial. Diabetologia. 2008;51:1457-1465. [DOI] [PubMed] [Google Scholar]
- 36. Chokkalingam K, Tsintzas K, Norton L, Jewell K, Macdonald IA, Mansell PI. Exercise under hyperinsulinaemic conditions increases whole-body glucose disposal without affecting muscle glycogen utilisation in type 1 diabetes. Diabetologia. 2007;50:414-421. [DOI] [PubMed] [Google Scholar]
- 37. MacDonald MJ. Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care. 1987;10:584-588. [DOI] [PubMed] [Google Scholar]
- 38. Hernandez JM, Moccia T, Fluckey JD, Ulbrecht JS, Farrell PA. Fluid snacks to help persons with type 1 diabetes avoid late onset postexercise hypoglycemia. Med Sci Sports Exerc. 2000;32:904-910. [DOI] [PubMed] [Google Scholar]
- 39. Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Handbook Clin Neurol. 2013;117:295-307. [DOI] [PubMed] [Google Scholar]
- 40. Diabetes Research in Children Network Study G, Tsalikian E, Tamborlane W, et al. Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care. 2009;32:1954-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of antecedent hypoglycemia on counterregulatory responses to subsequent euglycemic exercise in type 1 diabetes. Diabetes. 2003;52:1761-1769. [DOI] [PubMed] [Google Scholar]
- 42. Bao S, Briscoe VJ, Tate DB, Davis SN. Effects of differing antecedent increases of plasma cortisol on counterregulatory responses during subsequent exercise in type 1 diabetes. Diabetes. 2009;58:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murillo S, Brugnara L, Novials A. One year follow-up in a group of half-marathon runners with type-1 diabetes treated with insulin analogues. J Sports Med Phys Fitness. 2010;50:506-510. [PubMed] [Google Scholar]
- 44. Dencker M, Andersen LB. Accelerometer-measured daily physical activity related to aerobic fitness in children and adolescents. J Sports Sci. 2011;29:887-895. [DOI] [PubMed] [Google Scholar]
- 45. Dencker M, Thorsson O, Karlsson MK, et al. Daily physical activity and its relation to aerobic fitness in children aged 8-11 years. Eur J Appl Physiol. 2006;96:587-592. [DOI] [PubMed] [Google Scholar]
- 46. Dobkin BH. Wearable motion sensors to continuously measure real-world physical activities. Curr Opin Neurol. 2013;26:602-608. doi: 10.1097/WCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788-798. doi: 10.1177/1545968311425908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yilmaz T, Foster R, Hao Y. Detecting vital signs with wearable wireless sensors. Sensors (Basel). 2010;10:10837-10862. doi: 10.3390/s101210837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown RJ, Wijewickrama RC, Harlan DM, Rother KI. Uncoupling intensive insulin therapy from weight gain and hypoglycemia in type 1 diabetes. Diabetes Technol Ther. 2011;13:457-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care. 2013;36:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smart CE, Evans M, O’Connell SM, et al. Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care. 2013;36:3897-3902. doi: 10.2337/dc13-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bell KJ, Gray R, Munns D, et al. Estimating insulin demand for protein-containing foods using the food insulin index. Eur J Clin Nutr. 2014;9:126. [DOI] [PubMed] [Google Scholar]
- 53. Guerci B, Sauvanet JP. Subcutaneous insulin: pharmaco-kinetic variability and glycemic variability. Diabetes Metab. 2005;31:4S7-4S24. [DOI] [PubMed] [Google Scholar]
- 54. Maahs DM, Nadeau K, Snell-Bergeon JK, et al. Association of insulin sensitivity to lipids across the lifespan in people with type 1 diabetes. Diabet Med. 2011;28:148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Black LE, Swan PD, Alvar BA. Effects of intensity and volume on insulin sensitivity during acute bouts of resistance training. J Strength Cond Res. 2010;24:1109-1116. [DOI] [PubMed] [Google Scholar]
- 56. Bischof MG, Krssak M, Krebs M, et al. Effects of short-term improvement of insulin treatment and glycemia on hepatic glycogen metabolism in type 1 diabetes. Diabetes. 2001;50:392-398. [DOI] [PubMed] [Google Scholar]
