Abstract
Systems for continuous glucose monitoring (CGM) have been available for a number of years, and numerous clinical studies have been performed with them. Interestingly, in many of these studies patients with an increased risk of hypoglycemic events were excluded. In addition, in most studies subjects were using a pump for insulin delivery. Therefore our knowledge about the benefit of CGM in patients employing multiple daily injections (MDI) of insulin is limited, especially when it comes to a reduction in the risk of low glucose events in high-risk individuals. We are planning to run a 26-week randomized controlled study in Germany (HypoDE, Hypoglycemia in Deutschland) that is focused on evaluating if such a reduction can be observed in patients on MDI with an increased risk of low glucose events. In all, 160 patients will participate in the study, randomized into the intervention group and control group. Ideally one would study if the frequency of severe hypoglycemic events is different between both groups. However, this would require such a large sample size and study duration, so for pragmatic reasons we will use low glucose levels <55 mg/dl (measured by CGM) for at least 20 minutes as a risk marker for severe hypoglycemic events. The results from the HypoDE study shall help determine the advantage of using CGM in subjects with type 1 diabetes with an increased risk of low glucose events treated with MDI.
Keywords: insulin pumps, continuous glucose monitoring, hypoglycemic events, multiple daily injections
The evidence for continuous glucose monitoring (CGM) to improve HbA1c without increasing the risk of hypoglycemic events has been established. Yet, after more than a decade of availability, the evidence for usage of CGM to reduce the risk of hypoglycemic events (especially of severe hypoglycemic events [SH]) from a number of studies remains under debate.1-6 However, in many of these studies first generations CGM systems were used; the analytical performance, reliability, and usability of these systems were considerably worse than more recent CGM system generations. Although real-time CGM per se (that mean CGM only, without communicating with an insulin pump; subsequently the term CGM will be used in this sense) has been shown to reduce the amount of time a user spends at low glucose, it has not demonstrated to be effective in reducing such low glucose events in general and nocturnal events during sleep.5-8
In most studies performed to date, CGM systems were used by patients with type 1 diabetes that utilize continuous subcutaneous insulin infusion therapy (CSII) by means of insulin pumps, there is less data on patients using multiple daily injection (MDI) therapy by means of syringes or pens (Table 1). However, the majority of patients with type 1 diabetes around the world use MDI. Nevertheless, there are considerable differences between countries and age groups: The proportion of patients using MDI might be as low as 60-70% in the United States or it can be >90% in countries like the United Kingdom. It is of interest to note that there are no good publications available that provide reliable data backing up such numbers.9 Furthermore, only few studies have focused on hypoglycemia reduction, that is, their study design was adequate to show a reduction in hypoglycemia frequency with a sufficient study duration/sample size. In many of the CGM studies patients with an increased risk of hypoglycemic events or hypoglycemia unawareness were excluded from participating in the study.10-12 The number of studies which looked at patients with an increased risk of hypoglycemic events is rather small. However, in general the randomized controlled trials (RCTs) performed to date—along with observational studies/clinical practice—indicate that this complication is reduced by usage of CGM (see, eg, Pickup et al2). Nevertheless, the evidence from RCTs about the benefits of CGM usage in patients treated with MDI is scarce when it comes to hypoglycemic events. Although there is widespread reimbursement for CGM among commercial health plans in the United States for patients with type 1 diabetes, reimbursement challenges persist for this large patient group for example in Europe/Germany.
Table 1.
Publication in Which Results From Studies Are Presented That Study the Usage of CGM and Reduction of Hypoglycemic Events as 1 Outcome Parameter.
| Study/CGM system used | Significant inclusions/exclusions | Duration | Primary outcome | Secondary outcomes | Results | Comments |
|---|---|---|---|---|---|---|
| Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes62 Abbott Navigator |
Inclusion: HbA1c <7.5%, used pump or MDI N = 120 Control = 58 CGM = 62 |
26 weeks | Time spent in hypoglycemia (interstitial glucose concentration <63 mg/dL) | CGM data in both groups were used to estimate the amount of time per day the glucose level was hypoglycemic (<63 mg/dL, <70 mg/dL, or <55 mg/dL), hyperglycemic (>180 mg/dL or >250 mg/dL), and in the target range (70-180 mg/dL or 90-180 mg/dL) for each patient. The number of hypoglycemic excursions (<55 and <63 mg/dL) per day and separately during the night period of midnight to 6 am was also calculated. | Hypoglycemia was significantly shorter in the CGM group vs control group. CGM was associated with concomitant decrease in HbA1c. |
Used Navigator with marginal accuracy in the low glucose range. Record of SH in last year—control 12%, CGM 8%. Diagnosed with hypoglycemia unawareness—control 7%, CGM 10%. |
| The effect of continuous glucose monitoring in well-controlled type 1 diabetes7 Dexcom SEVEN MiniMed Paradigm RT Abbott Navigator |
Inclusion: HbA1c <7.0%, on pump or MDI N = 129 Control = 62 CGM = 67 |
26 weeks | Primary study outcomes: time with <70 mg/dL from baseline to 26 weeks | HbA1c level, and SH events (defined as an event that required assistance from another individual to administer carbohydrate, glucagon, or other resuscitative actions). | Time <70 mg/dL was less frequent in CGM group (not significantly different) Outcome measures combining HbA1c and hypo data favored CGM group vs control group |