- 57. Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J Clin Endocrinol Metab. 2004;89:4656-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Walsh J, Roberts R, Heinemann L. Confusion regarding duration of insulin action: a potential source for major insulin dose errors by bolus calculators. J Diabetes Sci Technol. 2014;8:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Admon G, Weinstein Y, Falk B, et al. Exercise with and without an insulin pump among children and adolescents with type 1 diabetes mellitus. Pediatrics. 2005;116:e348-e355. [DOI] [PubMed] [Google Scholar]
- 60. Hansen MV, Pedersen-Bjergaard U, Heller SR, et al. Frequency and motives of blood glucose self-monitoring in type 1 diabetes. Diabetes Res Clin Pract. 2009;85:183-188. [DOI] [PubMed] [Google Scholar]
- 61. Cauza E, Hanusch-Enserer U, Strasser B, et al. Continuous glucose monitoring in diabetic long distance runners. Int J Sports Med. 2005;26:774-780. [DOI] [PubMed] [Google Scholar]
- 62. Englert K, Ruedy K, Coffey J, Caswell K, Steffen A, Levandoski L. Skin and adhesive issues with continuous glucose monitors: a sticky situation [published online ahead of print April 24, 2014]. J Diabetes Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Joubert M, Reznik Y. Personal continuous glucose monitoring (CGM) in diabetes management: review of the literature and implementation for practical use. Diabetes Res Clin Pract. 2012;96:294-305. [DOI] [PubMed] [Google Scholar]
- 64. Iscoe KE, Davey RJ, Fournier PA. Is the response of continuous glucose monitors to physiological changes in blood glucose levels affected by sensor life? Diabetes Technol Ther. 2012;14:135-142. [DOI] [PubMed] [Google Scholar]
- 65. Facchinetti A, Sparacino G, Guerra S, et al. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Facchinetti A, Sparacino G, Cobelli C. Signal processing algorithms implementing the “smart sensor” concept to improve continuous glucose monitoring in diabetes. J Diabetes Sci Technol. 2013;7:1308-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lucini D, Zuccotti GV, Scaramuzza A, Malacarne M, Gervasi F, Pagani M. Exercise might improve cardiovascular autonomic regulation in adolescents with type 1 diabetes. Acta Diabetol. 2013;50:341-349. doi: 10.1007/s00592-012-0416-z. [DOI] [PubMed] [Google Scholar]
- 68. Cichosz SL FJ, Hejlesen OK, Tarnow L, Fleischer J. A novel algorithm for prediction and detection of hypoglycemia based on continuous glucose monitoring and heart rate variability in patients with type 1 diabetes [published online ahead of print March 31, 2014]. J Diabetes Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Acute, same-day effects of antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab. 2006;290:E1331-E1338. [DOI] [PubMed] [Google Scholar]
- 70. Sandoval DA, Davis SN. Metabolic consequences of exercise-associated autonomic failure. Exerc Sport Sci Rev. 2006;34:72-76. [DOI] [PubMed] [Google Scholar]
- 71. Galassetti P, Tate D, Neill RA, Richardson A, Leu SY, Davis SN. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290:E1109-E1117. [DOI] [PubMed] [Google Scholar]
- 72. Sandoval DA, Guy DL, Richardson MA, Ertl AC, Davis SN. Effects of low and moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes. Diabetes. 2004;53:1798-1806. [DOI] [PubMed] [Google Scholar]
- 73. Ertl AC, Davis SN. Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab Res Rev. 2004;20:124-130. [DOI] [PubMed] [Google Scholar]
- 74. Galassetti P, Mann S, Tate D, et al. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab. 2001;280:E908-E917. [DOI] [PubMed] [Google Scholar]
- 75. Kishore P, Gabriely I, Cui MH, et al. Role of hepatic glycogen breakdown in defective counterregulation of hypoglycemia in intensively treated type 1 diabetes. Diabetes. 2006;55:659-666. [DOI] [PubMed] [Google Scholar]
- 76. Walsh J, Roberts R, Bailey T. Guidelines for optimal bolus calculator settings in adults. J Diabetes Sci Technol. 2011;5:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jones MC. Therapies for diabetes: pramlintide and exenatide. Am Fam Physician. 2007;75:1831-1835. [PubMed] [Google Scholar]
- 78. Parkman HP, Fass R, Foxx-Orenstein AE. Treatment of patients with diabetic gastroparesis. Gastroenterol Hepatol (N Y). 2010;6:1-16. [PMC free article] [PubMed] [Google Scholar]
- 79. Ma J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis: diagnosis and management. Drugs. 2009;69:971-986. [DOI] [PubMed] [Google Scholar]
- 80. West DJ, Stephens JW, Bain SC, et al. A combined insulin reduction and carbohydrate feeding strategy 30 min before running best preserves blood glucose concentration after exercise through improved fuel oxidation in type 1 diabetes mellitus. J Sports Sci. 2011;29:279-289. [DOI] [PubMed] [Google Scholar]
- 81. Temple MY, Bar-Or O, Riddell MC. The reliability and repeatability of the blood glucose response to prolonged exercise in adolescent boys with IDDM. Diabetes Care. 1995;18:326-332. [DOI] [PubMed] [Google Scholar]
- 82. Silveira AP, Bentes CM, Costa PB, et al. Acute effects of different intensities of resistance training on glycemic fluctuations in patients with type 1 diabetes mellitus. Res Sports Med. 2014;22:75-87. [DOI] [PubMed] [Google Scholar]
- 83. Dube MC, Lavoie C, Weisnagel SJ. Glucose or intermittent high-intensity exercise in glargine/glulisine users with T1DM. Med Sci Sports Exerc. 2013;45:3-7. [DOI] [PubMed] [Google Scholar]
- 84. Campbell MD, Walker M, Trenell MI, et al. Large pre- and postexercise rapid-acting insulin reductions preserves glycemia and prevents early- but not late-onset hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2013;36:2217-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang M, Donaghue KC, Cho YH, Craig ME. Autonomic neuropathy in young people with type 1 diabetes: a systematic review. Pediatr Diabetes. 2013;14:239-248. [DOI] [PubMed] [Google Scholar]
- 86. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:1701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors. Diabetes Care. 2013;36:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ward WK, Castle JR, El Youssef J. Safe glycemic management during closed-loop treatment of type 1 diabetes: the role of glucagon, use of multiple sensors, and compensation for stress hyperglycemia. J Diabetes Sci Technol. 2011;5:1373-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013;185:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zisser H, Renard E, Kovatchev B, et al. Multicenter closed-loop insulin delivery study points to challenges for keeping blood glucose in a safe range by a control algorithm in adults and adolescents with type 1 diabetes from various sites. Diabetes Technol Ther. 2014;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789-1796. doi: 10.2337/dc13-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Finan DA, McCann TW, Jr, Rhein K, et al. Effect of algorithm aggressiveness on the performance of the Hypoglycemia-Hyperglycemia Minimizer (HHM) system. J Diabetes Sci Technol. 2014;18:1932296814534589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Doyle FJ, III, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37:1191-1197. doi: 10.2337/dc13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nimri R, Muller I, Atlas E, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes. 2014;15:91-99. [DOI] [PubMed] [Google Scholar]
- 97. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824-833. [DOI] [PubMed] [Google Scholar]
- 99. Schmidt S, Finan DA, Duun-Henriksen AK, et al. Effects of everyday life events on glucose, insulin, and glucagon dynamics in continuous subcutaneous insulin infusion-treated type 1 diabetes: collection of clinical data for glucose modeling. Diabetes Technol Ther. 2012;14:210-217. doi: 10.1089/dia.2011.0101. [DOI] [PubMed] [Google Scholar]
- 100. Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16:506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15:386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Turksoy K, Quinn LT, Littlejohn E, Cinar A. An integrated multivariable artificial pancreas control system. J Diabetes Sci Technol. 2014;8:498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Turksoy K, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive identification and control for artificial pancreas systems. IEEE Trans Biomed Eng. 2014;61:883-891. [DOI] [PubMed] [Google Scholar]