10% in the control group and 11% in the CGM group had a SH (SH was defined as an event that required assistance from another person to administer carbohydrate, glucagon, or other resuscitative actions) in the preceding 6 months |
| Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens63 MiniMed Paradigm RT |
Inclusion: HbA1c >8.0% used CSII (N = 94) or MDI(N = 84) N = 178 Pt Led = 62 Physician Led = 55 Control = 61 |
1 year | Primary outcome: reduction in A1c from baseline to 12 months. | Improvement in glucose control, frequency of mild (defined as an SMBG value, 70 mg/dL or symptoms of low BG) and SH (defined as an event requiring assistance from another person). Changes in QoL Efficacy of CGM in CSII and MDI |
HbA1c improved in CGM groups vs control group | There were differences in baseline SH in the groups: patient-led 24.2%, physician-led 9.1%, and control group 8.2%. |
| The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomized controlled trial64 MiniMed Paradigm RT |
Inclusion: adults (N = 81) and pediatric (N = 72) Total N = 153 Age 6-70 years HbA1c 7.5-9.5%. Excluded: Subjects with >3 incidents of SH (episode requiring assistance from another person or neurological recovery in response to restoration of plasma glucose to normal) in the past 12 months or history of IAH. Must have used CSII for > 6 months. |
6 months in each cross over time period with a 4-month washout between | Primary outcome was difference in sensor on vs sensor off arms HbA1c after 6 months of treatment | Secondary outcomes: Change in glycemic pattern expressed by 24-hour glucose and 24-hour AUC. Time hypoglycemia (<3.9 mmol/l), time hyperglycemia (> 10 mmol/l), and time euglycemia (3.9-10 mmol/l). |
HbA1c improved with CGM use. Hypoglycemia time reduced with CGM use. Hyperglycemia time was reduced. Significance (P < .001) achieved for both of those |
CGM improved outcomes, control and time in range for adults and pediatric patients. Funding from MDT. |
| Sensor-augmented pump therapy lowers HbA1c in suboptimally controlled type 1 diabetes; a randomized controlled trial (Eurythmics Trial)65 MiniMed Paradigm RT |
Inclusion:HbA1c ≥8.2%;currently treated with optimized MDI T1DM Aged 18-65 years N = 83 Sensor-augmented pump = 44 MDI = 39 |
26 weeks | Primary outcome: assess change in HbA1c between baseline and 26 weeks, change between 13 and 26 weeks. Proportion of patients reaching <7% | Other outcomes: minutes in hypoglycemia (< 4.0 mmol/l or 72 mg/dL), number of hypoglycemia events/day (an event was counted when the sensor glucose value crossed the hyper- or hypoglycemia threshold, followed by a 30-minute period between 4.0 and 11.1 mmol/l) | SAP therapy significantly lowered HbA1c after 26 weeks by 1.21%, as compared with MDI. Not powered to detect differences in SH. |
Authors concerned that patients in SAP arm benefitted from increased time with training for use of SAP compared to MDI. |
| Glycemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomized controlled trial66 MiniMed Paradigm RT |
Inclusion:T1DM—age 13-40 years HbA1c <8.5% CSII for > 6 months with use of bolus calculator SMBG at least 4 × a day Willing to wear sensor > 70% of the time N = 62/31 SAP, 31 control Exclusion: History of SH (defined as an episode of hypoglycemia resulting in seizure or coma or requiring third-party assistance or the use of glucagon or intravenous glucose for recovery) on CSII |
3 months | Primary outcome: Difference of time in target range during study period (4-10 mmol/l) | Secondary outcome: Difference in HbA1c, time in hypoglycemic (≤3.9 mmol/l) and hyperglycemic ranges (≥10.1 mmol/l). Glycemic variability. | HbA1c reduced 0.43% but no reduction in time spent low was noted | No additional training to SAP group on use of CGM data to enhance therapy was provided. Included MARD testing of the CGM—17.5% hypo, 14.5% euglycemic, 9% hyperglycemic. Median use of CGM was 62.5% of study time. |
| Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes (Real Trend study)67 MiniMed Paradigm RT |
Inclusion: N = 132; adults (n = 81) and children (n = 51) HbA1c: >8% On MDI randomized to SAP (N = 55) or standard CSII (N = 60) |
6 months | Primary outcome: Change in HbA1c after 6 months compared to standard CSII in patients with poorly controlled HbA1c. |
Secondary outcomes: Mean glucose change and descriptive parameters for biochemical hyperglycemia (190 mg/dl) and hypoglycemia (70 mg/dl). Daily insulin use was also compared. | Significant drops in HbA1c in both groups. No between group significance. If only study completers considered, SAP had a significant change compared to CSII. | Significant dropouts—total completers per protocol was 91—largest dropout in SAP group (32/55 completed). |
Subsequently we present the study concept for the HypoDE study. The aim of this RCT is to demonstrate that usage of a stand-alone CGM system reduces the frequency of low CGM-recorded glucose episodes in patients using MDI with an increased risk for hypoglycemia.
Risk of Hypoglycemic Events in Patients With Type 1 Diabetes and Usage of CGM
Treatment guidelines for people with type 1 diabetes agree that glycemic control should be optimized to prevent diabetes complications without simultaneously increasing the risk of SH.13,14 Unfortunately tight glycemic control in people with type 1 diabetes has been reported to be associated with an elevated risk of SH,15-17 making the attainment of the above treatment goals challenging. However, a number of more recent studies have not confirmed the strong association between optimization of glycemic control and an increase in risk of SH in children, adolescents, and adults.18-24 Nevertheless, CGM might be a technical solution assisting the achievement of both treatment goals. It is of interest to note that in a recent publication no indication was found that CGM usage reduces SH, fear of hypoglycemia, hypoglycemia awareness, or even biochemical hypoglycemia.25 The issue with this study is the study design, that is, patients in the control group received much more attention (= higher number of visits) than they would get during usual care. Therefore, the number of visits between both study groups is balanced in our study as good as possible (see below).
A closer look to the prevalence of SH shows that the likelihood for SH is not equally distributed. In fact, data suggest the existence of risk groups of people with type 1 diabetes, who account for the majority of SH events.16,23,24,26,27 A key risk factor for SH in patients with type 1 diabetes is reduced hypoglycemia awareness. Depending on the definition of hypoglycemia and of hypoglycemia unawareness used, about 30% of patients with type 1 diabetes have impaired awareness of hypoglycemia.28 These patients are at a 3- to 6-fold greater risk for experiencing SH events.29 Clearly other risk factors like duration of diabetes, age and intensity of diabetes management are of relevance in this context as well. The most important risk factor for hypoglycemia unawareness and subsequent hypoglycemia problems in people with type 1 diabetes is a prior history of hypoglycemia.30
Frequent hypoglycemia is associated with a downshift of glycemic threshold for endocrine and symptomatic counterregulatory responses toward low blood glucose, causing hypoglycemia unawareness and subsequently an increase of hypoglycemia problems. Due to the downshift of glycemic thresholds for endocrine counterregulation, the effects of glucose deprivation of the central nervous system (neuroglycopenia) are often the first detectable symptoms of low blood glucose for the affected subject. Neuroglycopenia narrows the window of opportunity for an effective self-treatment of low blood glucose values, enhancing the risk of SH, which has to be treated by the assistance of a third person or medical assistance.31,32 Early detection and scrupulous avoidance of low glucose values can restore hypoglycemia awareness and reduce the risk of SH by an upshift of glycemic thresholds.33-35
A diagnostic tool like CGM has great potential for the treatment of people with type 1 diabetes and an increased frequency of SH, since it alerts the affected user if critical low glucose values are reached and prompt them to treat low glucose. The most recent versions of CGM systems alert their user even before low values are reached by means of algorithms that predict that such event would take place in the near future.36 It can be expected that avoidance of SH by usage of CGM contribute to an upshift of glycemic threshold for symptomatic and endocrine counterregulation. This may lead to an improvement of hypoglycemia awareness, and thereby reduce the risk for SH. Based on this assumption the beneficial effects of CGM on hypoglycemic events were studied right away from the beginning of the usage of CGM systems in practice and evaluating them in RCTs some 15 years ago. Unfortunately, as stated above, the potential of CGM use for the treatment of hypoglycemia problems was not systematically evaluated with RCTs designed to evaluate the benefit of CGM usage on hypoglycemic events as a primary endpoint in patient groups with hypoglycemia problems (Table 1). The results obtained in, for example, the Juvenile Diabetes Research Foundation (JDRF) CGM trial were mixed, that is, although they demonstrated benefits in improving glucose control with CGM use, they failed to show a reduction in (severe) hypoglycemic events.37 However, the trial was not sufficiently powered to detect a reduction in SH events. Nevertheless, in the 6-month continuation phase of the JDRF trial a 46% reduction in mild-moderate hypos (not severe) was observed.38 It is worth to mention that many subjects in the trial used MDI and had similar A1C reduction. However, the majority of subjects used CSII therapy.
Even in studies performed more recently with a focus on hypoglycemic events, using a current CGM system along with an insulin pump with low glucose suspend, the benefits of hypoglycemia event reduction were significant in some, but not all studies. The observed reduction of SH to zero reported in 1 study39 and the publication of this study in a highly respected journal was regarded as quite positive for this approach. However, from a critical point of view it has to be acknowledged that the randomization resulted in massive differences in the starting values of the 2 study groups. Without an adjustment for the starting values, the advantage is much less prominent. This study also shows that recruiting patients for such studies is difficult.
There are different iterations of CGM and a pump (sensor-enabled or sensor-augmented pumping) combinations that have been used in these studies. However, benefits seen with the usage of these more advanced systems, that pave the way toward a fully automated closed-loop system, cannot be attributed to CGM alone but to the combination of different technologies.
Reasons for Missing Evidence of CGM to Reduce the Frequency of Hypoglycemic Events
In view of the obvious advantages of CGM versus self-monitoring of blood glucose (SMBG) with respect to the amount of information provided about the current glucose control one might be surprised why no clear benefit has been demonstrated in previous RCTs.4,40 Reasons for this observation are the following:
- The hypoglycemia rate among patients entered into the trials was low. Due to the exclusion criteria used in a number of trials patients with an increased frequency of hypoglycemic events were not included.
- Alarms generated by the CGM systems when glucose levels decline to prespecified levels do not always induce appropriate reactions by the patients. If a CGM has resulted in frequent false positive alarms, patients may develop alert fatigue and ignore alarms.
- The alert is not heard. The volume and tones, and duration of the alerts differ between CGM systems. The alert volume can also be muffled under blankets. One study using GlucoWatch showed that patients sleep through 75% of the alarms.41 Only 1 manufacturer (Dexcom) has a device with a fixed low glucose alarm at 55 mg/dl that can be temporarily silenced, but will continue to alarm until the user acknowledges the alert or the glucose values rise above this threshold.
- Inaccuracies of the CGM systems used at the time most probably have contributed to failure to detect hypoglycemic events and/or false-positive low glucose readings. This can be from analytical error (median or mean absolute relative difference [MARD] has gone down from approximately 20% to 10% today, combined with a better analytical performance also in the low blood glucose range), tissue compression (from sleeping on a sensor), or physiologically induced by the delay between rapid glucose changes in interstitial fluid and blood.
- If CGM misses or fails to alert or the alert is delayed, the patient might be cognitively impaired by the time the patient is alerted.
Definition of a Low Glucose Event and Primary Endpoint
For the planned study a clear definition of a “low glucose event” is mandatory (Table 2). The discussion about the definition of such an event is as long as studies were performed in which differences in frequencies of hypoglycemic events were studied.13 The most straightforward definition for a SH event is this: requires help of third parties (which may or may not include transfer into a hospital). In the planned study, each event that requires assistance by another person is defined as SH and will be documented. Ideally each of these hypoglycemic events is confirmed by a conventional blood glucose measurement.
Table 2.
Definition of Hypoglycemic Events in the HypoDE Study.
| Event | Blood/CGM glucose | Comment |
|---|---|---|
| Hypoglycemia | <70 mg/dl | ADA definition from 200568 |
| Advanced hypoglycemia | <55 mg/dl | Neuroglycopenia symptoms, CGM system will provide a hypo alarm |
| Severe hypoglycemia | Definition based on 1 of 4 criteria: | |
| Help from a third party is needed | ||
| Glucagon or glucose administration | ||
| Unconsciousness | ||
| Seizures | ||
The primary endpoint of this study is evaluation of the hypothesis that CGM users experience a greater reduction in the total number of low glucose episodes (<55 mg/dl) that occur over the past 4 weeks of this study measured by CGM in patients in the CGM group versus the control group compared to baseline. We believe that this definition reflects the best compromise between different aspects: Our choice of definition (glucose levels <55 mg/dl) was motivated by findings that SH is rarely observed at glucose values >55 mg/dl. Since severe neuroglycopenia has the potential to endanger patients during daily activities (eg, driving a vehicle) or could hamper self-treatment of the hypoglycemic episodes, the definition of glucose values <55 mg/dl is of clinical significance.42-44 Therefore we choose this definition.