- 104. Stenerson M, Cameron F, Payne SR, et al. The impact of accelerometer use in exercise-associated hypoglycemia prevention in type 1 diabetes [published online ahead of print September 17, 2014]. J Diabetes Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Davey RJ, Jones TW, Fournier PA. Effect of short-term use of a continuous glucose monitoring system with a real-time glucose display and a low glucose alarm on incidence and duration of hypoglycemia in a home setting in type 1 diabetes mellitus. J Diabetes Sci Technol. 2010;4:1457-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peyser T, Dassau E, Breton M, Skyler JS. The artificial pancreas: current status and future prospects in the management of diabetes. Ann N Y Acad Sci. 2014;1311:102-123. [DOI] [PubMed] [Google Scholar]
- 107. Doyle FJ, III, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37:1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cobelli C, Renard E, Kovatchev B. The artificial pancreas: a digital-age treatment for diabetes. Lancet Diabetes Endocrinol. 2014;2:679-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Turksoy K, Cinar A. Adaptive control of artificial pancreas systems—a review. J Healthc Eng. 2014;5:1-22. [DOI] [PubMed] [Google Scholar]
- 110. Sherr JL, Cengiz E, Palerm CC, et al. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36:2909-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Harmer AR, Chisholm DJ, McKenna MJ, et al. Sprint training increases muscle oxidative metabolism during high-intensity exercise in patients with type 1 diabetes. Diabetes Care. 2008;31:2097-2102. doi: 10.337/dc08-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bevier WC, Fuller SM, Fuller RP, et al. Artificial pancreas (AP) clinical trial participants’ acceptance of future AP technology. Diabetes Technol Ther. 2014;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kohl LF, Crutzen R, de Vries NK. Online prevention aimed at lifestyle behaviors: a systematic review of reviews. J Med Internet Res. 2013;15:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Davies CA, Spence JC, Vandelanotte C, Caperchione CM, Mummery WK. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int J Behav Nutr Phys Act. 2012;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nakhasi A, Shen AX, Passarella RJ, Appel LJ, Anderson CA. Online social networks that connect users to physical activity partners: a review and descriptive analysis. J Med Internet Res. 2014;16:e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res. 2014;16:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hutchesson MJ, Collins CE, Morgan PJ, Callister R. An 8-week web-based weight loss challenge with celebrity endorsement and enhanced social support: observational study. J Med Internet Res. 2013;15:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bailey BW, McInnis K. Energy cost of exergaming: a comparison of the energy cost of 6 forms of exergaming. Arch Pediatr Adolesc Med. 2011;165:597-602. [DOI] [PubMed] [Google Scholar]
- 119. Graves LE, Ridgers ND, Williams K, Stratton G, Atkinson G, Cable NT. The physiological cost and enjoyment of Wii Fit in adolescents, young adults, and older adults. J Phys Act Health. 2010;7:393-401. [DOI] [PubMed] [Google Scholar]
- 120. Graf DL, Pratt LV, Hester CN, Short KR. Playing active video games increases energy expenditure in children. Pediatrics. 2009;124:534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miyachi M, Yamamoto K, Ohkawara K, Tanaka S. METs in adults while playing active video games: a metabolic chamber study. Med Sci Sports Exerc. 2010;42:1149-1153. [DOI] [PubMed] [Google Scholar]
- 122. Guderian B, Borreson LA, Sletten LE, et al. The cardiovascular and metabolic responses to Wii Fit video game playing in middle-aged and older adults. J Sports Med Phys Fitness. 2010;50:436-442. [PubMed] [Google Scholar]
- 123. El-Gayar O, Timsina P, Nawar N, Eid W. Mobile applications for diabetes self-management: status and potential. J Diabetes Sci Technol. 2013;7:247-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. 2013;15:e235. doi: 10.2196/jmir.588. [DOI] [PMC free article] [PubMed] [Google Scholar]