The frequency of low glucose events (= primary endpoint) will be regarded as a surrogate marker for SH. The frequency is defined as CGM measured glucose values <55 mg/dl for at least 20 minutes with a minimum of 3 sensor recordings (to account for missed values); subsequent events require a minimum of 30 minutes with glucose values >55 mg/dl (at least 5 sensor values) before another event is counted. This evaluation will be done automatically by means of special software.
The rationale for selecting low glucose values (advanced hypoglycemia) as a surrogate marker for SH is this:
- SH events are the clinical endpoint of greatest interest to a payer; however, the use of a SH as a primary outcome lacks feasibility in a clinical trial. The occurrence rate is so low in patients with diabetes, even in high-risk populations, that the required sample size is unfeasibly large and/or the trial would need to be extremely long.45
- Neuroglycopenic symptoms and cognitive impairments usually occur at advanced hypoglycemia and not at those levels commonly used to diagnose/define hypoglycemia (70 mg/dl).
- Even with appropriate carbohydrate intake to treat hypoglycemia, glucose may continue to fall until the carbohydrates are absorbed and result in transiently advanced hypoglycemia. Glucose value <55 mg/dl or severe neuroglycopenia has the potential to limit self-treatment of low glucose46 and to increase the risk for helplessness, disorientation, or seizure and coma requiring medical or third-party assistance for recovery from hypoglycemia.
- The duration for which glucose has to be below the selected threshold was also used in the ASPIRE study.47
Secondary Endpoints
Patients in both study groups will be instructed to document (advanced) hypoglycemic or SH events during the study duration as close as possible; they will be asked to note the date, time, symptoms, measured blood glucose values, and treatment administered (self-reported recall of episodes). Each patient will receive a diary that allows him or her to note each event occurring from the screening visit through the final study visit.
A number of secondary endpoints will be evaluated, but no combined endpoint:
- Number of low (<55 mg/dl) glucose values during night/day
- Duration of low glucose values/time spent in hypoglycemia
- Number of low glucose values using other limits (eg, 70 mg/dl, AUCs)
- Frequency of low glucose values that requires third-party intervention such as glucagon/glucose administration or emergency services and/or hospitalization
- Change in HbA1c
- CGM glycemic measures: time in range (70-180 mg/dl), time in hyperglycemic range (>180 mg/dl), variability measures (including risk measures like low blood glucose index)
- SMBG measurement frequency
- Patient-related outcomes/QoL surveys
- Hypoglycemia unawareness scale (HUS)
- Diabetes Distress Scale (DDS)
- Hypoglycemia Fear Survey (HFS-II)
- Satisfaction with CGM (CGM-Sat)
- Quality of life (EQ5D)
- Diabetes Treatment Satisfaction Questionnaire (DTSQ)
Study Logistics
This study is designed and will be designed, executed, analyzed, and published as an investigator-initiated trial (IIT); the official investigator is the Working Group for Diabetes Technology (AGDT) of the German Diabetes Association (DDG). Performance of this study is supported by an unrestricted research grant by 1 large manufacturer of CGM systems (Dexcom). For planning and supervision of the study a Steering Committee was established: The 4 German members of this committee (a clinician, a practitioner, a psychologist, and a scientist who acts as coordinator of this study) have profound background in this type of research; the 2 US members of the committee (a diabetologist and a PhD with experience in health economics) also have pertinent expertise in study design and use of CGM and are employees of the company providing the grant for this study. The study will be performed by an experienced CRO.
Selection of Patients
As hypoglycemic events, especially SH events, are relatively infrequent and unpredictable, the sample size required for such study to demonstrate a statistical reduction in such a clinical endpoint would be large and the study duration excessive long.48 This is most probably the reason why no such study has been performed to date. Therefore, this study focuses on patients with an expected increased frequency of hypoglycemic events and uses a biochemical measurement (frequency of CGM measured glucose values <55 mg/dl) as a surrogate for SH as a primary endpoint. Subjects will be preselected according to their clinical history, that is, this study will focus on patients who have a high baseline frequency of self-reported hypoglycemic events, including SHs.
The physicians who performs the recruitment examination with potential study participants shall use their clinical judgment to decide if this subject might fit or not into this study. To support this decision, hypoglycemia unawareness scores (HUS) will be used. This score allows evaluating the extent that awareness of hypoglycemia is impaired.
Study Design and Study Duration
In this prospective, un-blinded, randomized, parallel, controlled study the utility of CGM use in patients with type 1 using MDI that are at an increased risk for low glucose values will be evaluated (Figure 1, Table 3). Appropriate patients will be identified in phase 0 based on their clinical history of an increased risk of hypoglycemic events and enrolled/randomized prior to phase 1 (at visit 1). Subjects will be randomized to either CGM usage (intervention group, called CGM group subsequently) or usual care (control group). In this study a current Dexcom CGM system will be used, this system has a CE mark and is available on the market in Germany and can be used either real-time by patients or can mask the CGM data to patients and allow use as a data collection device. Patients will be trained appropriately (see below) in CGM usage for MDI patients depending on the group they were randomized to.
Figure 1.
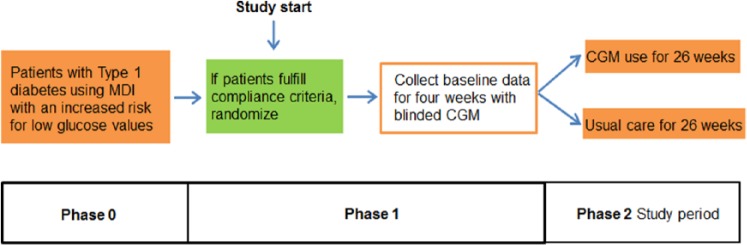
HypoDE—study design.
Table 3.
Study Visits.
| Phase 0 (prestudy) |
| Visit 0: Informed consent, baseline HbA1c measurement, clinical history/complete PRO/QoL/collect concurrent meds questionnaires, optimization of treatment, review applicable inclusion and exclusion criteria, if possible, randomize to RT-CGM or continue usual care (T = 0) and start study. |
| Phase 1 (baseline evaluation) |
| Visit 1: Sometime after visit 0 patients will use blinded CGM for 4 weeks to obtain baseline data and to evaluate compliance with using CGM. Patients in both groups will participate in a short CGM training course to be able to calibrate the device. |
| Phase 2 (study performance) |
| Visit 2: Patients who fulfill adherence criteria (eg, 80% wear time of the CGM system during baseline evaluation, calibrate appropriately) will continue in the study. Patients randomized to the CGM group will start their CGM training course. Patients randomized to the control group will continue their usual treatment. Check compliance with any visit: at least 6 CGM days per week in the CGM group and at least 4 SMBGs/per day in the control group. |
| Visit 3 (1 week [±3 days] after randomization/start of CGM): Patients from intervention group return to the site to check therapy (only visit that is different between both groups). |
| Visit 4 (after 4 weeks [±4 days]): Download CGM and SMBG devices and review data in both groups. |
| Visit 5 (after 12 weeks [±4 days]): Download CGM and SMBG devices and review data, HbA1c measurement in both groups. |
| Visit 6 (after 26 weeks [±4 days]): Download all CGM and SMBG devices, HbA1c measurement, administer PRO/QoL surveys. |
| Blinded CGM will be deployed to the control group after 22 weeks (±4 days) without any further interaction. Additional study procedures/phone calls are allowed to keep subjects in the study, to support patients in optimal CGM usage, provide them with study material and documentation of hypoglycemic events. Assessment of AEs (device, study, or disease-related) at each visit. |
To collect baseline data of the frequency of low glucose events in the patients in the 2 groups, blinded CGM recordings will be recorded in phase 1 for 4 weeks. This will also allow evaluating their compliance with CGM usage and willingness to use CGM. It is assumed that each patient has at least 1 event of low glucose per week (4 events/4 weeks) on average.
During phase 2, subjects in the control group will wear blinded CGM for a 4-week period in weeks 22 to 26 to allow evaluation if the frequency of low glucose values is different between the 2 groups. Subjects in the interventional group will use CGM data as part of their diabetes management during phase 2. This study should have a sufficient study duration (26 weeks in phase 2, 30 weeks in total) to minimize the Hawthorne effect/study effect. For a given subject the study duration will be 6 months; the total study duration will be approximately 15 months.
Patients will receive monthly phone calls to assess excessive health care resource utilization, including moderate and SH. The aim of this approach (mixture of visits and phone calls is) to have a good balance between safety of subjects in a study (also to keep them interested and thereby mitigate loss of retention) and the usual treatment of patients in Germany.
At the end of the study subjects in the control group will get a CGM system plus sensors as an incentive for their study participation; patients in the CGM group can keep their systems. A systematic follow-up of all study participants 12 months after study completion with respect to a subset of outcome parameter is intended.
It is clinical reality in Germany that many potential participants have participated in a hypoglycemia training program before entering the study. In a sense such a training program is standard of care. It will be offered to all patients who fit into the study. Depending on the clinical programs available at the given study site, patients may or may not participate in this training.
Differences Between Study Groups
Patients will receive ongoing advice regarding their individual diabetes treatment only by their treating physician and the diabetes team. The patients in the CGM group can contact the local distributor of the CGM system in Germany in case they require technical assistance on the CGM device. Patients in both study groups will be instructed to continue their usual self-monitoring of blood glucose (SMBG) schedule. However, there are distinct differences between the 2 groups in a number of other aspects:
- As CGM as a diagnostic tool has no direct impact per se on the primary endpoint but requires that the patients themselves modify their behavior, it is crucial that the behavioral change that is needed for optimal usage of CGM is provided with training and that the patients know how to self-manage their glucose control actively. Thus, participation in a CGM training program with 3 sessions of 1.5-hour duration is important for an effective usage of CGM (for details, see Table 3).
- Patients will be instructed to use the direction and rate of change of glycemia (as shown by the trend arrow) to adjust diabetes management decisions immediately. In addition, alert levels in the CGM system will be set by the treating physician at the study site and modified throughout the study to maximize benefit and to minimize subject nuisance. An alarm level of 75 mg/dl will be used to allow the patients to react in due time to avoid a hypoglycemic event. Assuming a decline in glycemia of 1 mg/dl/min this provides 20 minutes for absorption of glucose to bring glycemia up again. No algorithm will be used for this response, and clinicians can deviate from this alert level in a given patient according to their own judgment.
- Patients will be encouraged to review their CGM data via computer downloads on a regular basis to detect patterns of hypoglycemia that occur over time and facilitate treatment modifications.
- Patients in the control group will also undergo a minimum training to be able to calibrate the blinded CGM system during the recordings weeks.
- A critical aspect is to make sure that the patients in the control group continue to get “usual care.” The number of visits/patients contacts in both groups is as low as this appears to be acceptable from a clinical point of view. As no monthly contacts with the patients in the CGM group are planned, the adherence of the patients to CGM usage could not be evaluated as the CGM receiver holds data for 30 days only. However, at least for 3 months (in the 4 weeks before visits 4, 5, and 6) such an evaluation can be performed.
Economic Aspects
As treatment of hypoglycemic events can become costly if hospitalization is required, the data collected in this study will be used to evaluate the health economic benefits of CGM, that is, a cost effectiveness analysis will be performed. Although the study is not powered to demonstrate a reduction in SH events requiring hospitalization, the assumption is that reduction of low glucose events by using CGM may lead to reduction of costs for hypoglycemic treatment. In study self-reported health utilization will be tracked, for example, emergency room, hospital visits, loss of working hours, hours of absenteeism, and so on. Also equipment costs, office visits, phone calls, education visits, use of glucagon at home will be documented.
Quality of Life
Patient-reported outcomes (PROs) assessing diabetes distress using the Diabetes Distress Scale (DDS),49 fears of hypoglycemia using the Hypoglycemia fear survey-II (HFS-II),50 diabetes treatment satisfaction with the DTSQ questionnaire,51 satisfaction with CGM use using the CGM satisfaction questionnaire (CGM Sat),52,53 and generic aspects of quality of life using the EQ5D54 and the Hypoglycemia unawareness scale (HUS)55 will be obtained at the beginning and end of the study when appropriate.
Inclusion/Exclusion Criteria
Patients with overt nephropathy, longer duration of diabetes, lipohypertrophy, anti-insulin antibodies and more entrenched health beliefs that may predispose to hypoglycemia will not be excluded. The aim is to enrich the study with such patients who represent high-risk patients who might benefit of CGM usage.
Inclusion criteria for the patients to be enrolled are the following:
- Type 1 diabetes for at least 12 months
- Age 18-70 years
- HbA1c ≤9.0%
- MDI is defined as prandial insulin injections at each major meal (excludes premixed insulin) with doses determined by SMBG/carbohydrate counting and basal insulin injections
- Increased risk for low glucose events (defined as a score of 4 or higher on a HUS or a history of at least 1 SH event in the last 12 months)
- Have access to a computer with the necessary software
- Willing to not use paracetamol (acetaminophen) or drugs containing it continuously
Exclusion criteria are these:
- Use of personal real-time-CGM 6 months prior to study entry (professional CGM use (= episodic use of CGM as provided by HCP and owned by physician’s practice) is allowed, whether blinded or unblinded)
- Alcoholism or drug abuse
- Unable to comply with the protocol to the investigators discretion, such as known psychiatric diagnosis, cognitive/physical decline
- Pregnancy
Sample Size, Randomization
One hundred sixty subjects will be included in this trial in total. This sample size calculation is based on the assumption that CGM use, compared to the control group can reduce the incidence of “<55 mg/dl glucose events” by 0.5 standard deviations. To demonstrate a significant effect of CGM use with a statistical power of 1 – ß = 0.80 and a 2-sided error of α = 0.05 a group size of 64 participants per treatment group is needed. Anticipating a drop-out rate of 20%, 80 patients per group shall be included, that is, 160 patients in total. Drop-outs can be patients who withdrew their consent or are lost to follow-up.
This reduction has to be demonstrated in comparison of the CGM recordings during the baseline period of 4 weeks versus the recordings during weeks 22-26. It is assumed that there will not be a similar reduction observed in the usual care group. The recording during the run-in phase will be helpful in confirming how variable the number of events with low glucose levels is across the study population. Patients in both groups should wear the CGM system in these recording phases for >85% of the time (or 6 of 7 days). If a given patient have reasons for not be able to do this in this time frame, 1 additional week of wearing may be allowed. Subjects will be regarded as drop-outs if noncompliant. The investigator and team will assess subject’s eligibility to continue in the study based on adherence to CGM and study procedures. They should perform a minimum of 3 finger sticks daily—which includes 2 daily calibrations. Also during the course of the study participants should avoid use of paracetamol-containing medications during study performance as this has an impact on the CGM measurement result.
Patients will be randomized with an individual randomization stratified by per center. The randomization is done centrally. A randomization sequence will be generated using SYSTAT 12.0 (Systat Software, Inc, Chicago, IL) with a 1:1 allocation; the study center will be a stratifying variable. Each center will receive a sealed envelope per patient with the randomized treatment allocation.
Study Sites
The study will be performed by a number of specialized diabetes practices and clinical centers in Germany. All sites have experience with study performance and usage of CGM; that is, they have >5 patients with type 1 diabetes that are currently treated with CGM and >25 patients who potentially fit into this study. In addition, they have a diabetes team that is interested and experienced in CGM. Each site should be able to recruit and enroll at least 15 patients within 4 month, with a maximal number of 30 patients per site. Thereby the number of sites needed should be <15 sites.
Data Download
The data in the CGM system and glucose meter used by the study participants will be downloaded at each study visit. The patients shall continue to use their individual blood glucose meter; there will be no “study meters.” However, in case a patient uses a meter that is shown in respective evaluation studies to have an insufficient measurement quality, this might be switched to one with an adequate quality.56
Statistics
A statistical analysis plan (SAP) will be established for this study. In this it will also be described in detail how the study will be analyzed (Intention to treat/per protocol).
Quality of CGM
It is clear that the quality of the CGM system used is crucial for the success of such study, that is, the sensor used must be able to reliably measure glucose values in the range <70 mg/dl. It is also clear, that the CGM systems that were used when this technology was introduced to the market were not able to fulfill this requirement. Even more recent CGM systems had issues when it comes to lower glucose levels.57 However, the performance of some current CGM systems in the hypoglycemic range has significantly improved: The CGM system that will be used in this study will include the Dexcom Gen 4 Platinum sensor and a receiver with the modified 505 algorithm (G4); this is an improved version of the G4 system currently used in Germany. For the G4 system it has been shown that the evidence for detection of hypoglycemic events is 88% detection at 70 mg/dl (without delay), with the modified G4algorithm this goes up to 95%.58 The mean absolute difference (MAD) at BG <70 mg/dL was 6.4 mg/dl.59 However, at the time of this publication, this system has not yet been evaluated in a head-to-head comparison to other CGM systems. The receiver of the CGM system holds the data until the subjects return for a study visit, regardless of whether the receiver was charged or not.
Summary
In the past 15 years numerous clinical trials with CGM have been performed. However, there remain many important unanswered questions. The HypoDE study will increase our understanding of the value shall help to understand the advantage of using CGM in subjects with type 1 diabetes with an increased risk of low glucose events treated with MDI. To our knowledge this study will be the first RCT to selectively recruit such a patients group. With the study design chosen it should be possible to evaluate if CGM use has such an impact, that coverage with evidence determination (CED) for CGM per se can be achieved. The study will not answer the questions if all patients treated with MDI (but have no increased risk of low glucose events) benefit from CGM usage; however, it can help to understand to what extent this might be the case. The results of this study will also help answering the question for health technology assessment organizations and for payers: If there is a limited budget, what is more important, CGM or CSII?60,61
Footnotes
Abbreviations: AGDT, Working Group for Diabetes Technology; CED, coverage with evidence determination; CGM, continuous glucose monitoring; CGM-Sat, satisfaction with CGM; CRO, clinical research organization; CSII, continuous subcutaneous insulin infusion; DDG, German Diabetes Association; DDS, Diabetes Distress Scale; DTSQ, Diabetes Treatment Satisfaction Questionnaire; EQ5D, quality of life; HFS-II, Hypoglycemia Fear Survey; HUS, hypoglycemia unawareness score; HypoDE, Hypoglycemia in Deutschland; IIT, investigator-initiated trial; JDRF, Juvenile Diabetes Research Foundation; MAD, mean absolute difference; MARD, median or mean absolute relative difference; MDI, multiple daily injections; PRO, patient-reported outcome; QoL, quality of life; RCT, randomized controlled trial; SAP, statistical analysis plan; SH, severe hypoglycemic events; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LH holds shares in the Profil Institute for Metabolic Research, Neuss, Germany, and the Profil Institute for Clinical Research, San Diego, USA. LH is consultant for a range of companies that develop new diagnostic and therapeutic options for the treatment of diabetes. LH receives support by Dexcom for preparing the HypoDE study. DD received speaker honoraria from Dexcom, Lilly, Novo Nordisk, and Medtronic. NH is member of the Global Diabetes Educator Advisory Board of Eli Lilly and the Global DAWN 2 Study International publication committee. He is also member of the national advisory board of DAWN 2 (Novo Germany), Abbott, Germany, and the Hypode study (Dexcom). He receives speaker honoraria from Berlin Chemie, Astra Zenecca, Novo Nordisk, Germany. CG is an employee of Dexcom Inc and holds shares in the company. AL is advisor for and receives speaker honoraria or research support from Dexcom, Roche, Medtronic, Becton Dickinson, Astra Zeneca, Boehringer Ingelheim, MSD, Novo Nordisk, Sanofi, Eli Lilly. DP is an employee of Dexcom Inc and holds shares in the company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dexcom supports this investigator-initiated trial with an unrestricted research grant. This study will be supported by an unrestricted research grant by Dexcom, San Diego. This study will be sponsored by an unrestricted research grant by Dexcom Inc.
References
- 1. Langendam MW, Luijf YM, Hooft L, DeVries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;1:CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Facchinetti A, Sparacino G, Guerra S, et al. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinemann L, DeVries JH. Evidence for continuous glucose monitoring: sufficient for reimbursement? Diabet Med. 2014;31:122-125. [DOI] [PubMed] [Google Scholar]
- 5. Floyd B, Chandra P, Hall S, et al. Comparative analysis of the efficacy of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes mellitus. J Diabetes Sci Technol. 2012;6:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golden SH, Sapir T. Methods for insulin delivery and glucose monitoring in diabetes: summary of a comparative effectiveness review. J Manag Care Pharm. 2012;18:S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck RW, Hirsch IB, Laffel L, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311-320. [DOI] [PubMed] [Google Scholar]
- 9. White HD, Goenka N, Furlong NJ, et al. The UK service level audit of insulin pump therapy in adults. Diabetic Med. 2014;31:412-418. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10:377-383. [DOI] [PubMed] [Google Scholar]
- 11. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824-833. [DOI] [PubMed] [Google Scholar]
- 13. ADA. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:S11-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Böhm BO, Dreyer M, Fritsche A, Füchtenbusch M, Gölz S, Martin S. Therapie des Diabetes mellitus Typ 1 (S3-Leitlinie). 2011. Available at: http://www.awmf.org/uploads/tx_szleitlinien/057-013l_S3_Therapie_des_Typ_1_Diabetes_2012-03.pdf. Accessed June 30, 2014.
- 15. DCCT Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968-983. [PubMed] [Google Scholar]
- 16. DCCT Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271-286. [PubMed] [Google Scholar]
- 17. Maltoni G, Zucchini S, Scipione M, et al. Severe hypoglycemic episodes: a persistent threat for children with type 1 diabetes mellitus and their families. J Endocrinol Invest. 2013;36:617-621. [DOI] [PubMed] [Google Scholar]
- 18. Fredheim S, Johansen A, Thorsen SU, et al. Nationwide reduction in the frequency of severe hypoglycemia by half [published online ahead of print December 21, 2014]. Acta Diabetol. [DOI] [PubMed] [Google Scholar]
- 19. Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c Levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLOS Med. 2014;11:e1001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Connell SM, Cooper MN, Bulsara MK, Davis EA, Jones TW. Reducing rates of severe hypoglycemia in a population-based cohort of children and adolescents with type 1 diabetes over the decade 2000-2009. Diabetes Care. 2011;34:2379-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherubini V, Pintaudi B, Rossi MC, et al. Severe hypoglycemia and ketoacidosis over one year in Italian pediatric population with type 1 diabetes mellitus: a multicenter retrospective observational study. Nutr Metab Cardiovasc Dis. 2014;24:538-546. [DOI] [PubMed] [Google Scholar]
- 22. Peterson A, Hanberger L, Akesson K, Bojestig M, Andersson GB, Samuelsson U. Improved results in paediatric diabetes care using a quality registry in an improvement collaborative: a case study in Sweden. PLOS ONE. 2014;9:e97875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. J Clin Endocrinol Metab. 2013;98:3411-3419. [DOI] [PubMed] [Google Scholar]
- 24. Cooper MN, O’Connell SM, Davis EA, Jones TW. A population-based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia. 2013;56:2164-2170. [DOI] [PubMed] [Google Scholar]
- 25. Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37:2114-2122. [DOI] [PubMed] [Google Scholar]
- 26. Bott S, Bott U, Berger M, Mühlhauser I. Intensified insulin therapy and the risk of severe hypoglycaemia. Diabetologia. 1997;40:926-932. [DOI] [PubMed] [Google Scholar]
- 27. Schiel R, Müller UA. Die Stoffwechselqualität von insulinbehandelten Patienten mit Diabetes mellitus einer mitteldeutschen Stadt 1989/90 bis 1999/2000. Die JEVIN Studie. Medizinische Klinik. 2003;98:303-312. [DOI] [PubMed] [Google Scholar]
- 28. Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2008;25:501-504. [DOI] [PubMed] [Google Scholar]
- 29. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697-703. [DOI] [PubMed] [Google Scholar]
- 30. Nathan D. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750-756. [DOI] [PubMed] [Google Scholar]
- 32. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013;369:362-372. [DOI] [PubMed] [Google Scholar]
- 33. Fanelli CG, Epifano L, Rambotti AM, et al. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes. 1993;42:1683-1689. [DOI] [PubMed] [Google Scholar]
- 34. Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet. 1994;344:283-287. [DOI] [PubMed] [Google Scholar]
- 35. Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426-1434. [DOI] [PubMed] [Google Scholar]
- 36. Danne T, Tsioli C, Kordonouri O, et al. The PILGRIM study: in silico modeling of a predictive low glucose management system and feasibility in youth with type 1 diabetes during exercise. Diabetes Technol Ther. 2014;16:338-347. [DOI] [PubMed] [Google Scholar]
- 37. JDRF. Randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10:310-321. [DOI] [PubMed] [Google Scholar]
- 38. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310:1240-1247. [DOI] [PubMed] [Google Scholar]
- 40. Heinemann L, DeVries JH. Evidence for continuous glucose monitoring: sufficient for reimbursement? Diabet Med. 2014;31:122-125. [DOI] [PubMed] [Google Scholar]
- 41. Buckingham B, Block J, Burdick J, et al. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7:440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maran A, Lomas J, Macdonald IA, Amiel SA. Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia. 1995;38:1412-1418. [DOI] [PubMed] [Google Scholar]
- 43. Amiel SA, Pottinger RC, Archibald HR, et al. Effect of antecedent glucose control on cerebral function during hypoglycemia. Diabetes Care. 1991;14:109-118. [DOI] [PubMed] [Google Scholar]
- 44. Deary IJ. Symptoms of hypoglycaemia and effects on mental performance and emotions. In: Frier BM, Fisher M, eds. Hypoglycaemia in Clinical Diabetes. 2nd ed. Chichester, UK: John Wiley; 2007:25-48. [Google Scholar]
- 45. Beck RW, Kollman C, Xing D, Buckingham BA, Chase HP. Outcome measures for outpatient hypoglycemia prevention studies. J Diabetes Sci Technol. 2011;5:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fanelli C, Pampanelli S, Epifano L, et al. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37:797-807. [DOI] [PubMed] [Google Scholar]
- 47. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224-232. [DOI] [PubMed] [Google Scholar]
- 48. Beck RW, Buckingham B, Miller K, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. [DOI] [PubMed] [Google Scholar]
- 50. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care. 2011;34:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bradley C. Diabetes Treatment Satisfaction Questionnaire (DTQS). In: Bradley C, ed. Handbook of Psychology and Diabetes. Amsterdam, Netherlands: Harwood; 1994:111-132. [Google Scholar]
- 52. DirecNet Study Group. Youth and parent satisfaction with clinical use of the GlucoWatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care. 2005;28:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hermanns N, Kulzer B, Gulde C, et al. Short-term effects on patient satisfaction of continuous glucose monitoring with the GlucoDay with real-time and retrospective access to glucose values: a crossover study. Diabetes Technol Ther. 2009;11:275-281. [DOI] [PubMed] [Google Scholar]
- 54. Greiner W, Claes C. Der EQ-5D der EuroQoL-Gruppe. In: Schöffski O, Graf v.d., Schulenburg JM, eds. Gesundheitsökonomische Evaluationen. Heidelberg, Germany: Springer; 2007:403-414. [Google Scholar]
- 55. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517-522. [DOI] [PubMed] [Google Scholar]
- 56. Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6:1060-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zijlstra E, Heise T, Nosek L, Heinemann L, Heckermann S. Continuous glucose monitoring: quality of hypoglycaemia detection. Diabetes Obes Metab. 2013;15:130-135. [DOI] [PubMed] [Google Scholar]
- 58. Garcia A, Rack-Gomer AL, Bhavaraju NC, et al. Dexcom G4AP: an advanced continuous glucose monitor for the artificial pancreas. J Diabetes Sci Technol. 2013;7:1436-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bailey T, Nakamura K, Chang A, Christiansen M, Price D, Balo A. CGM is not a limiting factor in artificial pancreas systems. Diabetes. 2014;63(suppl 1):A18. [Google Scholar]
- 60. Moreno-Fernandez J, Gomez FJ, Gazquez M, et al. Real-time continuous glucose monitoring or continuous subcutaneous insulin infusion, what goes first? Results of a pilot study. Diabetes Technol Ther. 2013;15:596-600. [DOI] [PubMed] [Google Scholar]
- 61. Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336-347. [DOI] [PubMed] [Google Scholar]
- 62. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riveline JP, Schaepelynck P, Chaillous L, et al. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care. 2012;35:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hermanides J, Norgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled type 1 diabetes: a randomized controlled trial. Diabet Med. 2011;28:1158-1167. [DOI] [PubMed] [Google Scholar]
- 66. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52:1250-1257. [DOI] [PubMed] [Google Scholar]
- 67. Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32:2245-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes. Diabetes Care. 2005;28:1245-1249. [DOI] [PubMed] [Google Scholar]


